Introduction
Atherosclerosis is the main underlying cause of many
cardiovascular diseases, and has seriously harmful effects on human
health (1,2). As living standards improve, the
incidence of cardiovascular and cerebrovascular disease is
increasing. Atherosclerosis is a most important and commonly
observed vascular disease, with the characteristic of initiation
from the intima in the affected artery (3). Lesion development usually involves the
initial accumulation of lipids and complex carbohydrates, followed
by bleeding, thrombus formation, fibrosis and calcinosis (4). Gradually, the artery becomes calcified
and the elasticity of medium and large arteries is lost (5,6). Once
the lesions have developed to a size large enough to block the
arterial lumen, the tissues or organs that are supplied by the
arteries become ischemic or necrotic (7,8). There
are several hypotheses for the cause of atherosclerosis, including
lipid infiltration, smooth muscle cell (SMC) proliferation, and the
thrombosis source theory (9–11). However, to date, the pathogenesis of
atherosclerosis has not been clearly explained and clarified.
However, investigation of the mechanism has revealed that
atherosclerosis is always accompanied by an inflammatory response
during the progression of the disease. Thus, Ross proposed that
atherosclerosis is an inflammatory disease (12,13).
Apolipoprotein E (ApoE) is a glycoprotein that was
first identified in 1973 as a constituent of very-low-density
lipoprotein (VLDL) in normal humans. It is a single peptide of 299
amino acids with a molecular weight of 34 kDa, and has subsequently
been found in all lipoprotein classes (14–16).
ApoE is an important component of plasma apolipoprotein. In humans,
ApoE is mainly present in VLDL, low-density lipoprotein (LDL),
chylomicrons (CMs) and CM remnants. It is present in the subclasses
of β-VLDL and high-density lipoprotein. In 1992, Shimano et
al first established a transgenic mouse expressing rat ApoE, in
which a >4-fold higher level of plasma ApoE, and significantly
lower concentrations of VLDL and LDL cholesterol were observed,
when compared with normal mice (17,18).
Moreover, the animals with a high concentration of ApoE exhibited
an alleviation of high-fat diet-induced hypercholesterolemia.
Conversely, the ApoE knock-out mouse model was also established in
1992 (19,20). Mice homozygous for the ApoE mutation
can normally survive and have reproductive ability. If
ApoE-deficient mice are fed with a high-fat diet, the level of
cholesterol can become elevated to as high as 46.5 mmol/l in the
plasma, mainly distributed in VLDL and intermediate-density
lipoproteins, suggesting that the mice seriously lack the
cholesterol-removing ability of ApoE lipoprotein and the ApoE
receptor-binding function (21,22).
Atherosclerotic plaques undergo a >3-fold increase in size when
a high-fat diet is administered for >1 month (22). The pathological features and
histological distributions in these model mice are very similar to
those in human atherosclerotic disease, which suggests that ApoE
plays an important role in the pathogenesis of coronary heart
disease in humans (23,24). Thus, in the present study, ApoE
knock-out mice were used as a model of atherosclerosis in which to
investigate the effects of triptolide (TPL) on the pathogenesis of
this condition.
TPL is an active compound extracted from the Chinese
medicinal herb Tripterygium wilfordii Hook.f., which has
been demonstrated to possess anti-inflammatory activities in
various cells (25,26). However, the effects of TPL on
atherosclerosis have not yet been investigated. Thus, the goal of
the present study was to determine the effects of TPL on
ApoE−/− mice with a high-fat diet and to
analyze the changes of proteins associated with lipid metabolism
and inflammatory cytokines to clarify the underlying molecular
mechanisms.
Materials and methods
Antibodies
Anti-ATP-binding cassette transporter A1 (ABCA1)
antibody (cat. no. NB400-105) was obtained from Novus Biologicals,
LLC (Littleton, CO, USA). Primary rabbit polyclonal LXRα (cat. no.
ab3585) was purchased from Abcam (Cambridge, UK). Primary mouse
monoclonal β-actin antibody (cat. no. sc-47778) and secondary
antibodies, including horseradish peroxidase (HRP)-conjugated goat
anti-rabbit IgG (cat. no. sc-2004) and goat anti-mouse IgG-HRP
(cat. no. sc-2005) were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Animals
A total of 12 eight-week-old ApoE knock-out
(ApoE−/−) mice (male, 5; female, 7; weight,
18–20 g) were obtained from the Laboratory Animal Center of Peking
University Health Science Center (Beijing, China) and housed in
specific pathogen-free conditions. The
ApoE−/− mice were given a high-fat diet from
weeks 8–20, and were used as atherosclerosis models. In addition,
30 8-week-old male wild-type C57BL/6 mice, weighing 18–20 g, with
the same genetic background were used as blank controls. The feed
was constructed with 21% fat, 0.15% cholesterol and 19.5% casein.
All mice were free to eat and drink and the ambient temperature was
kept at 22±2°C with a humidity of 50–60%. The lighting was
artificially controlled in the room and alternating cycles of 12
h-light (8:00–20:00) and 12 h-darkness (20:00–8:00 the next day)
were used.
Genotype identification of the
ApoE−/− mice
The tail tips of the mice were cut and DNA samples
were prepared using a DNA extraction kit (Bioteke Corporation,
Beijing, China). The genotype of ApoE was detected by polymerase
chain reaction (PCR) assay using an 2X EasyTaq PCR SuperMix
(Quanshijin Corp., Beijing, China). Three PCR primers were used as
follows: Primer 1: 5′-GCCTAGCCGAGGGAGAGCCG-3′; primer 2:
5′-TGTGACTTGGGAGCTCTGCAGC-3′; and primer 3:
5′-GCCGCCCCGACTGCATCT–3′.
Thermal cycling conditions were 95°C for 10 min,
followed by 40 cycles of 30 sec at 95°C, 30 sec at 67°C and 30 sec
at 72°C, and then 10 min at 72°C for final elongation. The length
of the DNA fragments of wild-type, ApoE−/−
type and the heterozygous type mice are expected to be 155 bp/155
bp, 245 bp/155 bp and 155 bp/155 bp, respectively.
Western blot analysis
Cells were collected and lyzed using RIPA buffer.
Total protein (30 µg/well) was separated by SDS-PAGE and
transferred to nitrocellulose membrane at 400 mA for 2 h. The
membrane was blocked with 5% skim milk at room temperature prior to
incubation with the primary antibodies (all 1:1,000) overnight at
4°C. Subsequently, the membrane was washed three times with 1X
Tris-buffered saline with Tween 40 (0.1%; TBST) for 5 min and
incubated with the secondary antibodies for 30 min at room
temperature. The membrane was washed three times with TBST and the
bands were developed using an enhanced chemiluminescence kit.
BioRad GelDoc XR and Quantity One 1-D analysis software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) were used for band detection
and visualization, respectively.
Animal experiment
The 8-week-old male ApoE−/−
mice and C57BL/6 mice (control group) were housed in specific
pathogen-free conditions. The ApoE−/− mice were randomly
divided into four groups: ApoE−/− model (untreated) and
three TPL treatment groups. The mice in the TPL groups were
intraperitoneally injected with 25, 50 or 100 µg/kg TPL every 3
days, from week 12 to week 20 after birth. Each group contained 6
mice.
Measurement of total cholesterol and
total triglyceride levels in plasma
The mice were divided into five groups and were
treated as described above. At weeks 16 and 20, blood samples were
collected from the tails of the mice. Total cholesterol and total
triglyceride levels in plasma were determined using kits from
Mingfeng Biotechnology Co., Ltd. (Shanghai, China) according to the
protocols provided with the kits.
Cytokine production by
macrophages
Mice were sacrificed via cervical dislocation and 5
ml of 1640 medium was subsequently injected into the abdominal
cavity with a syringe to harvest peritoneal fluid. Following
centrifugation at 200 rpm for 10 min, the macrophages were
isolated. Peritoneal macrophages from the five groups were prepared
and adjusted to 3×105 cells/ml with 1640 medium for
stimulation by TPL. After adherence, the cells were treated with 25
µg/ml oxidized LDL (oxLDL; Luwen Biocorporation, Shanghai, China)
for 48 h. The supernatants were harvested and the concentration of
inflammatory cytokines, namely tumor necrosis factor (TNF)-α,
interleukin (IL)-1β, IL-6 and IL-8, were determined with
enzyme-linked immunosorbent assay (ELISA) kits (Neobioscience,
Beijing, China) according to the manufacturer's protocol and a
Benchmark Microplate Reader (Bio-Rad Laboratories, Inc.). In the
present experiment, all samples were analyzed in duplicate for
determination of the cytokine levels.
Statistical analysis
The data were analyzed using SPSS 20.0 software (IBM
SPSS, Armonk, NJ, USA). Cytokine production data were compared
using Student's t-tests. Data are expressed as means ± standard
errors of the means. P<0.05 was considered to indicate a
statistically significant difference.
Results
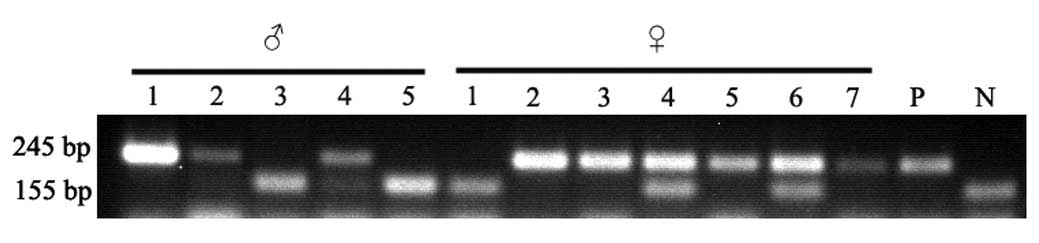
ApoE−/− mice are
identified and selected by PCR
Mouse ApoE primers were used to identify the
wild-type allele (155 bp) and ApoE null allele (245 bp). The
heterozygote with wild-type and ApoE null alleles had a mixture of
155-bp and 245-bp fragments. Homozygotes of wild-type mice only had
the wild-type allele of 155 bp and homozygotes of knock-out mice
only had the ApoE null allele of 245 bp. Two male
ApoE−/− mice (lanes 1 and 2) and four female
ApoE−/− mice (lanes 2, 3, 5 and 7) were
screened. Additionally, the corresponding wild-type mice were also
identified with same genetic background to
ApoE−/− mice. PCR results are shown in
Fig. 1.
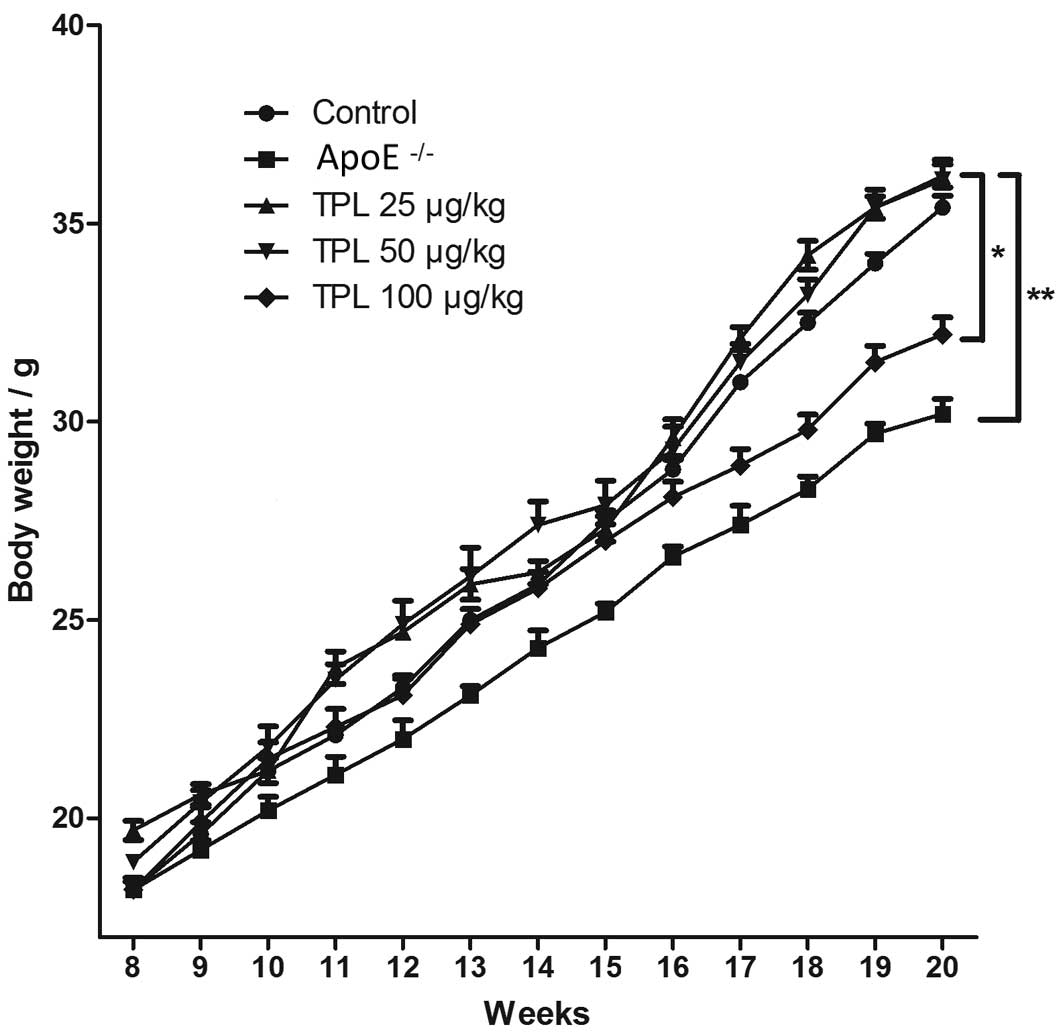
Body weights of
ApoE−/− knock-out mice are lower than those
of mice in the other groups
Body weight is a key indicator of toxicity for
drug-treated mice. Mice in the different groups were given a
high-fat diet from week 8 after birth until week 20, and were
allowed to eat and drink freely. The body weights of the mice were
determined once every week during the high-fat diet. As shown in
Fig. 2, the body weights of the mice
in the ApoE−/− knock-out group were gradually
and significantly decreased compared with those in the wild-type
control group (P<0.01). However, ApoE−/−
mice treated with 25 or 50 µg/kg TPL exhibited no marked variation
in body weight compared with the wild-type control group. Notably,
the body weights of the ApoE−/− mice treated
with 100 µg/kg TPL were less than those of the wild-type control
mice, suggesting that 100 µg/kg TPL may be toxic to the mice, and
thus have caused them to lose weight.
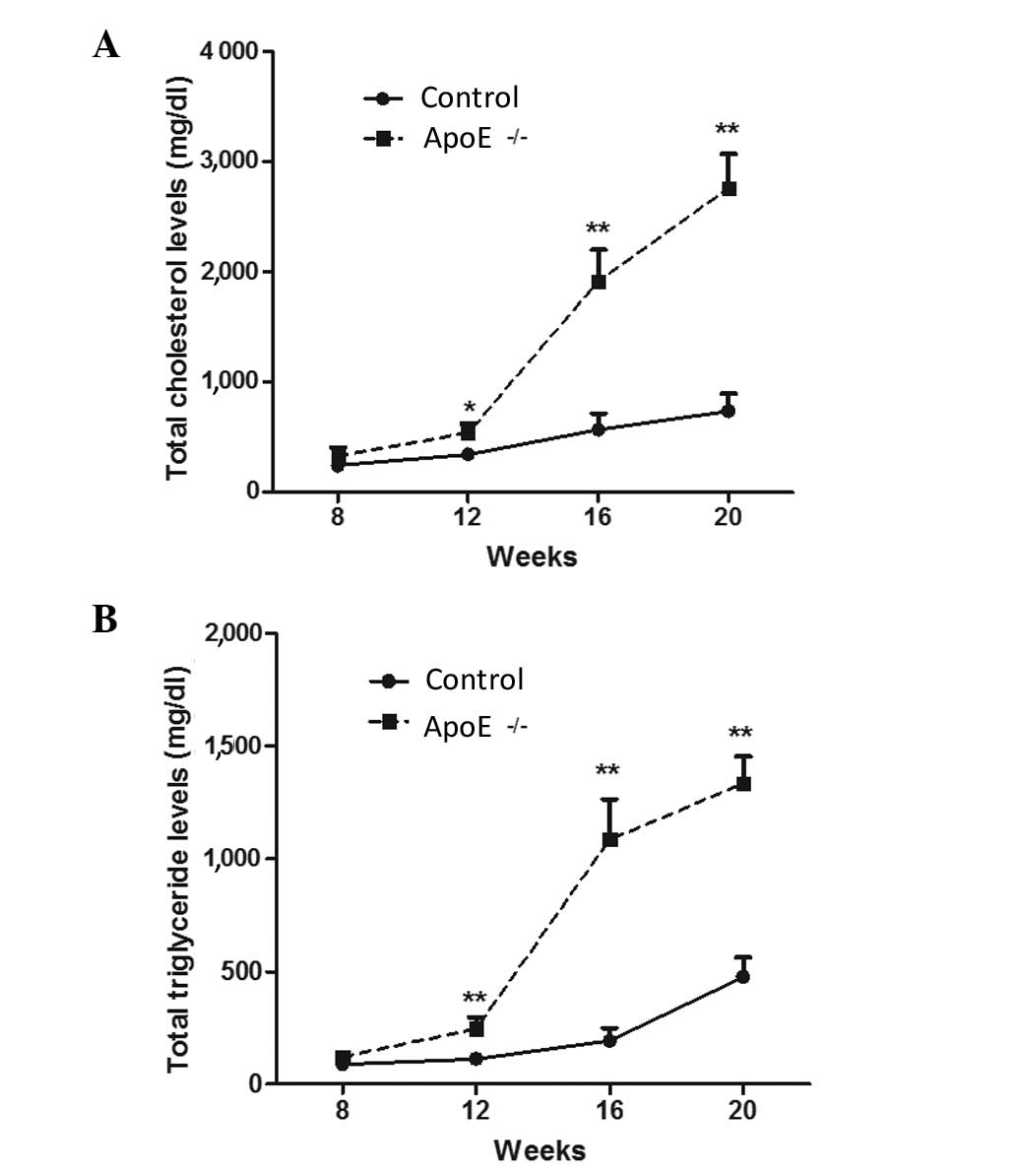
Levels of total cholesterol and total
triglyceride are highly increased in ApoE−/−
mice models
Atherosclerosis is inextricably linked with abnormal
lipid metabolism. Abnormal lipid metabolism is mainly characterized
by higher levels of plasma cholesterol, which is an important risk
factor for the injury induced by atherosclerosis (27,28). In
the present study, the levels of total cholesterol and total
triglyceride in ApoE−/− mice and normal
control mice were measured. As shown in Fig. 3, after 1 month of high-fat diet, the
total cholesterol and total triglyceride levels were gradually and
significantly increased in the ApoE−/− mice compared
with those in wild-type mice (P<0.05 and P<0.01,
respectively), and the difference between the levels in the two
groups increased as the duration of the high-fat diet extended
(P<0.01). These data indicate that the atherosclerotic mouse
model was successfully constructed.
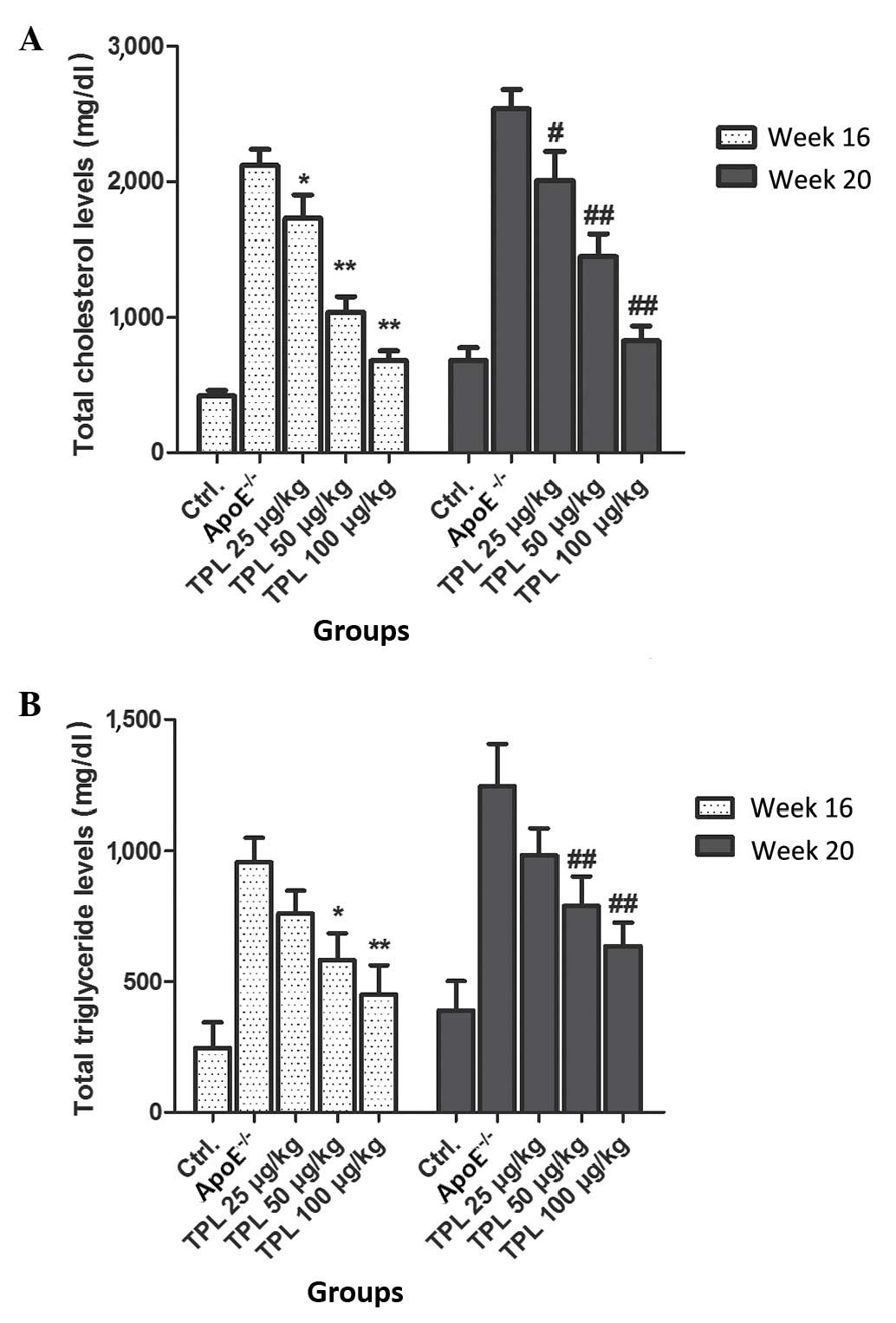
Treatment with TPL contributes to the
downregulation of total cholesterol and total triglyceride levels
in ApoE−/− mice
In order to detect the effects of TPL on the
production of total cholesterol and total triglyceride, various
concentrations of TPL were used to treat
ApoE−/− mice fed with a high-fat diet. From
week 8 after birth, mice with an ApoE−/−
genotype were fed a high-fat diet, and treated with 25, 50 or 100
µg/kg TPL. The total cholesterol and total triglyceride levels were
detected at 16 and 20 weeks, respectively. The results demonstrated
that the levels of total cholesterol and total glyceride were
decreased by TPL treatment in a dose-dependent manner (Fig. 4).
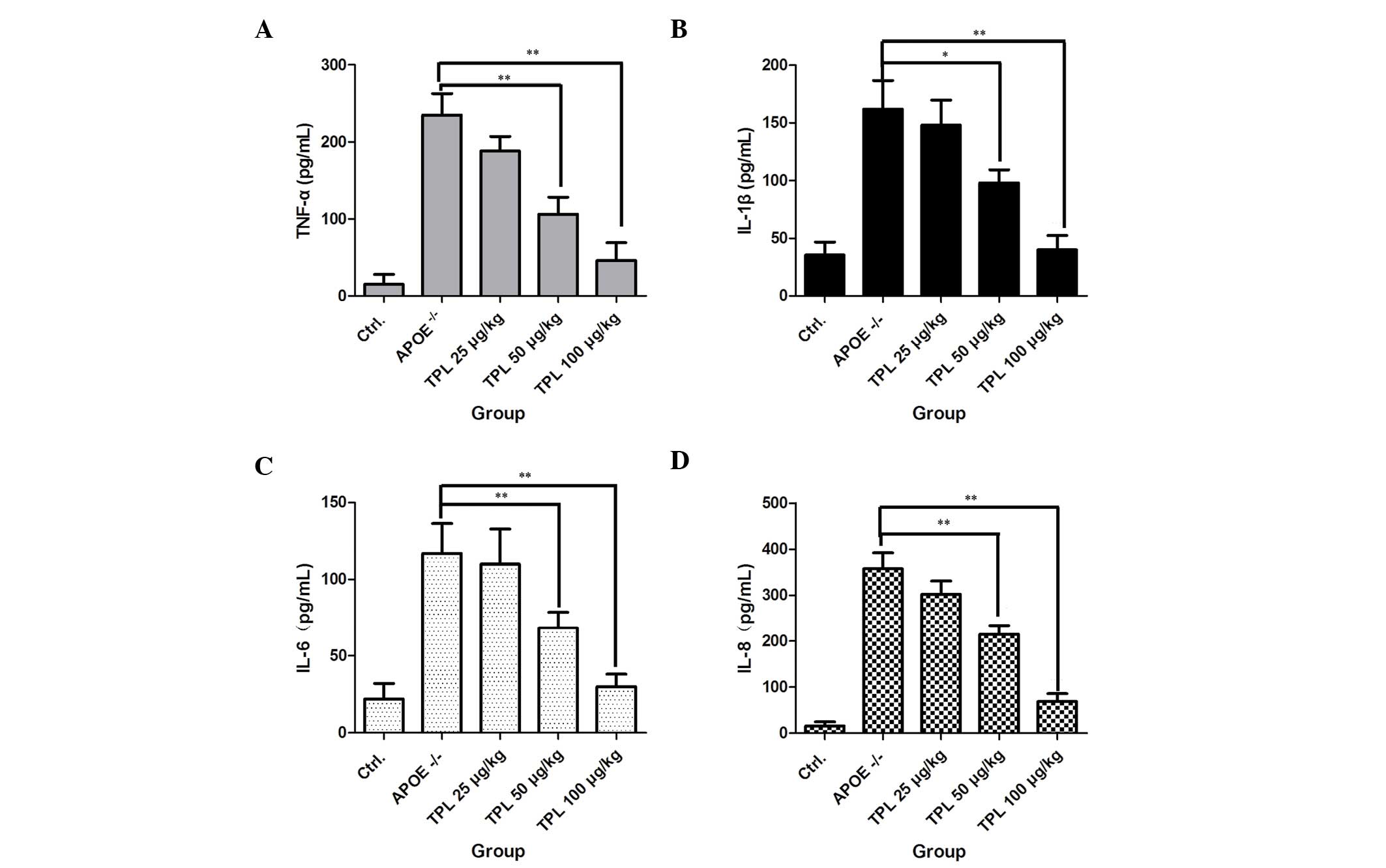
TPL inhibits the secretion of
inflammatory cytokines in macrophages
Foam cells, which are fat-laden macrophages, are the
characteristic pathological cells in atherosclerotic plaques, and
are mainly derived from blood mononuclear cells and vascular smooth
muscle cells (29). Whether TPL is
able to inhibit cytokine production in macrophages during the
progression of atherosclerosis was investigated in the present
study. As shown in Fig. 5,
macrophages from ApoE−/− mice produced high
levels of the inflammatory cytokines TNF-α, IL-1β, IL-6 and IL-8.
However, treatment with TPL at concentrations of 25, 50 and 100
µg/kg markedly reduced the levels of the cytokines in a
dose-dependent manner, which demonstrated that TPL inhibited the
progression of inflammation.
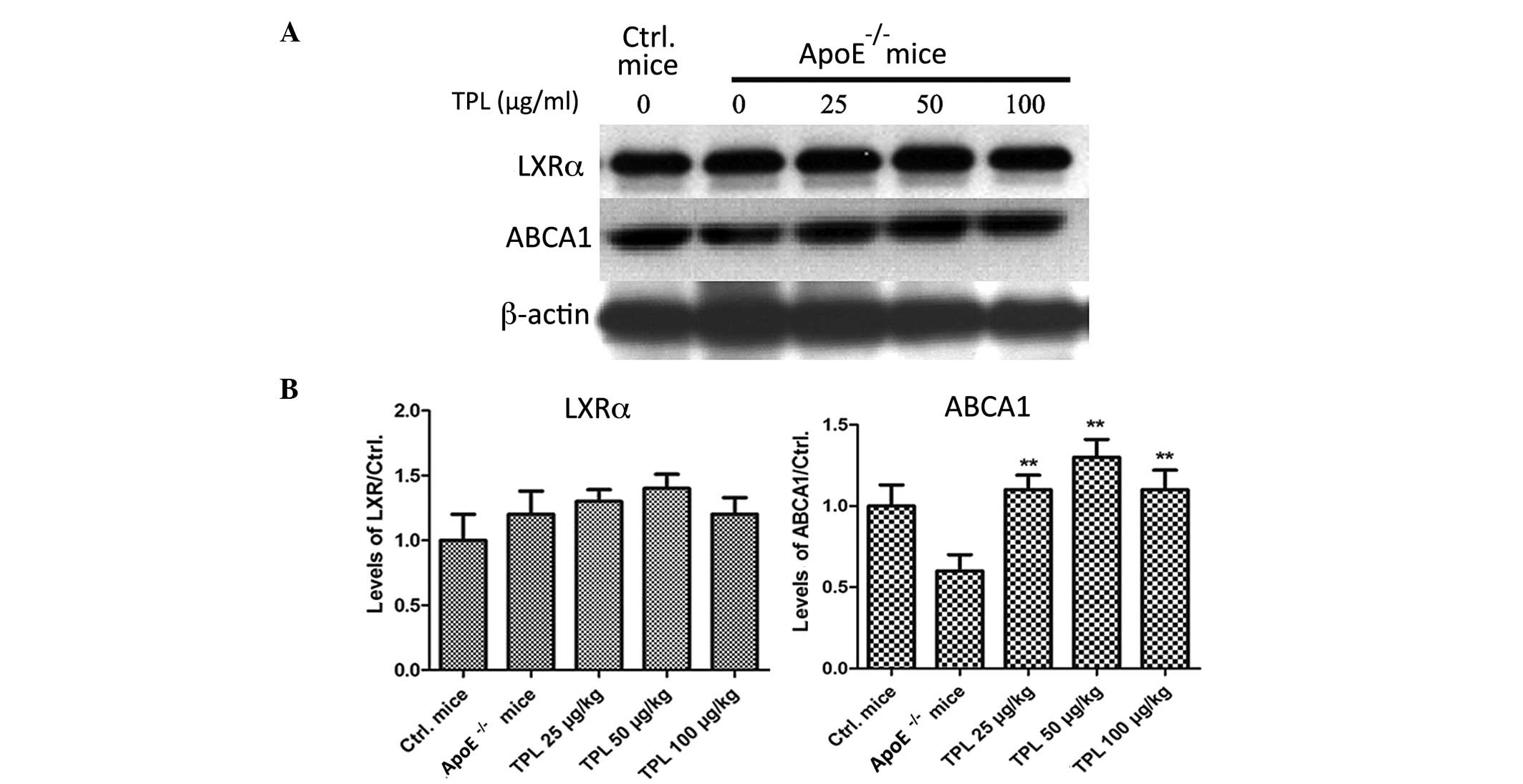
TPL treatment increases the expression
of ABCA1 but has no effect on liver X receptor α (LXRα)
ABCA1 is a membrane-associated protein and mediates
the transmission of the intracellular cholesterol. It contributes
to reducing the formation of foam cells and preventing the
occurrence of atherosclerosis (30).
The expression of ABCA1 can be induced by increased levels of HDL
stimulated by LXR and retinoid X receptor (30). The expression levels of LXRα and
ABCA1 were determined in the present study. As shown in Fig. 6, the expression levels of ABCA1 in
the macrophages of ApoE−/− mice were
significantly increased in the mice treated with TPL. However, the
expression levels of LXRα were not significantly affected by
treatment with different concentrations of TPL.
Discussion
Atherosclerosis is the main pathology for
cardiovascular and cerebrovascular diseases, and it progresses with
chronic inflammation (31–33). TPL is a compound originally extracted
from the plant T. wilfordii Hook.f., which has potent
anti-inflammatory activity (34).
The aim of the present study was to identify whether TPL was able
to inhibit the progression of atherosclerosis by an
anti-inflammatory mechanism. Thus, ApoE−/−
mice were given high-fat diet to construct animal models of
atherosclerosis, and different doses of TPL were used to treat the
mice. The observation and determination of the effects of TPL in
the progression of atherosclerosis may provide new insights useful
for the therapy of atherosclerotic disease.
Lipoproteins are predominantly synthesized in the
liver and intestines. ApoE is a type of apolipoprotein found in
VLDL, CMs and their debris; it is a ligand of the LDL receptor in
the body and has an important role in lipid metabolism. ApoE
deficiency can cause the accumulation of cholesterol-rich
lipoproteins in the blood, which induces the formation and
development of atherosclerotic lesions (35). Abnormal lipid metabolism, in
particular that leading to high levels of plasma cholesterol, is an
important risk factor for the development of atherosclerosis
(36). In the present study, ApoE
gene knock-out mice were used the animal models to explore the
mechanism of atherosclerosis.
TPL has been demonstrated to be the main active
component of the herb T. wilfordii Hook.f., which is used in
traditional Chinese medicine, and it has been revealed to exert
anti-inflammatory activity by inhibiting the NF-κB signaling
pathway (25). Lee et al
found that TPL confers neuroprotection against traumatic brain
injury, at least in part, via its anti-inflammatory activity
(37). Han et al demonstrated
that TPL contributes to the treatment of psoriasis and other
immune-mediated inflammatory diseases (38). An autoimmune disease, rheumatoid
arthritis, is associated with increased production of
pro-inflammatory cytokines. Matta et al found that TPL
alleviated the progression of rheumatoid arthritis not only by
inhibiting NF-κB-regulated reporter transcription, but also by
blocking the activity of other transcription factors (39). Atherosclerosis is also a disease
associated with chronic inflammation. In the present study, various
concentrations of TPL were used to treat
ApoE−/− mice fed with a high-fat diet for
longer than 2 months. The results demonstrated that TPL inhibited
the secretion of inflammatory cytokines in
ApoE−/− mice, which was consistent with the
previous studies and confirmed that TPL has anti-inflammatory
activity.
In conclusion, the results of the present study
demonstrate that the abnormal lipid metabolism and a chronic
inflammatory response contributed to the progression of
atherosclerosis in ApoE−/− mice, and that
treatment with TPL regulated lipid metabolism and inhibited
inflammation in ApoE−/− mice. The clarification of the
mechanism of atherosclerosis and the effects of TPL is helpful and
instructive in the clinical treatment of this disease.
References
|
1
|
Watanabe T, Sato K, Itoh F, Iso Y,
Nagashima M, Hirano T and Shichiri M: The roles of salusins in
atherosclerosis and related cardiovascular diseases. J Am Soc
Hypertens. 5:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zeng Y, Song JX and Shen XC: Herbal
remedies supply a novel prospect for the treatment of
atherosclerosis: A review of current mechanism studies. Phytother
Res. 26:159–167. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Suzuki M, Minami A, Nakanishi A, Kobayashi
K, Matsuda S, Ogura Y and Kitagishi Y: Atherosclerosis and tumor
suppressor molecules (review). Int J Mol Med. 34:934–940.
2014.PubMed/NCBI
|
|
4
|
Hulthe J, Wikstrand J, Mattsson-Hulten L
and Fagerberg B: Circulating ICAM-1 (intercellular cell-adhesion
molecule 1) is associated with early stages of atherosclerosis
development and with inflammatory cytokines in healthy 58 year-old
men: The atherosclerosis and insulin resistance (AIR) study. Clin
Sci (Lond). 103:123–129. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maranhão RC and Leite AC Jr: Development
of anti-atherosclerosis therapy based on the inflammatory and
proliferative aspects of the disease. Curr Pharm Des. 21:1196–1204.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shing CM, Fassett RG, Peake JM and Coombes
JS: Voluntary exercise decreases atherosclerosis in nephrectomised
ApoE knockout mice. PloS One. 10:e01202872015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
González-Gay MA, González-Juanatey C,
Llorca J and Castañeda S: The influence of inflammation in the
development of subclinical atherosclerosis in psoriatic arthritis:
Comment on ‘Cardiovascular comorbidities in patients with psoriatic
arthritis: A systematic review’ by Jamnistki etal. Ann Rheum Dis.
73:e272014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hansson GK: Immune and inflammatory
mechanisms in the development of atherosclerosis. Br Heart J.
69:(Suppl). S38–S41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pizzi J, Silva LR, Moser D and Leite N:
Relationship between subclinical atherosclerosis, blood pressure
and lipid profile in obese children and adolescents: A systematic
review. Arq Bras Endocrinol Metabol. 57:1–6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kreisberg RA and Oberman A: Clinical
review 141: Lipids and atherosclerosis: Lessons learned from
randomized controlled trials of lipid lowering and other relevant
studies. J Clin Endocrinol Metab. 87:423–437. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L and Yang L: Anti-inflammatory
effects of vinpocetine in atherosclerosis and ischemic stroke: A
review of the literature. Molecules. 20:335–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ross R: Atherosclerosis is an inflammatory
disease. Am Heart J. 138:S419–S420. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ross R: Atherosclerosis - an inflammatory
disease. N Engl J Med. 340:115–126. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schlag B, Winkler L, Plonné D, Dürer U and
Dargel R: Preparation of apoE-free rat low density lipoprotein for
catabolic studies. J Lipid Res. 28:1521–1524. 1987.PubMed/NCBI
|
|
15
|
Martinez-Oliván J, Arias-Moreno X,
Velazquez-Campoy A, Millet O and Sancho J: LDL receptor/lipoprotein
recognition: Endosomal weakening of ApoB and ApoE binding to the
convex face of the LR5 repeat. FEBS J. 281:1534–1546. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kostogrys RB, Franczyk-żarów M, Maślak E,
Gajda M, Mateuszuk L, Jackson CL and Chłopicki S: Low carbohydrate,
high protein diet promotes atherosclerosis in apolipoprotein
E/low-density lipoprotein receptor double knockout mice
(apoE/LDLR−/−). Atherosclerosis. 223:327–331.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shimano H, Yamada N, Katsuki M, Yamamoto
K, Gotoda T, Harada K, Shimada M and Yazaki Y: Plasma lipoprotein
metabolism in transgenic mice overexpressing apolipoprotein E.
Accelerated clearance of lipoproteins containing apolipoprotein B.
J Clin Invest. 90:2084–2091. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shimano H, Yamada N, Katsuki M, Shimada M,
Gotoda T, Harada K, Murase T, Fukazawa C, Takaku F and Yazaki Y:
Overexpression of apolipoprotein E in transgenic mice: Marked
reduction in plasma lipoproteins except high density lipoprotein
and resistance against diet-induced hypercholesterolemia. Proc Natl
Acad Sci USA. 89:1750–1754. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dallongeville J, Lussier-Cacan S and
Davignon J: Modulation of plasma triglyceride levels by apoE
phenotype: A meta-analysis. J Lipid Res. 33:447–454.
1992.PubMed/NCBI
|
|
20
|
Zhang SH, Reddick RL, Piedrahita JA and
Maeda N: Spontaneous hypercholesterolemia and arterial lesions in
mice lacking apolipoprotein E. Science. 258:468–471. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Weingärtner O, Ulrich C, Lütjohann D,
Ismail K, Schirmer SH, Vanmierlo T, Böhm M and Laufs U:
Differential effects on inhibition of cholesterol absorption by
plant stanol and plant sterol esters in
apoE−/− mice. Cardiovasc Res. 90:484–492.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sato M, Yamada Y, Matsuoka H, Nakashima S,
Kamiya T, Ikeguchi M and Imaizumi K: Dietary pine bark extract
reduces atherosclerotic lesion development in male ApoE-deficient
mice by lowering the serum cholesterol level. Biosci Biotechnol
Biochem. 73:1314–1317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gopal K, Kumar K, Nandini R, Jahan P and
Kumar MJ: High fat diet containing cholesterol induce aortic
aneurysm through recruitment and proliferation of circulating
agranulocytes in apoE knock out mice model. J Thromb Thrombolysis.
30:154–163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou Y, Du H, Yin M, Zhang L, Mao L, Xiao
N, Ren G, Zhang C and Pan J: Effects of high dietary fat and
cholesterol on expression of PPAR alpha, LXR alpha and their
responsive genes in the liver of apoE and LDLR double deficient
mice. Mol Cell Biochem. 323:195–205. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Zhang L, Duan W, Liu B, Gong P,
Ding Y and Wu X: Anti-inflammatory effects of triptolide by
inhibiting the NF-κB signalling pathway in LPS-induced acute lung
injury in a murine model. Mol Med Rep. 10:447–452. 2014.PubMed/NCBI
|
|
26
|
Chen BJ: Triptolide, a novel
immunosuppressive and anti-inflammatory agent purified from a
Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma.
42:253–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calabresi L, Gomaraschi M, Simonelli S,
Bernini F and Franceschini G: HDL and atherosclerosis: Insights
from inherited HDL disorders. Biochim Biophys Acta. 1851:13–18.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao X, Zhong J, Maiseyeu A, Gopalakrishnan
B, Villamena FA, Chen LC, Harkema JR, Sun Q and Rajagopalan S:
CD36-dependent 7-ketocholesterol accumulation in macrophages
mediates progression of atherosclerosis in response to chronic air
pollution exposure. Circulation Res. 115:770–780. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Woollard KJ: Immunological aspects of
atherosclerosis. Clinical Sci. 125:221–235. 2013. View Article : Google Scholar
|
|
30
|
Chen JH, Wang CJ, Wang CP, Sheu JY, Lin CL
and Lin HH: Hibiscus sabdariffa leaf polyphenolic extract inhibits
LDL oxidation and foam cell formation involving up-regulation of
LXRalpha/ABCA1 pathway. Food Chem. 141:397–406. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang W, Lee Y and Lee CH: Review: The
physiological and computational approaches for atherosclerosis
treatment. Int J Cardiol. 167:1664–1676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rodondi N, Auer R, de Bosset Sulzer V,
Ghali WA and Cornuz J: Atherosclerosis screening by noninvasive
imaging for cardiovascular prevention: A systematic review. J Gen
Intern Med. 27:220–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hartman J and Frishman WH: Inflammation
and atherosclerosis: A review of the role of interleukin-6 in the
development of atherosclerosis and the potential for targeted drug
therapy. Cardiol Rev. 22:147–151. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ziaei S and Halaby R: Immunosuppressive,
anti-inflammatory and anti-cancer properties of triptolide: A mini
review. Avicenna J Phytomed. 6:149–164. 2016.PubMed/NCBI
|
|
35
|
Hoeke G, Kooijman S, Boon MR, Rensen PC
and Berbee JF: Role of brown fat in lipoprotein metabolism and
atherosclerosis. Circ Res. 118:173–182. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith BW, Miller RJ, Wilund KR, O'Brien WD
Jr and Erdman JW Jr: Effects of tomato and soy germ on lipid
bioaccumulation and atherosclerosis in ApoE-/- mice. J Food Sci.
80:H1918–1925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee HF, Lee TS and Kou YR:
Anti-inflammatory and neuroprotective effects of triptolide on
traumatic brain injury in rats. Respir Physiol Neurobiol. 182:1–8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Han R, Rostami-Yazdi M, Gerdes S and
Mrowietz U: Triptolide in the treatment of psoriasis and other
immune-mediated inflammatory diseases. Br J Clin Pharmacol.
74:424–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matta R, Wang X, Ge H, Ray W, Nelin LD and
Liu Y: Triptolide induces anti-inflammatory cellular responses. Am
J Transl Res. 1:267–282. 2009.PubMed/NCBI
|