Introduction
In the United States, mothers with epilepsy give
birth to more than 30,000 children/year. Antiepileptic drugs (AEDs)
are a class of drugs that are used to treat epilepsy in patients.
AEDs are administered to most epileptic patients, including
pregnant women; however, they can have negative consequence to
fetuses in utero (1). AEDs
are also used to treat other diseases as well such as neuropathic
pain, migraines and psychiatric disorders, which means that AEDs
can affect other pregnancies as well (2). Therefore, many pregnant women are being
exposed to the potential dangerous effects of AEDs to the fetuses.
However, AEDs are important for the treatment of epilepsy in
pregnant mothers. Most women with epilepsy require AEDs to control
seizures the entire length of the pregnancy. The most concerning
issue in pregnant women with epilepsy is the development of
tonic-clonic seizures, which can lead to dangerous issues for the
fetus such as intracranial hemorrhage, transient hemorrhage and
heart beat abnormalities (3,4).
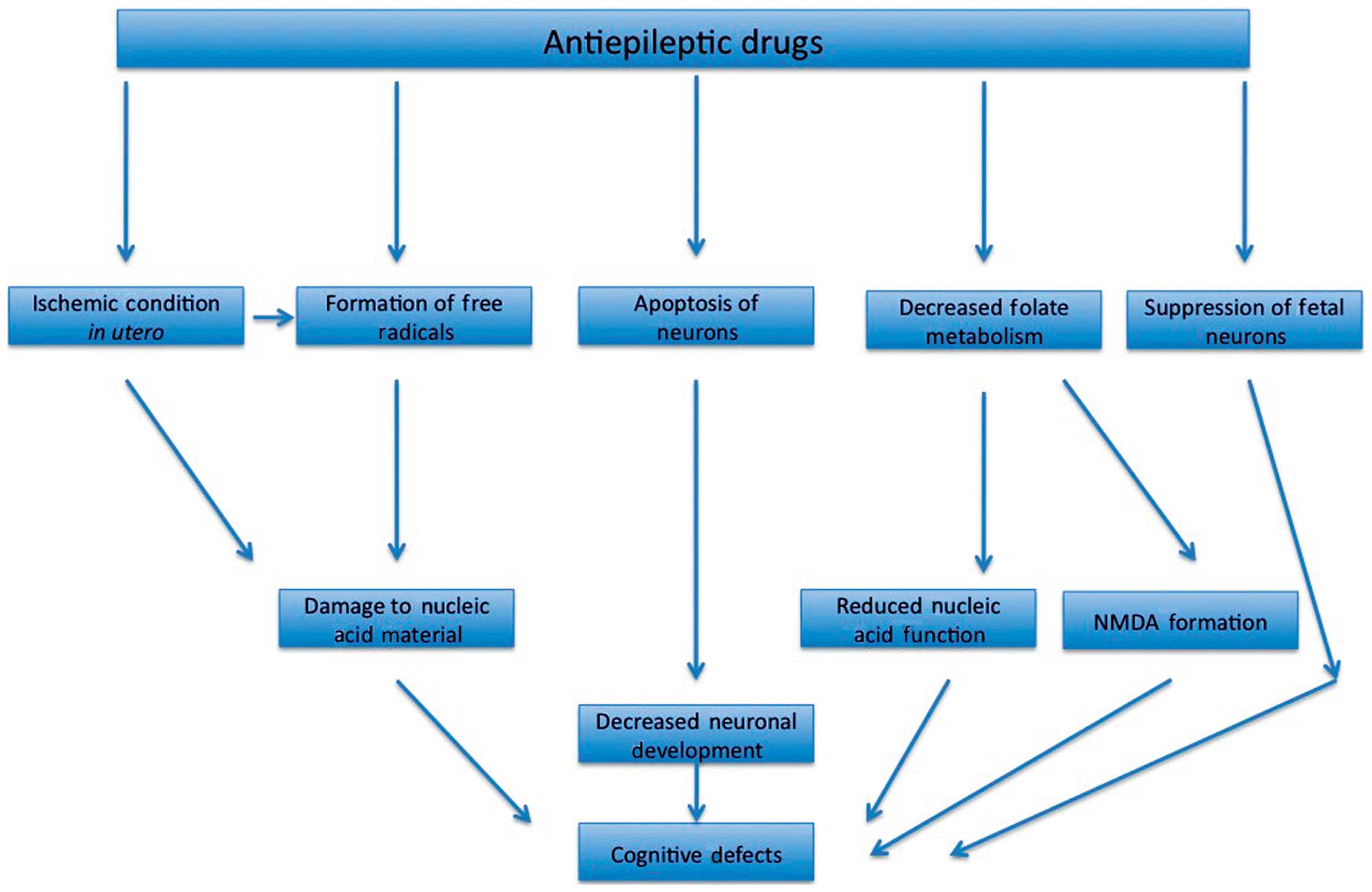
There may be multiple mechanisms through which AEDs
affect fetal neurodevelopment. While scientists have still not
properly characterized the pathways through which this may occur, a
number of studies have investigated the mechanism through which
this may occur. Cognitive defects can still occur in fetuses that
are exposed to AED dosages that are lower than those that produce
structural abnormalities. There are various hypothesis that have
been set forward that may explain the ability of AEDs to produce
functional defects; these may include suppression of neurons,
ischemic condition in utero, formation of free radicals,
apoptosis in neurons and decreased folate metabolism (Fig. 1) (5).
AED induced birth defects
The statistics for pregnant women taking AEDs are
morbid, as 30% of pregnancies result in maternal mortality and 50%
result in fetus mortality (6).
Despite these odds, the majority of doctors encourage the use of
AEDs during pregnancy. This is because, generally, women with
epilepsy have normal childbirths and the offspring are born without
any structural abnormalities. However, compared to a normal
pregnancy, there is still a higher chance of the children to be
born with birth defects (4).
A meta-analysis found that pregnant women with
epilepsy that are on AEDs have a 6.1% chance of the development of
major congenital malformations (7).
Different AEDs have a different major congenital malformation rate
(Fig. 1). Women with epilepsy that
are not on AEDs have a 2.8% rate of major congenital malformation,
which is slightly higher than the control group at 2.2% (8). When only treated with one AED (AED
monotherapy), the prevalence of major congenital malformations is
3.7% while it is 6% in pregnant women that are treated with AED
polytherapy. The types of malformations that are observed due to
AED exposure in utero include cardiac malformations,
hypospadias and facial clefts (9).
The potential effects of some AEDs have been well-established but
the effects of others remain unclear. One particular example of a
specific malformation is the use of the drug valproate, which
causes a 1–2% risk of neural tube defects (10) (Table
I).
 | Table I.Major congenital malformation rate
due to in utero exposure to various AEDs. |
Table I.
Major congenital malformation rate
due to in utero exposure to various AEDs.
| Antiepileptic
drug | MCM rate (%) |
|---|
| Carbamazepine | 2.20 |
| Valproic acid | 6.20 |
| Lamotrigine | 3.20 |
| Phenytoin | 3.70 |
| Gabapentin | 3.20 |
| Topiramate | 7.10 |
Neurobehavioral endpoints with AEDs
Evidence from a variety of studies has shown that
there is a definite correlation between the in utero
exposure to various AEDs and the risk of cognitive and behavioral
problems in children (6,11). The Kerala Registry of Epilepsy and
Pregnancy conducted a study and determined that children that were
exposed to AEDs in utero had the lowest mental development
scores when compared to healthy controls (12). The mental development scores were
found to be the lowest in children that were exposed to valproic
acid. The mental development scores in children exposed to valproic
acid were decreased by 40.8% when compared to controls. Other AEDs
such as phenytoin, carbamazepine and phenobarbital also led to
decreased mental development scores by 37, 29 and 26%, respectively
(12).
Another study conducted by Meador et al
demonstrated that at the age of 3, children that were exposed to
the AED valproic acid in utero had the lowest IQ score. The
mean IQ score for fetuses exposed to valproic acid was 92, which
was 7 and 9 points lower, respectively, than the children that were
exposed to carbamazepine and lamotrigine (5). In fact, the IQ of the offspring
correlated with the mother's IQ in every case except for the
exposure to valproic acid. Thirteen percent of children that were
exposed in utero to valproic acid presented with IQs that
were in the impaired range; this is contrasted to 5% of children
that were exposed to phenytoin, 3% of children that were exposed to
carbamazepine and 2% of children that were exposed to lamotrigine
(5,13). Another study tested the ability of
children exposed in utero to AEDs to generate quantity and
quality of ideas. The study showed that both the quantity and
quality of ideas were lower in children that were exposed to
valproic acid when compared to the children that were exposed to
lamotrigine and carbamazepine. Even in studies done in older
children, AED in utero exposure was found to lead to altered
cognitive function (14). Gaily
et al found that older children that were exposed to
monotherapy in utero had decreased performance on
attentional tasks while being exposed to polytherapy led to
decreased performance in auditory attention, sentence repetition
and fine motor task (15). Long-term
studies have shown that all AEDs, except for cabamazepine, led to a
negative result in terms of intellectual function (16).
Effects of different AEDs on neural
defects
There are various AEDs that have been investigated
due to their potential for teratogenicity in pregnant women. Due to
the fact that AEDs should not be discontinued during pregnancy,
this is an issue that needs to be elaborated on extensively. The
AEDs that we discuss in this review are phenobarbital, valproate,
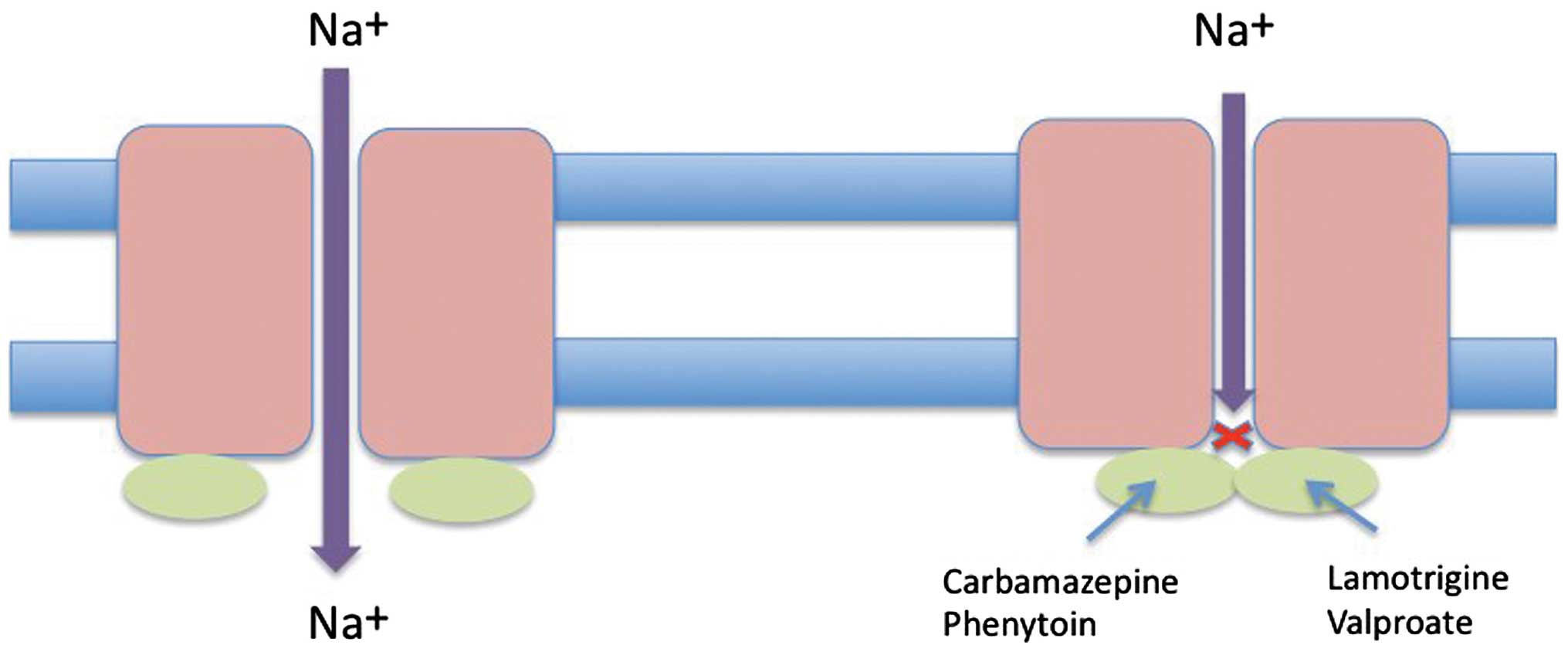
carbamazepine, lamotrigine, phenytoin and topiramate. The mechanism
by which these drugs impart their action is by inhibiting the
voltage-gated sodium channel (Fig.
2) (17).
Phenobarbital
Phenobarbital is one of the oldest AEDs that have
been used to treat epilepsy in all patient cohorts. The effect of
phenobarbital on fetuses was not evaluated until the 1970s. A study
performed on pregnant mice using phenobarbital suggests that it
leads to a 4.3% increase in incidences of cleft palates in fetuses
(18). Phenobarbital exposure to
pregnant rats has also led to an increased incidence of fetus
mortality, impaired growth and delayed motor development. The
mechanism behind phenobarbital-induced neural issues in the fetus
may be related to the upregulation of cytochrome P450s of the 2B
family. It may act to produce oxidative stress by generating
superoxide radicals, which leads to the production of hydroxyl
radicals. This causes GC to TA transversions, which may eventually
lead to developmental defects (19,20).
Valproic acid
Valproic acid and its various analogs and
metabolites have been researched regarding their ability to assert
a teratogenic effect. In order to be teratogenic, the drug needs to
have the following components: A free carboxyl group, an α-hydrogen
atom, branching of carbon chains, no double bonds on C-2 or C-3 and
an alkyl substituent on the C-2 that is larger than the methyl
groups (21). Valproic acid and its
effects have been analyzed across various animal systems such as
zebrafish, mouse, rat, hamster and others (22–26).
When valproic acid is administered at high enough dosages, such as
those between 200–800 mg/day in mice, valproic acid induces various
developmental defects, such as skeletal defects in craniofacial
bones, in a dose-dependent manner. Increased doses of valproic acid
were also found to lead to intra-uterine growth retardation,
craniofacial, skeletal and cardiac defects (27).
In addition, rodents that were exposed to valproic
acid were found to have fetuses that presented with neural tube
defects. Valproic acid administration, when administered during
early neural tube formation, can lead to exencephaly. Neural tube
defects were found to be caused by >225 µg/ml of valproic acid,
which is above the concentration recommended for humans. The
mechanism of the neural tube defect induced by valproic acid may be
a result of altered folate metabolism in the embryo by increasing
the tetrahydrofolate levels and decreasing levels of 5-formyl- and
10-formyltetrahydrofolates (28).
Carbamazepine
Carbamazepine, which is used as treatment for
epilepsy and bipolar disorder, has been reported to lead to
congenital abnormalities when used during pregnancies. Many
articles have reviewed the first trimester exposure to
carbamazepine and found that there was an increase in malformations
such as neural tube defects, developmental delays, craniofacial
defects and behavioral changes (29). A study by Diav-Citrin et al
found that a cohort of pregnant women treated with carbamazepine
showed a lack of neural tube defects, though that may be due to
sample size limitation (30).
Another study showed that offspring of epileptic pregnant women
that were treated with carbamazepine led to a 10-mm decrease in
fetal head circumference, which did not become normal by the age of
18 months (31). Other studies have
also found that carbamazepine exposure in utero has led to
craniofacial defects and developmental delays. Therefore,
carbamazepine is considered a human teratogen as it leads to major
and minor abnormalities in fetuses and babies such as developmental
problems, abnormal IQ and growth retardation (32).
Lamotrigine
Lamotrigine is one the newer AEDs and therefore,
there is not much long-term information available on its effect on
fetus development. GlaxoSmithKline (London, UK) began a database of
women on lamotrigine in 1992, which is where much of the current
data on this drug originates. The database recognized that women on
lamotrigine presented with fetal malformations at a rate of 2.7%
when compared to the general population, which is 1.62% (33). Another database in the UK suggests
that there is a dose-dependent effect on fetal tetralogy at the
concentration of 200 mg/ml. The two databases confirmed that there
is increased occurrence of facial cleft. The incidence of facial
cleft in newborns with pregnant women treated with lamotrigine is
at the rate of 9.9/1,000 people, which is significantly higher when
compared to the control rate of 2/1,000 people. A multitude of
registries and databases have confirmed that there is a 2- to
3-fold increase in major malformations when there is in
utero exposure to lamotrigine (34).
Phenytoin
Phenytoin is a hydantoin component which, when
exposed in utero, can cause fetal hydantoin syndrome (FHS)
in fetuses and newborns. This disorder causes and leads to multiple
dysmorphic findings such as epicanthal folds, hypertelorism, broad
flat nasal bridges, an upturned nasal tip, wide lips, distal
digital hypoplasia, intrauterine growth retardation and mental
retardation (35). A recent study
showed that there was a prevalence of FHS in 11% of the children
exposed to phenytoin in utero with 30% of the exposed
children expressing at least some of the features of FHS.
Therefore, the teratogenicity of phenytoin has been
well-established (36).
Topiramate
Topiramate is a drug that is used for the treatment
of epilepsy as well as migraines. While the data regarding the
teratologic effects of topiramate is limited, a study has reported
that the malformations resulting from its administration were at a
rate of 4.8%, and when administered with other drugs (polytherapy),
that number went up to 11.2%. The types of malformations resulting
from topiramate include oral clefts and hypospadias, which were 11-
and 14-fold higher, respectively, than the control (37). In addition, multiple cases of
hypospadias, which is a birth defect of the urethra in males, have
been reported for children that were exposed to topiramate in
utero (38). The rates of major
congenital malformations are different from one study to the next.
In a study conducted by Ornoy (29)
9.8% of fetuses were found to have malformation rate, which is
double the rate reported by other studies (39,40).
Researchers also found that pregnant women that were exposed to
topiramate were found to have infants of lower birth weight as well
as have a higher rate of spontaneous abortions (41). However, according to current
literature, the rate of MCMs with exposure to topiramate is found
to be similar to other AEDs.
Vigabatrin
Vigabatrin is an AED that is used specifically to
treat specific refractoriness; its mechanism is to inhibit
GABA-transaminase. While it is known that exposure to vigabatrin
causes irreversible loss of vision in adult years, there is little
research suggesting the consequence of its use in pregnancy. The
scarce data available convey inconsistent findings of monotherapy
exposure of vigabatrin (41). It was
reported that approximately 15% of fetuses that were exposed to
vigabatrin during pregnancy developed congenital malformations.
There were some confounding factors in this study, including the
fetal exposure to other AEDs. In addition, another study found that
exposure to vigabatrin led to the malformation in children
(42). However, once again, the
women studied were also exposed to other AEDs. Finally, a study
conducted by Turanli et al found no implications for
children that were exposed to vigabatrin as fetus (43).
Conclusion
Appropriate management of epileptic pregnant women
who are administered AEDs is important; seizure frequency may
change during times like pregnancy, and both seizures and AEDs can
have negative results for the fetus. We evaluated the effects of
multiple AEDs including phenobarbital, valproic acid,
carbamazepine, lamotrigine, phenytoin and topiramate. Some AEDs,
such as valproic acid, are worse for the fetus than others. Newer
AEDs, such as lamotrigine, need to be further evaluated in
long-term studies for their effect on developing fetuses. Studies
done on the use of phenobarbital suggests that it leads to a 4.3%
increase in incidences of cleft palates in fetuses. Valproic acid
administration was found to lead to neural tube defects, which lead
to exencephaly. Study on carbamazepine found that it led to a 10-mm
decrease in fetal head circumference. Administration of lamotrigine
during pregnancy led to a 2- to 3-fold increase in major
malformations. Phenytoin led to a 11% incidence of FHS in fetuses.
Topiramate led to oral clefts at a 11-fold higher rate in fetuses.
Putting aside valproate, the major congenital malformation rate for
pregnant women taking AEDs is generally double that of women not on
medication; the risk for women on AEDs for having children with
malformations is approximately 4%. Hence, most pregnant women who
are being administered AEDs will have no problems. Some ways to
decrease the risk of birth defects are the use of folic acid and
good obstetric care. Further studies are needed, however, to
address cognitive effects of other AEDs; especially if these agents
are used in increasing numbers during pregnancy (1,3–5).
References
|
1
|
Yerby MS: Clinical care of pregnant women
with epilepsy: neural tube defects and folic acid supplementation.
Epilepsia. 44:(Suppl 3). 33–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rogawski MA and Löscher W: The
neurobiology of antiepileptic drugs for the treatment of
nonepileptic conditions. Nat Med. 10:685–692. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schmidt D, Canger R, Avanzini G, Battino
D, Cusi C, Beck-Mannagetta G, Koch S, Rating D and Janz D: Change
of seizure frequency in pregnant epileptic women. J Neurol
Neurosurg Psychiatry. 46:751–755. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crawford P: Best practice guidelines for
the management of women with epilepsy. Epilepsia. 46:(Suppl 9).
117–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Meador KJ, Baker G, Cohen MJ, Gaily E and
Westerveld M: Cognitive/behavioral teratogenetic effects of
antiepileptic drugs. Epilepsy Behav. 11:292–302. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adab N, Kini U, Vinten J, Ayres J, Baker
G, Clayton-Smith J, Coyle H, Fryer A, Gorry J, Gregg J, et al: The
longer term outcome of children born to mothers with epilepsy. J
Neurol Neurosurg Psychiatry. 75:1575–1583. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meador K, Reynolds MW, Crean S, Fahrbach K
and Probst C: Pregnancy outcomes in women with epilepsy: a
systematic review and meta-analysis of published pregnancy
registries and cohorts. Epilepsy Res. 81:1–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jentink J, Loane MA, Dolk H, Barisic I,
Garne E, Morris JK and de Jong-van den Berg LT: EUROCAT
Antiepileptic Study Working Group: Valproic acid monotherapy in
pregnancy and major congenital malformations. N Engl J Med.
362:2185–2193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morrow J, Russell A, Guthrie E, Parsons L,
Robertson I, Waddell R, Irwin B, McGivern RC, Morrison PJ and Craig
J: Malformation risks of antiepileptic drugs in pregnancy: a
prospective study from the UK Epilepsy and Pregnancy Register. J
Neurol Neurosurg Psychiatry. 77:193–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Samrén EB, van Duijn CM, Koch S, Hiilesmaa
VK, Klepel H, Bardy AH, Mannagetta GB, Deichl AW, Gaily E,
Granström ML, et al: Maternal use of antiepileptic drugs and the
risk of major congenital malformations: a joint European
prospective study of human teratogenesis associated with maternal
epilepsy. Epilepsia. 38:981–990. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vinten J, Adab N, Kini U, Gorry J, Gregg J
and Baker GA: Liverpool and Manchester Neurodevelopment Study
Group: Neuropsychological effects of exposure to anticonvulsant
medication in utero. Neurology. 64:949–954. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomas SV, Indrani L, Devi GC, Jacob S,
Beegum J, Jacob PP, Kesavadas K, Radhakrishnan K and Sarma PS:
Pregnancy in women with epilepsy: preliminary results of Kerala
registry of epilepsy and pregnancy. Neurol India. 49:60–66.
2001.PubMed/NCBI
|
|
13
|
Baker GA, Bromley RL, Briggs M, Cheyne CP,
Cohen MJ, García-Fiñana M, Gummery A, Kneen R, Loring DW, Mawer G,
et al: Liverpool and Manchester Neurodevelopment Group: IQ at 6
years after in utero exposure to antiepileptic drugs: a controlled
cohort study. Neurology. 84:382–390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McVearry KM, Gaillard WD, VanMeter J and
Meador KJ: A prospective study of cognitive fluency and originality
in children exposed in utero to carbamazepine, lamotrigine, or
valproate monotherapy. Epilepsy Behav. 16:609–616. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaily E, Kantola-Sorsa E, Hiilesmaa V,
Isoaho M, Matila R, Kotila M, Nylund T, Bardy A, Kaaja E and
Granstrom ML: Normal intelligence in children with prenatal
exposure to carbamazepine. Neurology. 62:28–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hill DS, Wlodarczyk BJ, Palacios AM and
Finnell RH: Teratogenic effects of antiepileptic drugs. Expert Rev
Neurother. 10:943–959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rogawski MA and Löscher W: The
neurobiology of antiepileptic drugs. Nat Rev Neurosci. 5:553–564.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walker BE and Patterson A: Induction of
cleft palate in mice by tranquilizers and barbiturates. Teratology.
10:159–163. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ornoy A: Neuroteratogens in man: an
overview with special emphasis on the teratogenicity of
antiepileptic drugs in pregnancy. Reprod Toxicol. 22:214–226. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mantovani A and Calamandrei G: Delayed
developmental effects following prenatal exposure to drugs. Curr
Pharm Des. 7:859–880. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wlodarczyk BJ, Palacios AM, George TM and
Finnell RH: Antiepileptic drugs and pregnancy outcomes. Am J Med
Genet A. 158A:2071–2090. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bruni J and Wilder BJ: Valproic acid.
Review of a new antiepileptic drug. Arch Neurol. 36:393–398. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Herrmann K: Effects of the anticonvulsant
drug valproic acid and related substances on the early development
of the zebrafish (Brachydanio rerio). Toxicol In Vitro. 7:41–54.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rao S, Rajesh KR and Joseph T: Effect of
antiepileptic drugs valproic acid, carbamazepine and ethosuccimide
on exploratory behaviour in mice. Indian J Exp Biol. 29:127–130.
1991.PubMed/NCBI
|
|
25
|
Eyal S, Lamb JG, Smith-Yockman M, Yagen B,
Fibach E, Altschuler Y, White HS and Bialer M: The antiepileptic
and anticancer agent, valproic acid, induces P-glycoprotein in
human tumour cell lines and in rat liver. Br J Pharmacol.
149:250–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wlodarczyk B, Biernacki B and Minta M:
Teratogenic action of antiepileptic drug: 2-Propylpentanoic
acid-effects on rat and hamster embryos cultured in vitro. Ginekol
Pol. 72:955–960. 2001.PubMed/NCBI
|
|
27
|
Faiella A, Wernig M, Consalez GG, Hostick
U, Hofmann C, Hustert E, Boncinelli E, Balling R and Nadeau JH: A
mouse model for valproate teratogenicity: parental effects,
homeotic transformations, and altered HOX expression. Hum Mol
Genet. 9:227–236. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DiLiberti JH, Farndon PA, Dennis NR and
Curry CJ: The fetal valproate syndrome. Am J Med Genet. 19:473–481.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ornoy A: Valproic acid in pregnancy: how
much are we endangering the embryo and fetus? Reprod Toxicol.
28:1–10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Diav-Citrin O, Shechtman S, Arnon J and
Ornoy A: Is carbamazepine teratogenic? A prospective controlled
study of 210 pregnancies. Neurology. 57:321–324. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harding JJ and Timko JV: The use of
psychotropic medications during pregnancy and lactation. The
Foundation for The Global Library of Women's Medicine; Carlisle:
1999
|
|
32
|
Holmes LB, Harvey EA, Coull BA, Huntington
KB, Khoshbin S, Hayes AM and Ryan LM: The teratogenicity of
anticonvulsant drugs. N Engl J Med. 344:1132–1138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cunnington M and Tennis P: International
Lamotrigine Pregnancy Registry Scientific Advisory Committee:
Lamotrigine and the risk of malformations in pregnancy. Neurology.
64:955–960. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tennis P and Eldridge RR: International
Lamotrigine Pregnancy Registry Scientific Advisory Committee:
Preliminary results on pregnancy outcomes in women using
lamotrigine. Epilepsia. 43:1161–1167. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Allen RW Jr, Ogden B, Bentley FL and Jung
AL: Fetal hydantoin syndrome, neuroblastoma, and hemorrhagic
disease in a neonate. JAMA. 244:1464–1465. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adams J, Vorhees CV and Middaugh LD:
Developmental neurotoxicity of anticonvulsants: human and animal
evidence on phenytoin. Neurotoxicol Teratol. 12:203–214. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hunt S, Russell A, Smithson WH, Parsons L,
Robertson I, Waddell R, Irwin B, Morrison PJ, Morrow J and Craig J:
UK Epilepsy and Pregnancy Register: Topiramate in pregnancy:
preliminary experience from the UK Epilepsy and Pregnancy Register.
Neurology. 71:272–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lindhout D and Omtzigt JG: Teratogenic
effects of antiepileptic drugs: implications for the management of
epilepsy in women of childbearing age. Epilepsia. 35:(Suppl 4).
S19–S28. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tennis P, Chan KA, Curkendall SM, Li DK,
Mines D, Peterson C, Andrews EB, Calingaert B, Chen HY, Deshpande
G, Everage N, Holick CN, Meyer NM, Nkhoma ET, Quinn S, Rothman KJ
and Esposito DB: Topiramate use during pregnancy and major
congenital malformations in multiple populations. Birth Defects Res
A Clin Mol Teratol. 103:269–275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Green MW, Seeger JD, Peterson C and
Bhattacharyya A: Utilization of topiramate during pregnancy and
risk of birth defects. Headache. 52:1070–1084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lombardo SA, Leanza G, Meli C, Lombardo
ME, Mazzone L, Vincenti I and Cioni M: Maternal exposure to the
antiepileptic drug vigabatrin affects postnatal development in the
rat. Neurol Sci. 26:89–94. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kroes HY, Reefhuis J and Cornel MC: Is
there an association between maternal carbamazepine use during
pregnancy and eye malformations in the child? Epilepsia.
43:929–931. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Turanli G, Celebi A, Yalnizoglu D, Topcu
M, Topaloglu H, Banu A and Aysun S: Vigabatrin in pediatric
patients with refractory epilepsy. Turk J Pediatr. 48:25–30.
2006.PubMed/NCBI
|