Introduction
In patients with cancer, pain is a common symptom
and the major factor responsible for decreasing the quality of life
(1,2). A number of studies concerning the
prevalence of pain in cancer patients have shown that 24–60% of
patients undergoing active anticancer treatment (3,4) and
62–86% of terminal cancer patients (5,6) suffer
from burdensome pain symptoms. The unique pathophysiology of cancer
pain causes it to exceed that of a combination of inflammatory and
neuropathic pain (7). In addition,
there is evidence suggesting that patients with chronic pain always
exhibit immune suppression symptoms (8). It has been suggested that the levels of
CD4+ T cells in the serum of patients with cancer pain
are decreased (9). Therefore, cancer
pain and immune suppression are two main symptoms in cancer
patients.
Opioids are used widely to treat acute pain
following extensive surgery and many kinds of chronic pain,
particularly cancer pain (10–13). It
is known that opioids not only result in analgesia but also
modulate the immune system (14).
Opioids include endogenous opioid peptides and exogenous opiates.
There is growing evidence that acute and long-term administration
of exogenous opiates, especially morphine, which is a heterogenous
opioid, mediates immunosuppression (15). However, the effects of endogenous
opioids on the immune system remain a subject of debate, with some
reports that endogenous opioids promote the immune function and
others supporting the opposite view (15,16).
Although numerous studies have observed the effects
of opioid drugs on immune responses, the clinical relevance of
these observations for heterogenous and homogenous opioids remains
uncertain. Few studies have analyzed the association between
opioids and the immune system in vivo. To address this, in
the present study, Walker 256 cells were injected into a tibial
cavity in rats to establish a bone cancer pain model. Recombinant
rat β-endorphin (β-EP; 50 µg/kg) and plant-derived morphine (10
mg/kg) were administered by intraperitoneal injection and the
analgesic effects were compared. In addition, the effects of the
opioids on cellular immune function, specifically T lymphocyte
proliferation, natural killer (NK) cell cytotoxicity and the levels
of T cell subgroups in the bone cancer pain models were examined,
and the differences between the effects on cellular immune function
were compared between the heterogenous and homogenous opioid
treatment groups. The aim of this study was to provide scientific
evidence useful in the development of human opioids to treat cancer
pain.
Materials and methods
Animals
A total of 40 adult female Sprague-Dawley (SD) rats
weighing (150–170 g; Shanghai SLAC Laboratory Animal Co., Ltd,
Shanghai, China) and 10 female SD rats (weight, 70–80 g; Shanghai
SLAC Laboratory Animal Co., Ltd.) were raised in a 12-h light/dark
cycle with access to plentiful amounts of food and water. They were
housed five per cage and were acclimatized for 1 week prior to
behavioral studies. Efforts were made to minimize animal discomfort
and reduce the numbers of animals used. The animal protocols were
approved by the Animal Ethics Committee at Zhejiang Chinese Medical
University (Hangzhou, China).
Surgery
Walker 256 cells (1×107; The Cell Bank of
Type Culture Collection of Chinese Academy of Sciences, Shanghai,
China) were administered by intraperitoneal injection into the
abdominal cavity of juvenile rats (70–80 g). After 7 days, ascites
were generated in the peritoneal cavity and carcinoma cells were
harvested through sterile syringes. The percentage of cellular
activity was checked to ensure that it was >95%, as measured
using a TC10™ Automated Cell Counter (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Female SD rats were anesthetized by the
administration of 10% chloral hydrate (0.35 ml/100 g)
intraperitoneally, and then placed in a supine position. The left
leg of the rat was shaved and the skin sterilized with iodophor and
75% ethanol. A 1-cm rostro-caudal incision was then made in the
skin in the upper half of the tibia. The tibia was carefully
exposed with minimal damage to the muscle and blood vessels. A
21-gauge needle was inserted at the site of intercondylar eminence
at a 30–45° angle and pierced 5 mm below the knee joint into the
medullary cavity of the tibia. The needle was then removed and
replaced with a 10-µl syringe (Hamilton Co., Bonaduz, Switzerland)
containing the carcinoma cells (3×105) to be injected
into the tibial cavity. The syringe was kept in position for 2 min
prior to removal from the tibial cavity to prevent cells from
leaking out along the injection hole. The injection site was
quickly sealed using bone wax and the wound was closed with
stitches. Penicillin (20,000 units, intramuscular injection) was
given to avoid infection.
Rats of the sham surgery group were injected with
the same volume of phosphate-buffered saline (PBS) into the tibial
cavity, and the other protocols were the same as those used in the
surgery group.
Experimental groups
The rats were separated randomly into four groups:
i) Sham surgery group (n=10); ii) surgery group (n=10); iii)
morphine group (n=10); and iv) β-EP group (n=10).
Paw withdrawal thresholds (PWTs)
The PWTs were observed at six time points: Baseline
(prior to surgery), at 6 days after surgery and following 1, 3, 6,
and 8 treatments (as described below). As in a previous study
(17), rats were adapted to the new
environment by being placed on a metal mesh table. A mechanical
stimulus (force 0–50 g over a 20 sec time period) was delivered to
the plantar surface of the left hind paw using a Dynamic Plantar
Aesthesiometer (37450; Ugo Basile, Monvalle, Italy). When the
animal withdrew its hind paw, the mechanical stimulus was
automatically stopped, and the force at which the animal withdrew
its paw was recorded as the PWT. Withdrawal responses were taken
from four consecutive trials with ≥3 min between trials and
averaged.
Administration of treatments
Immediately after finishing the measurement of PWTs
on 6 day, rats in the morphine group were intraperitoneally
injected once every other day with 10 mg/kg morphine hydrochloride
injection (C81004-2; Northeast Pharmaceutical Group Shenyang No. 1
Pharmaceutical Co., Ltd., Shenyang, China) and rats in the β-EP
group were injected intraperitoneally with 50 µg/kg β-EP (H-284;
Bachem AG, Hauptstrasse, Switzerland) for 15 days, once every other
day. Sham surgery and surgery groups did not received any
treatment.
Body weight measurements
The body weights of the rats were measured at
baseline, at 6 days after surgery and following 1, 3, 6, and 8
treatments. The increase in body weight was calculated as follows:
Body weight growth rate (%) = measured value/basal value × 100.
Extraction of splenic monocytes
Rats were sacrificed by cervical dislocation after
the last PWT had been measured. The dead rats were soaked into 75%
alcohol, and then moved onto a super clean bench. The spleen was
excised, soaked in RPMI-1640 medium, HEPES [RPMI-1640 supplemented
with 10% fetal bovine serum (FBS), 10,000 U/ml penicillin G and
10,000 µg/ml streptomycin; Thermo Fisher Scientific, Inc., Waltham,
MA, USA] for 20 min. The spleen was placed on a 200-mesh stainless
steel screen, cut into pieces and then ground, using PBS (sterile)
to keep the tissue moist during the whole experiment. The obtained
cell suspension was combined with 3–5 volumes of red blood cell
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China),
and mixed gently. After standing for 2 min, the suspension was
centrifuged at room temperature for 10 min at 1,000 g, and the
supernatant was discarded. The obtained cell suspension was
combined with 5 volumes of PBS (sterile), mixed gently, then
centrifuged at room temperature for 10 min at 1,000 × g, twice.
Cells were suspended in RPMI-1640 (10% FBS, 100 U/ml penicillin G
and 100 µg/ml streptomycin) after cleaning, and the concentration
of the cell suspension was adjusted to 1×106/ml.
T lymphocyte proliferation assays
Splenic monocytes were seeded into 96-well plates at
2×105 cells/well in triplicate. For the test samples, 20
µl concanavalin A (ConA; 10 µg/ml; Sigma-Aldrich, St. Louis, MO,
USA) was added to each well. For the control, 20 µl RPMI-1640 was
added to three wells. The plates were incubated at 37°C for 72 h.
Cell viability was assessed using Cell Counting kit-8 (CCK-8;
Beyotime Institute of Biotechnology) and according to the
manufacturer's protocol. WTS-8 (20 µl) was added to each well and
plates were incubated at 37°C for 4 h. The absorbance of each
sample was measured at 450 nm using a microtiter plate reader
(SpectraMax M4; Molecular Devices, LLC, Sunnyvale, CA, USA). The
reference wavelength was >650 nm. The T lymphocyte proliferation
function was determined using the following equation: T lymphocyte
activity (%) = absorbance of test sample/absorbance of control ×
100.
NK cell cytotoxicity assays
YAC-1, a mouse lymphoma cell line, was purchased
from Shanghai Institutes for Biological Sciences, Chinese Academy
of Sciences (No. TCM28; Shanghai, China). Cells were grown in
suspension in a culture bottle (Corning Incorporated, Corning, NY,
USA), with RPMI-1640 medium, HEPES (RPMI-1640 supplemented with 10%
FBS, 100 U/ml penicillin G and 100 µg/ml streptomycin). Only cells
in the exponential growth phase were used for cytotoxicity assays.
The YAC-1 cells were used as sensitive target cells for the
evaluation of NK cell cytotoxicity in vitro (18). Determination of NK cell function was
implemented using an enzymatic colorimetric technique involving
lactate dehydrogenase (LDH) release (LDH-cytotoxicity assay kit;
BioVision, Inc., Milpitas, CA, USA).
Splenic monocytes as effector cells were incubated
in RPMI-1640 medium, HEPES (RPMI-1640 supplemented with 10% FBS,
100 U/ml penicillin G and 100 µg/ml streptomycin). Viability of
effector and target cells was determined by the trypan blue dye
exclusion test prior to the cytotoxicity test to confirm that the
viability was >95%. In the test sample, the effector cells at a
concentration of 1×106 in 100 µl culture medium were
mixed with 100 µl YAC-1 cells at a concentration of
2×104, resulting in an effector cell:target cell ratio
of 50:1. As the background control, 200 µl medium/well was added to
triplicate wells (the background value was subtracted from all
other values). As the low control, 1×106 cells/well in
200 µl culture medium were added to triplicate wells. As the high
control, 1×106 cells/well in 200 µl culture medium
containing 1% Triton X-100 were added to triplicate wells. Each
test sample and all controls were evaluated in triplicate in
96-micro-well plates, and incubated at 37°C in a thermostatic
incubator with 5% CO2 for 4 h. The micro-well plates
were centrifuged at 250 × g for 10 min, and the supernatant was
isolated. The LDH reaction mixture was added and maintained for 30
min at room temperature with the absence of light. The absorbance
was measured using the SpectraMax M4 reader at 490 nm and the
percentage of cytotoxicity was determined using the following
equation: Cytotoxicity (%) = [absorbance (test sample - background
control - low control)]/[absorbance (high control - low control)] ×
100.
Flow cytometry assay
Spleen cell suspensions were collected from the cell
culture bottles, and were adjusted to a concentration of
1–5×106 cells/ml. Fluorescein isothiocyanate
(FITC)-conjugated anti-rat CD3 monoclonal antibody (cat. no.
E00051-1631; eBioscience, Inc., San Diego, CA, USA),
allophycocyanin (APC)-conjugated anti-rat CD4 monoclonal antibody
((E07034-1632; eBioscience, Inc.) and phycoerythrin (PE)-conjugated
anti-rat CD8a monoclonal antibody (cat. no. E01045-1633;
eBioscience, Inc.) were used for T cell, helper T cell and
cytotoxic T cell detection respectively. Spleen cell suspensions
were added to the 100 µl paraformaldehyde solution and were
incubated with the above-mentioned antibodies for 15 min at 4°C.
The cells were then washed three times with PBS, and 500 µl PBS was
added to resuspend the cells. CD3+, CD4+ and
CD8a+ cell analysis was performed within the lymphocyte
cell range. The data were analyzed using BD FACSCanto II software
(BD Biosciences, Franklin Lakes, NJ, USA). PBS was substituted for
the antibody to serve as the control. Monoclonal mouse IgG3 Isotype
Control FITC (cat. no. E11772-1632; eBioscience, Inc.), mouse IgG2a
K Isotype Control APC (cat. no. E11418-1633; eBioscience, Inc.) and
mouse IgG1 K Isotype Control PE (cat. no. E11418-1633; eBioscience,
Inc.) were used as the respective isotype controls.
Enzyme linked immunosorbent assay
(ELISA) for plasma interleukin (IL)-2
Plasma was analyzed to determine IL-2 levels using a
Quantikine® ELISA kit (R&D Systems Europe, Ltd.,
Abingdon, UK) according to manufacturer's protocol. All samples
were run on one 96-well plate for each variable. Plasma IL-2 levels
were measured using the SpectraMax M4 microplate reader. Each
sample was measured in triplicate.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. The PWTs were analyzed using a two-way analysis of
covariance (ANOVA) with repeated measures. One-way ANOVA with post
hoc multiple comparisons was applied to identify differences
between experimental groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of opioids on PWTs in a rat
model of bone cancer pain
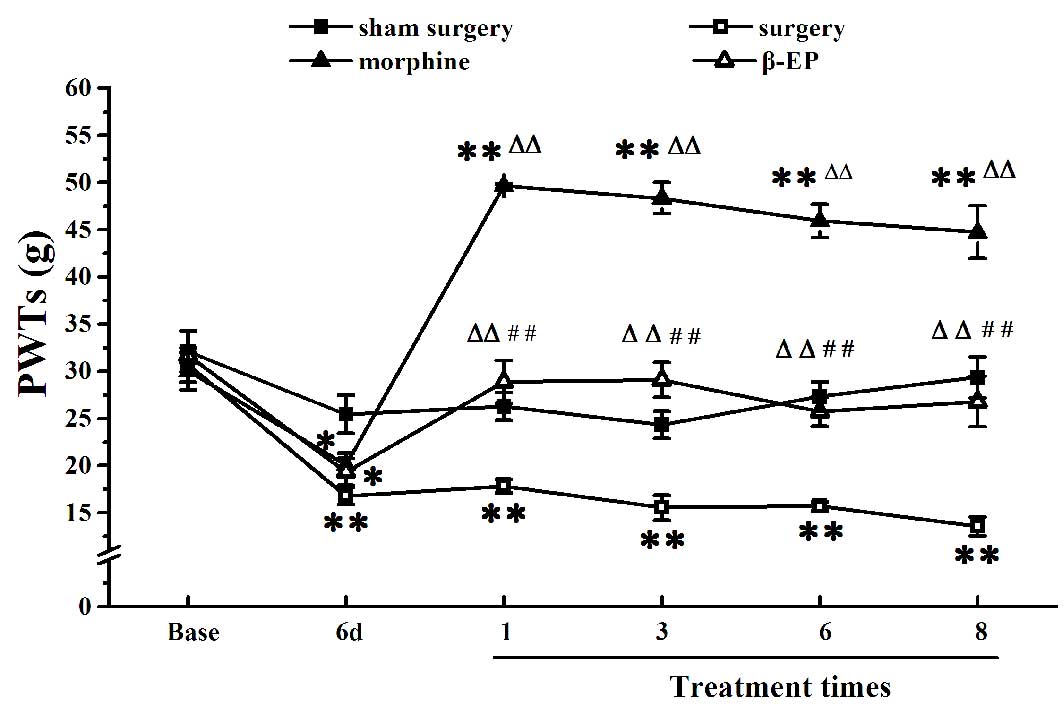
As shown in Fig. 1,
there were no differences of basal PWTs among the sham surgery,
surgery, morphine and β-EP groups (P>0.05). Compared with the
sham surgery group, the injection of Walker 256 cells into the
tibial cavity in the surgery group induced a marked reduction of
the PWT in the ipsilateral hind paw at 6 days (P<0.01). After
receiving treatment 1, 3, 6 and 8 times, the PWTs of the β-EP and
morphine group were markedly increased compared with those of the
surgery group (P<0.01). The PWTs of the morphine group were the
most increased, being higher than those of the β-EP group at any
time of treatment (P<0.01).
Effects of opioids on body weight
increase in a rat model of bone cancer pain
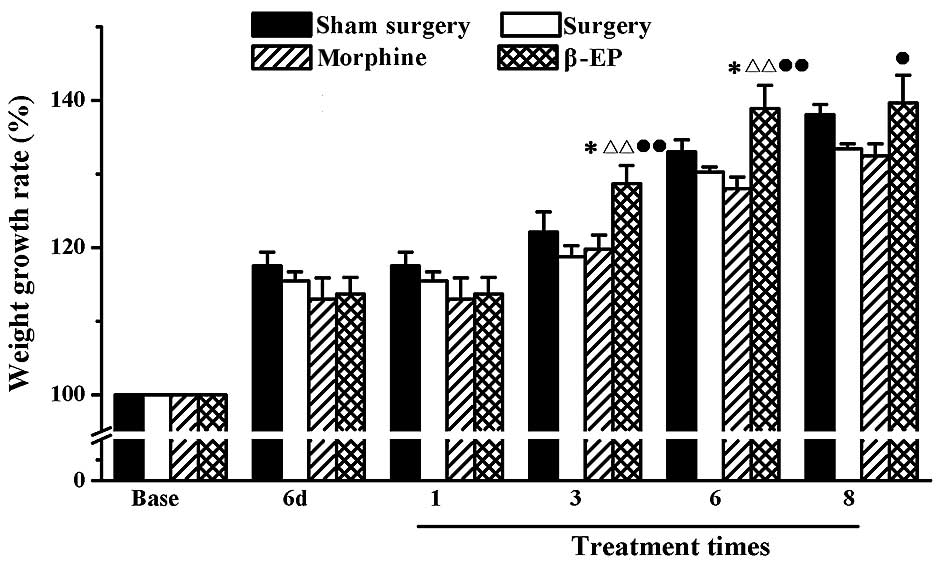
The percentage increase in body weight of the rats
in each group was determined. As shown in Fig. 2, the weight increase in the sham
surgery, surgery, morphine and β-EP groups were not different prior
to surgery, on 6 day after surgery, and following one treatment.
After treatment 3 and 6 times, the percentage increase in body
weight of the β-EP group was higher than that of the sham surgery
(P<0.05), surgery (P<0.01) and morphine groups (P<0.01).
After 8 treatments, the percentage increase of body weight in the
β-EP group was higher than that of the morphine group only
(P<0.05).
Effects of opioids on T cell
proliferation in the splenic lymphocytes of a rat model of bone
cancer pain
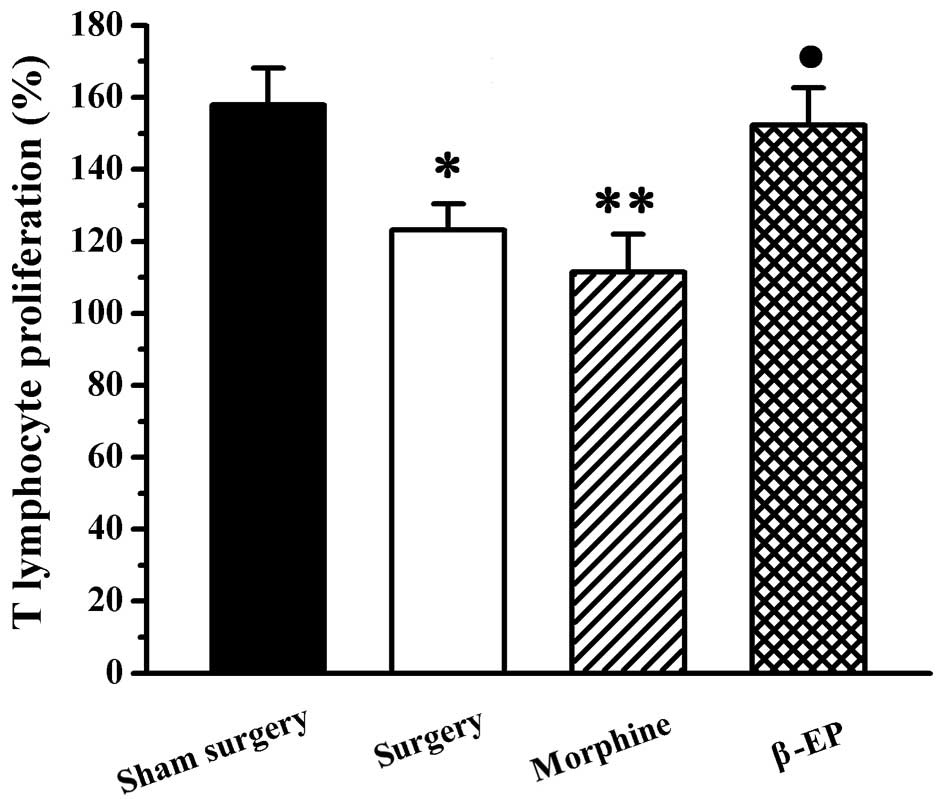
A Cell Counting kit-8 assay was conducted to observe
the T cell growth rate of the spleen, with measurement of the
optical density for splenic lymphocytes incubated with WST-8 for 4
h. As shown in Fig. 3, compared with
the sham surgery group, the T cell growth rates of the surgery
group and morphine group were significantly decreased (P<0.05
and P<0.01, respectively), and there was no significant
difference between the surgery and morphine groups (P>0.05).
Compared with morphine group, the T cell growth rate of the β-EP
group was significantly increased (P<0.05).
Effects of opioids on NK cell
cytotoxicity in splenic lymphocytes of a rat model of bone cancer
pain
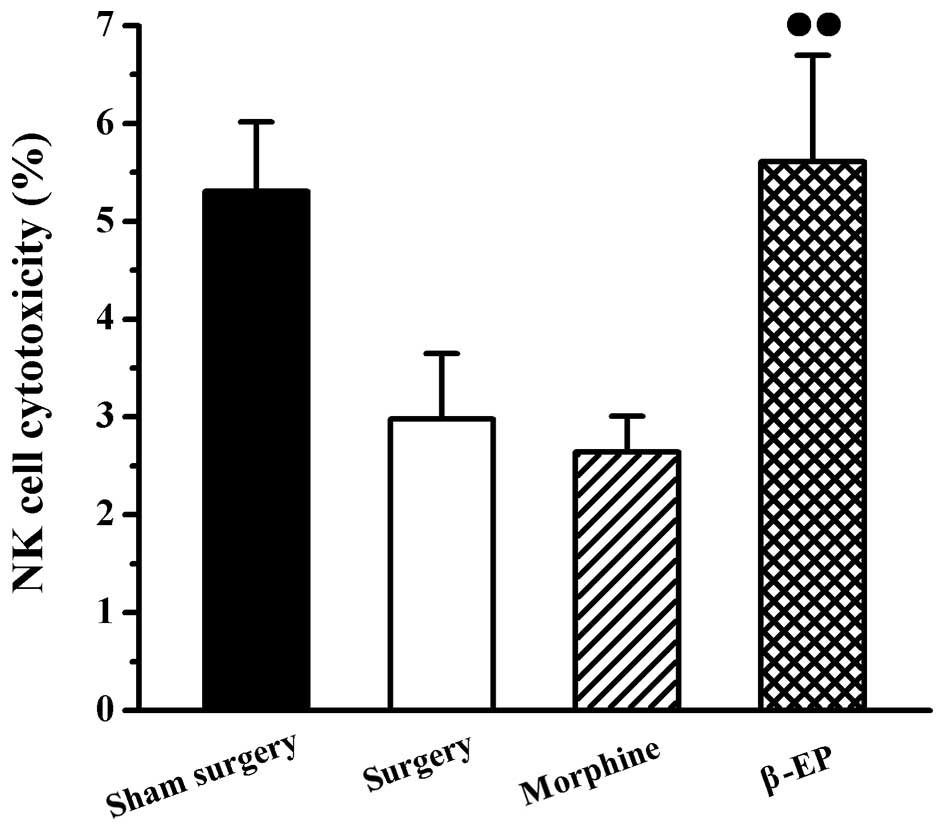
An assay involving LDH release was used to measure
the cytotoxicity of NK cells in the spleen. As shown in Fig. 4, there were no significant
differences of spleen NK cell cytotoxicity among the sham surgery,
surgery and morphine groups. The NK cell cytotoxicity of the spleen
in the β-EP group was stronger than that of the morphine group
(P<0.05).
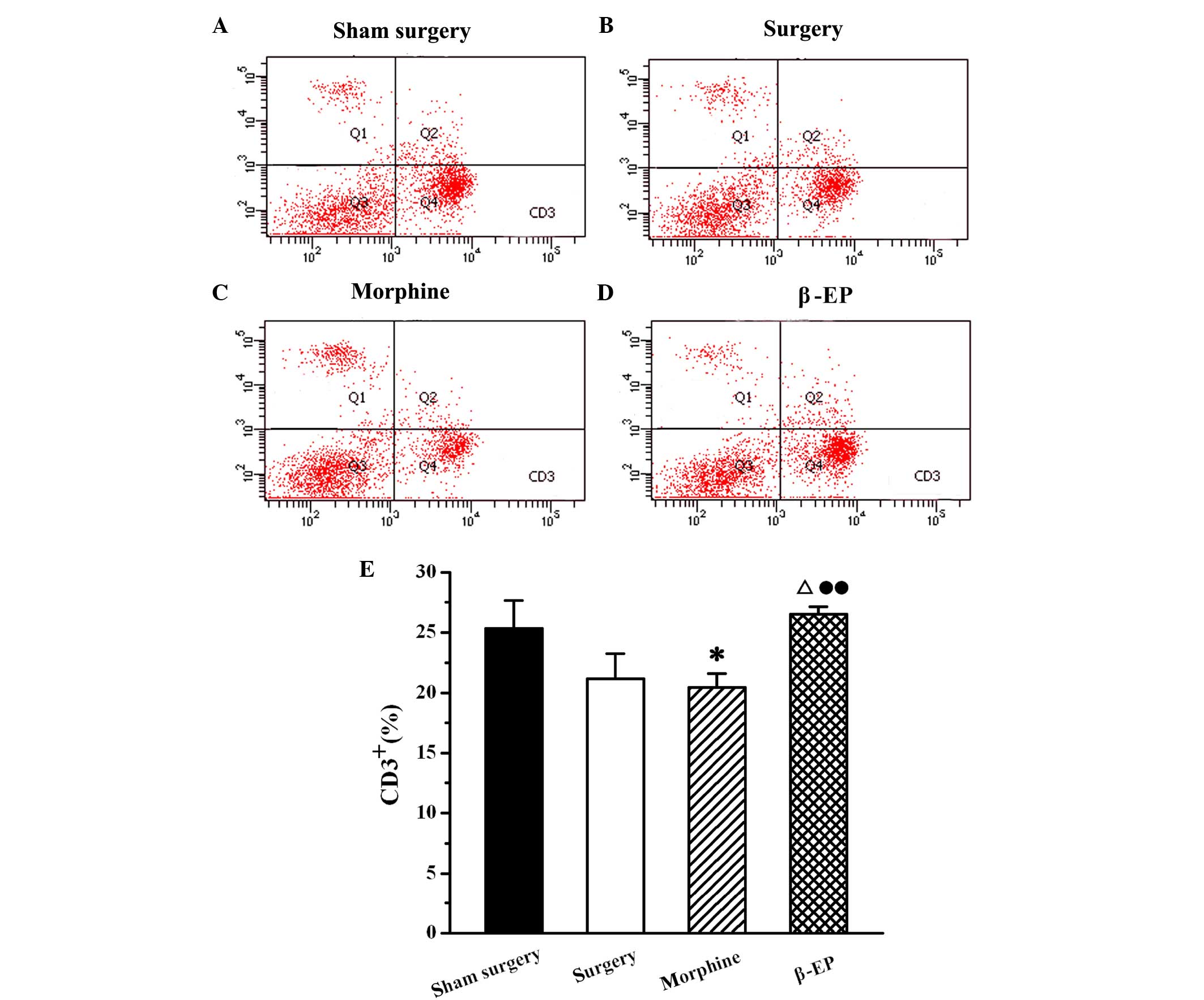
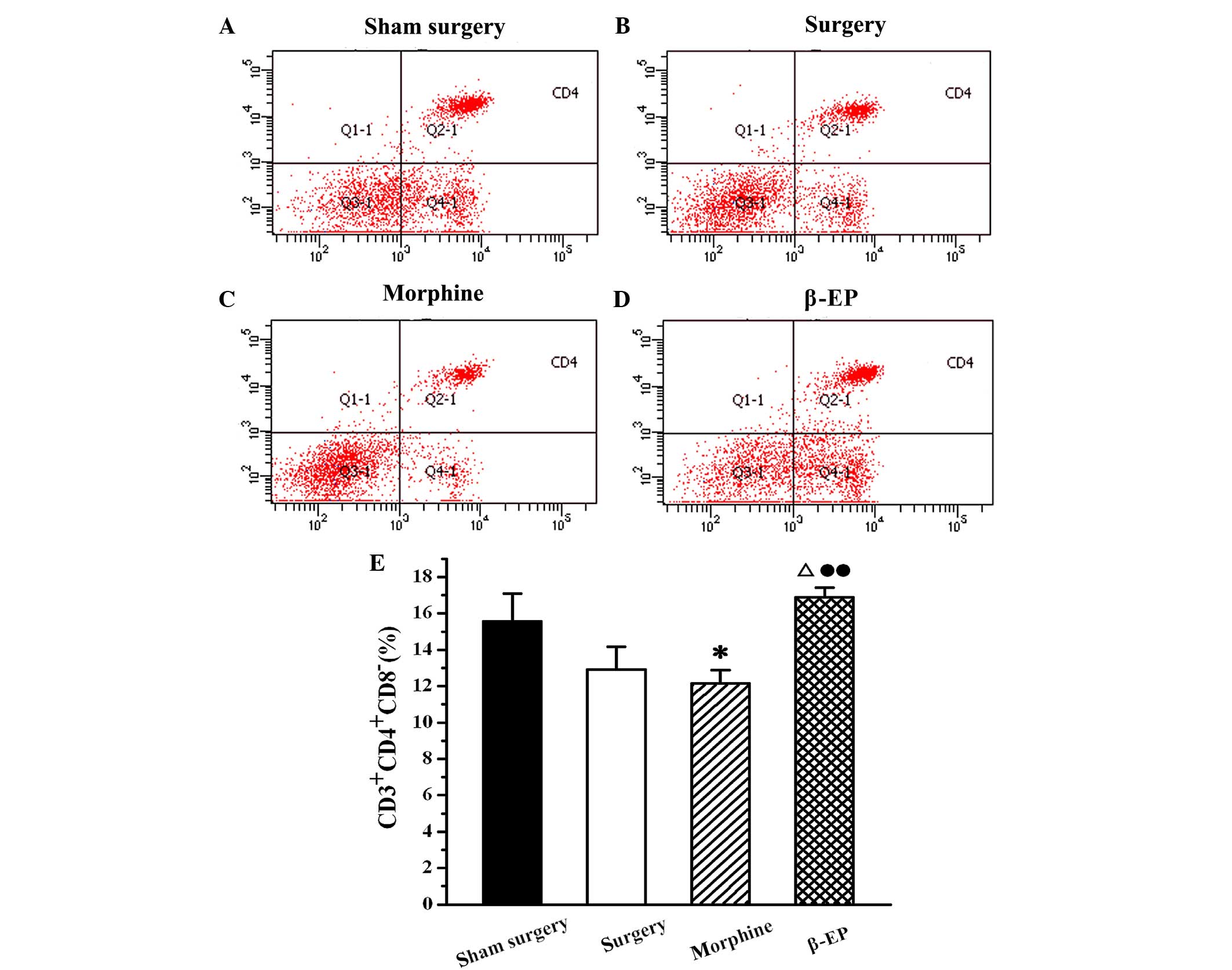
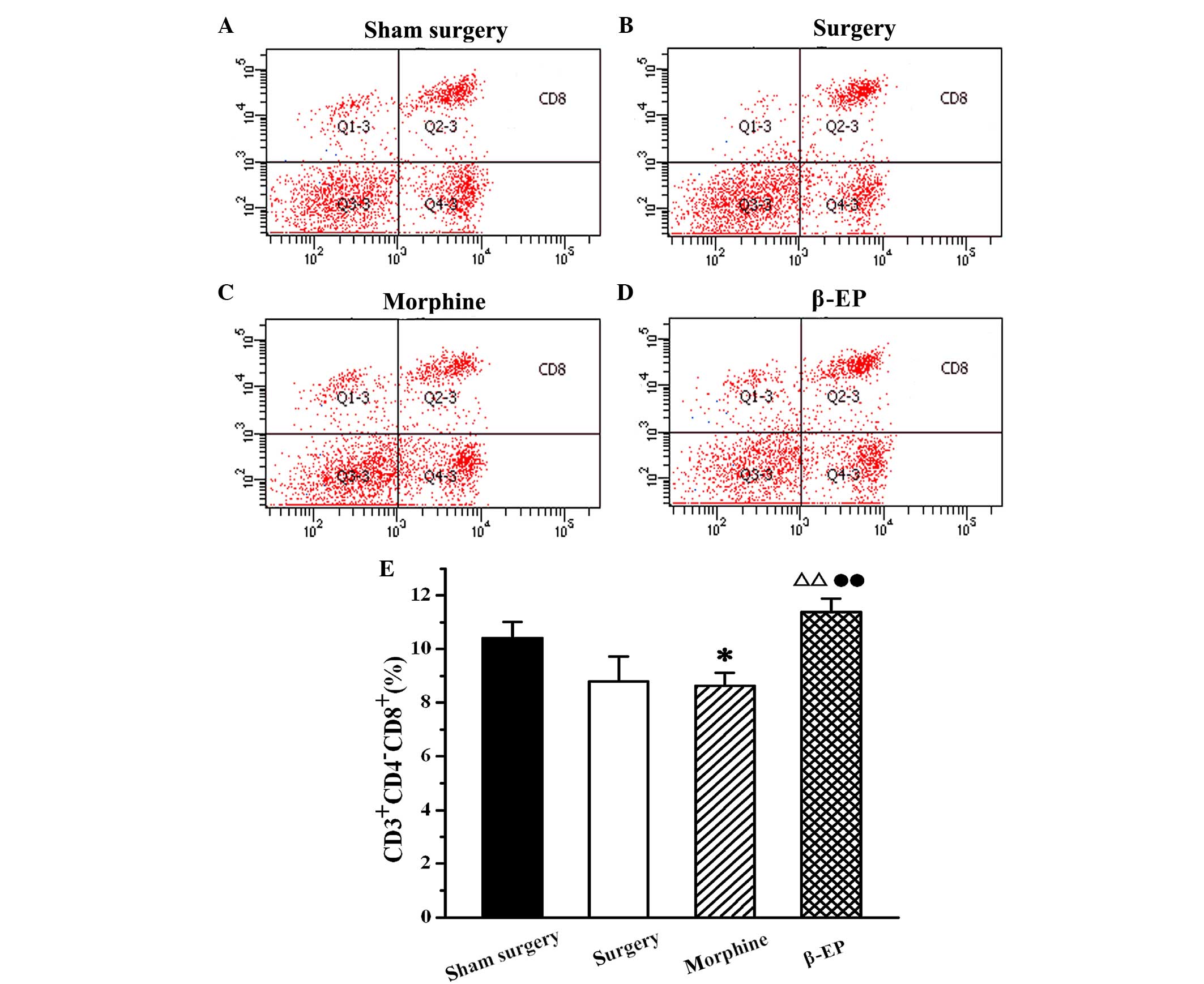
Effects of opioids on levels of T cell
subtypes (CD3+, CD4+ and CD8+ cells) in splenic lymphocytes of a
rat model of bone cancer pain
Flow cytometry was used to assay the content of
CD3+, CD4+ and CD8+ cells in
splenic lymphocytes among the sham surgery, surgery, morphine and
β-EP groups. As shown in Fig. 5, the
percentage of CD3+ cells in the splenocytes of the
surgery group was decreased compared with that in the sham surgery
group, but the reduction was not statistically significant. When
the surgery and morphine groups were compared with the β-EP group,
the differences in CD3+ cell percentages were
significant (P<0.05 and P<0.01, respectively). The percentage
of CD3+ cells in the β-EP group was greater than that in
the surgery and morphine groups, and there was no significant
difference between the surgery and morphine groups. The results for
CD4+ and CD8+ percentages in the splenic
lymphocytes were similar to those for CD3 (Figs. 6 and 7).
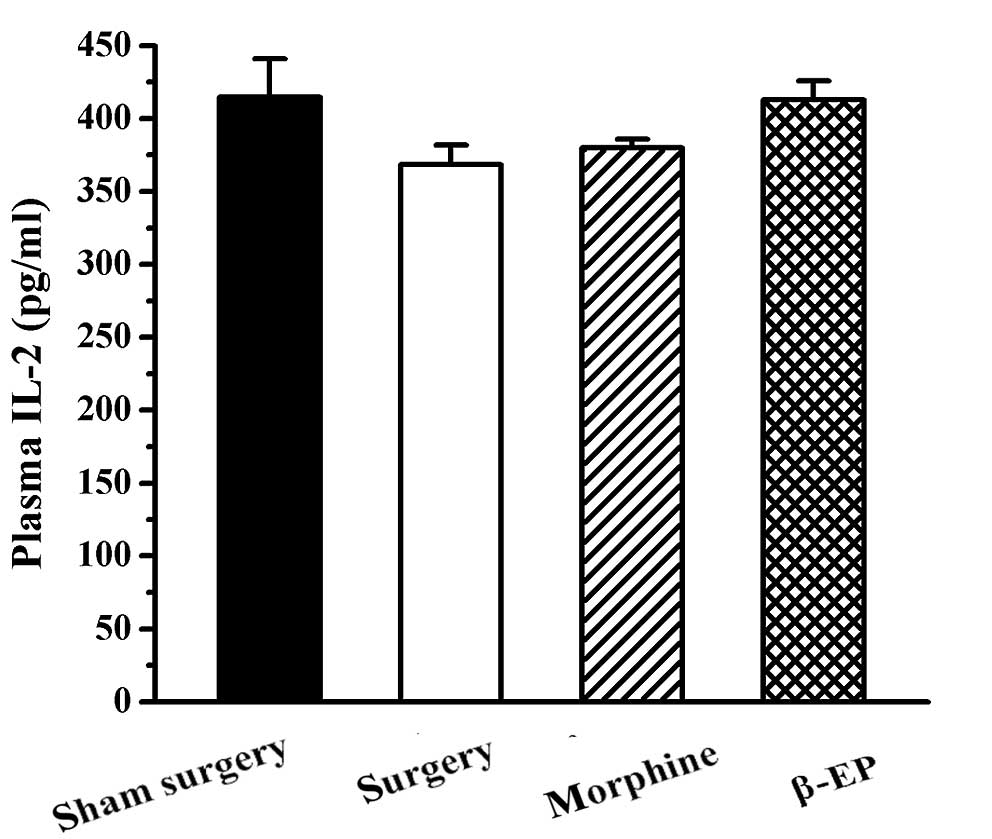
Effects of opioids on plasma IL-2
levels in a rat model of bone cancer pain
No significant difference was found among the sham
surgery, surgery, morphine and β-EP groups with regard to plasma
IL-2 level (P>0.05; Fig. 8).
Discussion
In the present study, the analgesic effect and
immune modulating function of a homogenous opioid peptide (rat
source β-EP) and heterogenous opioid (plant source morphine) were
compared in a bone cancer pain model. It was found that both β-EP
and morphine have good analgesic effects in this bone cancer pain
model, and that the analgesia provided by of morphine was stronger
than that of β-EP. Morphine treatment reduced the spleen T cell
growth rate and the content of T cell subtypes (CD3+,
CD4+ and CD8+ cells), whereas β-EP
administration had the opposite effects on those indices as well as
the NK cell cytotoxicity. Morphine and β-EP each had no effect on
plasma IL-2 levels. These results demonstrate that the homogenous
opioid had a positive effect on cancer pain.
Bone cancer pain in the most commonly used model of
cancer pain, and many researchers have successfully established
calcaneus, tibial and femur bone cancer pain models. Injection of
Walker 256 cells into a tibial cavity can be used to study the
mechanism of bone cancer pain. Mao-Ying et al reported that
bone cancer developed from intra-tibial Walker 256 cells induced
ambulatory pain and mechanical allodynia, and also reduced weight
bearing, but that thermal hyperalgesia was not observed after
Walker 256 cell inoculation (19). A
previous study by the present team had similar results; it found
that in a bone cancer model induced by the injection of Walker 256
cells into the tibial cavity, the rats had mechanical allodynia and
spontaneous pain on days 4–22, and thermal hyperalgesia appeared in
the intermediate stage (20). It was
also found that the tibial cavity injection of Walker 256 cells in
the bone cancer model induced a reduction in PWTs.
A number of studies have demonstrated that NK cells
are able to kill tumor target cells in vitro and in
vivo in animal models (21–23).
Studies have shown that decreased NK cell activity is associated
with the growth and development of a variety of cancers in humans
(24) and animals (25), and NK cells protect against the
metastatic spread of tumor cells (26). Multiple studies have provided
evidence that morphine, a typical exogenous opiate, is involved in
inhibiting the innate immune response (27). It has been reported that NK cell
immune function is downregulated by morphine in vivo
(28). NK cells are the first line
of defense of the immune system, with a key role in the host
defense against tumor cells (29).
NK cells represent a unique subset of lymphocytes that have no
restriction by major histocompatibility complex (MHC) antigens, are
important in the initiation of tumor development and have the
ability to lyse certain tumor cells without the requirement for
prior immune sensitization of the host (30,31). Not
all kinds of opioids share the same immunosuppressive effects. It
has been found that β-EP, an endogenous opioid peptide, promotes
innate immune function and reduces the incidence of cancer in rat
models (32). β-EP can also increase
peripheral NK cell activities; in vitro differentiated β-EP
cells when transplanted into the paraventricular nucleus improved
NK cell cytolytic function in model rats (33). In the present study, intraperitoneal
morphine and β-EP administration had different effects on NK cell
cytotoxic activity. The systemic injection of rat source β-EP, a
homogenous opioid peptide, increased the NK cell cytotoxicity of
the rats with bone cancer pain, while the systemic injection of
morphine, a heterogenous opiate, did not have such an effect.
In the present study, in addition to reducing the
immune function of NK cells, morphine treatment also inhibited T
cell function. It has been suggested that the immune system is able
to detect and reject incipient tumors, and that total T cells
(CD3+), helper T cells (CD4+) and cytotoxic T
cells (CD8+) play key roles in tumor immunology
(34). CD8+ cells are
traditionally considered to be the major mediators of an effective
antitumor response by T cells. This is based on the pronounced
cytotoxic activity of CD8+ T cells exhibited in
vitro, and the observation that tumors capable of evading
attack by CD8+ T cells may have altered or downregulated
expression of MHC class I antigens (35–37).
Furthermore, in a study involving a transgenic mouse with MHC class
I-restricted T cell receptors, it was found that CD8+ T
cells maintained an antitumor effect when CD4+ T cells
were absent (38). Conflicting with
these observations, other studies have indicated that the antitumor
effects of CD8+ T cells alone are limited (39,40).
CD4+ cells that were limited by MHC II of themselves
recognized exogenous antigen peptides (about 13–17 amino acids
long). Thus, the MHC class II status of tumor cells is of
importance in the immune response of CD4+ T cells to
tumors. However, a number of studies have indicated that
CD4+ T cells contribute to the eradication of tumors
even in the absence of CD8+ T cells (41,42).
Cytotoxic CD4+ T cells have been shown to be capable of
directly eliminating tumor cells that are MHC class II positive, in
addition to indirectly killing tumor cells that lack MHC class II
expression (43,44). In the present study, it was found
that the systemic injection of rat-derived β-EP, a homogenous
opioid peptide, increased CD3+, CD4+ and
CD8+ T cell subtype expression in a rat model of bone
cancer pain, while the systemic injection of morphine, a
heterogenous opioid compound, reduced their expression.
In conclusion, morphine and β-EP exhibited good
analgesic effects in the rat model of bone cancer pain, and the
analgesia provided by morphine was stronger than that of β-EP.
Morphine and β-EP administration in vivo have no significant
effect on the secretion of IL-2 by T cells. With regard to T cell
proliferation rate, the effects of the two different types of
opioids differed; morphine suppressed T cell proliferation and β-EP
increased it. The opioid compounds from different sources exhibited
different effects on the adaptive cell immune; the homogenous
opioid peptide β-EP increased the adaptive cell immune
function.
Acknowledgements
This study was supported by the National Natural
Science Fund of China (grant no. 81102643) and the State
Administrative Bureau of Traditional Chinese Medicine (Acupuncture)
of Key Subjects Construction Funding [grant no. (2009) 30].
References
|
1
|
Mercadante S and Fulfaro F: Management of
painful bone metastases. Curr Opin Oncol. 19:308–314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marcus DA: Epidemiology of cancer pain.
Curr Pain Headache Rep. 15:231–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pignon T, Fernandez L, Ayasso S, Durand
MA, Badinand D and Cowen D: Impact of radiation oncology practice
on pain: A cross-sectional survey. Int J Radiat Oncol Biol Phys.
60:1204–1210. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rietman JS, Dijkstra PU, Debreczeni R,
Geertzen JH, Robinson DP and De Vries J: Impairments, disabilities
and health related quality of life after treatment for breast
cancer: A follow-up study 2.7 years after surgery. Disabil Rehabil.
26:78–84. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilson KG, Graham ID, Viola RA, Chater S,
de Faye BJ, Weaver LA and Lachance JA: Structured interview
assessment of symptoms and concerns in palliative care. Can J
Psychiatry. 49:350–358. 2004.PubMed/NCBI
|
|
6
|
van den Beuken-van Everdingen MH, de Rijke
JM, Kessels AG, Schouten HC, van Kleef M and Patijn J: Prevalence
of pain in patients with cancer: A systematic review of the past 40
years. Ann Oncol. 18:1437–1449. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Honore P, Rogers SD, Schwei MJ,
Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR and Mantyh PW:
Murine models of inflammatory, neuropathic and cancer pain each
generates a unique set of neurochemical changes in the spinal cord
and sensory neurons. Neuroscience. 98:585–598. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schwab JM, Zhang Y, Kopp MA, Brommer B and
Popovich PG: The paradox of chronic neuroinflammation, systemic
immune suppression, autoimmunity after traumatic chronic spinal
cord injury. Exp Neurol. 258:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HY, Li SG, Cho WC and Zhang ZJ: The
role of acupoint stimulation as an adjunct therapy for lung cancer:
A systematic review and meta-analysis. BMC Complement Altern Med.
13:3622013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sacerdote P: Opioid-induced
immunosuppression. Curr Opin Support Palliat Care. 2:14–18. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Smith RJ, Rhodes K, Paciotti B, Kelly S,
Perrone J and Meisel ZF: Patient perspectives of acute pain
management in the era of the opioid epidemic. Ann Emerg Med.
66:246–252, e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kostev K, Wartenberg F, Richter H,
Reinwald M and Heilmaier C: Persistence with opioid treatment in
Germany in patients suffering from chronic non-malignant or cancer
pain. Curr Med Res Opin. 31:1157–1163. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deyo RA, Von Korff M and Duhrkoop D:
Opioids for low back pain. BMJ. 350:g63802015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stefano GB, Fricchione G, Goumon Y and
Esch T: Pain, immunity, opiate and opioid compounds and health. Med
Sci Monit. 11:MS47–MS53. 2005.PubMed/NCBI
|
|
15
|
Vallejo R, de Leon-Casasola O and Benyamin
R: Opioid therapy and immunosuppression: A review. Am J Ther.
11:354–365. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sacerdote P: Opioids and the immune
system. Palliat Med. 20 Suppl 1:S9–S15. 2006.PubMed/NCBI
|
|
17
|
Fang JQ, Du JY, Liang Y and Fang JF:
Intervention of electroacupuncture on spinal p38 MAPK/ATF-2/VR-1
pathway in treating inflammatory pain induced by CFA in rats. Mol
Pain. 9:132013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poli A, Brons NH, Ammerlaan W, Michel T,
Hentges F, Chekenya M and Zimmer J: Novel method for isolating
untouched rat natural killer cells with higher purity compared with
positive selection and fluorescence-activated cell sorting.
Immunology. 131:386–394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mao-Ying QL, Zhao J, Dong ZQ, Wang J, Yu
J, Yan MF, Zhang YQ, Wu GC and Wang YQ: A rat model of bone cancer
pain induced by intra-tibia inoculation of Walker 256 mammary gland
carcinoma cells. Biochem Biophys Res Commun. 345:1292–1298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jun-Ying D, Yi L, Yi-Tian C, et al:
Functional evaluation of spleen T lymphocytes in the rat model of
Walker-256 bone cancer pain. Zhong Guo Bi Jiao Yi Xue Za Zhi.
24:8-13MS2014.(In Chinese).
|
|
21
|
Wennerberg E, Kremer V, Childs R and
Lundqvist A: CXCL10-induced migration of adoptively transferred
human natural killer cells toward solid tumors causes regression of
tumor growth in vivo. Cancer Immunol Immunother. 64:225–235. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ardolino M, Azimi CS, Iannello A, Trevino
TN, Horan L, Zhang L, Deng W, Ring AM, Fischer S, Garcia KC and
Raulet DH: Cytokine therapy reverses NK cell anergy in
MHC-deficient tumors. J Clin Invest. 124:4781–4794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zitvogel L and Kroemer G: Cytokines
reinstate NK cell-mediated cancer immunosurveillance. J Clin
Invest. 124:4687–4689. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shereck E, Satwani P, Morris E and Cairo
MS: Human natural killer cells in health and disease. Pediatr Blood
Cancer. 49:615–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saijo N, Ozaki A, Beppu Y, Takahashi K,
Fujita J, Sasaki Y, Nomori H, Kimata M, Shimizu E and Hoshi A:
Analysis of metastatic spread and growth of tumor cells in mice
with depressed natural killer activity by anti-asialo GM1 antibody
or anticancer agents. J Cancer Res Clin Oncol. 107:157–163. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sacerdote P: Opioid-induced
immunosuppression. Curr Opin Support Palliat Care. 2:14–18. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang MC, Fan SZ, Hsiao PN, Cheng WF and
Sun WZ: Influence of morphine on host immunity. Acta Anaesthesiol
Taiwan. 49:105–108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brown JN, Ortiz GM, Angel TE, Jacobs JM,
Gritsenko M, Chan EY, Purdy DE, Murnane RD, Larsen K, Palermo RE,
et al: Morphine produces immunosuppressive effects in nonhuman
primates at the proteomic and cellular levels. Mol Cell Proteomics.
11:605–618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kheav VD, Busson M, Scieux C, de Latour R
Peffault, Maki G, Haas P, Mazeron MC, Carmagnat M, Masson E, Xhaard
A, et al: Favorable impact of natural killer cell reconstitution on
chronic graft-versus-host disease and cytomegalovirus reactivation
after allogeneic hematopoietic stem cell transplantation.
Haematologica. 99:1860–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacobs B and Ullrich E: The interaction of
NK cells and dendritic cells in the tumor environment: how to
enforce NK cell & DC action under immunosuppressive conditions?
Curr Med Chem. 19:1771–1779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao JX: Advances of enhancing
cytotoxicitiy of NK cells. Chinese Journal of Cancer Prevention
& Treatment. 16:1267–1271. 2009.
|
|
32
|
Schweizerhof M, Stösser S, Kurejova M,
Njoo C, Gangadharan V, Agarwal N, Schmelz M, Bali KK, Michalski CW,
Brugger S, et al: Hematopoietic colony-stimulating factors mediate
tumor-nerve interactions and bone cancer pain. Nat Med. 15:802–807.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sarkar DK, Murugan S, Zhang C and
Boyadjieva N: Regulation of cancer progression by β-endorphin
neuron. Cancer Res. 72:836–840. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Koebel CM, Vermi W, Swann JB, Zerafa N,
Rodig SJ, Old LJ, Smyth MJ and Schreiber RD: Adaptive immunity
maintains occult cancer in an equilibrium state. Nature.
450:903–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Antony PA, Piccirillo CA, Akpinarli A,
Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff
CA, Overwijk WW, et al: CD8+ T cell immunity against a
tumor/self-antigen is augmented by CD4+ T helper cells and hindered
by naturally occurring T regulatory cells. J Immunol.
174:2591–2601. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fujii H, Arakawa A, Utsumi D, Sumiyoshi S,
Yamamoto Y, Kitoh A, Ono M, Matsumura Y, Kato M, Konishi K, et al:
CD8+ tumor-infiltrating lymphocytes at primary sites as
a possible prognostic factor of cutaneous angiosarcoma. Int J
Cancer. 134:2393–2402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Y, Ayaru L, Mathew S, Morris E,
Pereira SP and Behboudi S: Expansion of anti-mesothelin specific
CD4+ and CD8+ T cell responses in patients with pancreatic
carcinoma. PLoS One. 9:e881332014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hanson HL, Donermeyer DL, Ikeda H, White
JM, Shankaran V, Old LJ, Shiku H, Schreiber RD and Allen PM:
Eradication of established tumors by CD8+ T cell adoptive
immunotherapy. Immunity. 13:265–276. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arancibia-Carcamo CV, Osawa H, Arnett HA,
Háskova Z, George AJ, Ono SJ, Ting JP and Streilein JW: A
CIITA-independent pathway that promotes expression of endogenous
rather than exogenous peptides in immune-privileged sites. Eur J
Immunol. 34:471–480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Boon T, Coulie PG, Van den Eynde BJ and
van der Bruggen P: Human T cell responses against melanoma. Annu
Rev Immunol. 24:175–208. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Segal BM, Glass DD and Shevach EM: Cutting
Edge: IL-10-producing CD4+ T cells mediate tumor rejection. J
Immunol. 168:1–4. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Quezada SA, Simpson TR, Peggs KS, Merghoub
T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA, et
al: Tumor-reactive CD4(+) T cells develop cytotoxic activity and
eradicate large established melanoma after transfer into
lymphopenic hosts. J Exp Med. 207:637–650. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Noyan F, Lieke T, Taubert R, Sievers M,
Dywicki J, Hapke M, Falk CS, Manns MP, Jaeckel E and
Hardtke-Wolenski M: Naive tumour-specific CD4+ T cells were
efficiently primed in acute lymphoblastic leukaemia. Scand J
Immunol. 80:161–168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Snook AE, Magee MS, Schulz S and Waldman
SA: Selective antigen-specific CD4(+) T-cell, but not CD8(+) T- or
B-cell, tolerance corrupts cancer immunotherapy. Eur J Immunol.
44:1956–1966. 2014. View Article : Google Scholar : PubMed/NCBI
|