Introduction
In recent years, Acinetobacter baumannii
(A. baumannii) has become a major nosocomial pathogen and
frequent cause of hospital-acquired pneumonia, surgical wound
infections and sepsis (1–3). The mortality of A. baumannii-VAP
cases in critical care units is 42% (2), rendering A. baumannii a serious
threat to ICU populations.
Toll-like receptors (TLRs), which belong to
receptors of type I transmembrane proteins, were widely
constitutively expressed in several cell types in lung tissue
(4,5). TLRs identified many types of
pathogen-associated molecular patterns (PAMPs); transfer PAMP, such
as LPS, into the cells through its transmembrane structure; produce
complicated signaling cascade, which may induce the activation of
nuclear factor-κB (NF-κB); induce the synthesis and release of a
variety of inflammatory mediators; accelerate the elimination of
A. baumannii by primary alveolar macrophages; and start the
anti-apoptotic mechanism of lung epithelial cells (6,7). TLR4
expression was downregulated by LPS isolated from
multidrug-resistant A. baumannii by 0.6-fold (8). Additionally, through the establishment
of a mouse model of TLR4 gene knockout it was observed that
the bacterial-killing ability against A. baumannii was
impaired in TLR4-deficient bone marrow-derived macrophages (BMDMs)
(9,10). Thus, the physiological significance
of TLR4 is to defend micro-organisms, and mediate innate immune
responses, in which appropriate inflammation responses were useful
in the defense of A. baumannii.
The mechanisms of the induced expression of TLR4 and
the intensity of its signal transduction pathway have yet to be
clarified. Smoking and high-dose LPS can enhance TLR4 expression of
the alveolar macrophages (11,12). In
addition, the absence of endotoxin of A. baumannii, the
circulatory disturbance of TLR4 receptors on the Golgi
apparatus-cell membrane-lysosomal compartment of cells, and the
effects of lung microenvironment on the subcellular localization of
TLR4 may cause reduced TLR4 expression and the elimination of
gram-negative bacillus (13–15). Thus, a different expression of TLR4
is associated with the cell type and state of bronchoalveolar and
lung cells, the used reagents, and primers (7).
Clinically, patients with low immune function are
particularly prone to A. baumannii infection, although the
reason for this susceptibility remains to be determined. In ICU
patients, because of the high prevalence of A. baumannii
drug resistance, most A. baumannii infections result in
severe bacterial infection, respiratory failure or multiple organ
failure, with the mortality rate being equivalent to complex
cardiovascular and cerebrovascular diseases (2). Thus, the constitutive and induced
expression of TLR4 in immunocompromised patients with A.
baumannii infection is to be investigated.
In the present study, an immunocompromised rat model
with weakened phagocytic function of macrophage was established
using hydrocortisone subcutaneous injection for 2 weeks, and the
expression and changes of TLR4 in lung of rats with A.
baumannii infection were observed. The role of the TLR4-NF-κB
pathway in the pathogenesis and development of A. baumannii
lung infection in immunocompromised hosts was then determined by
detecting the expression of interleukin (IL)-6 and tumor necrosis
factor (TNF)-α in bronchoalveolar lavage. Control rats efficiently
cleared A. baumannii, concurrent with the upregulated
expression of TLR4 and a rapid elaboration of IL-6 and TNF-α in
bronchoalveolar lavage and a corresponding mobilization of
intrapulmonary neutrophils and macrophages. Immunocompromised rats
deficient in TLR4 demonstrated a substantial delay in the clearance
of A. baumannii with a decreased expression of IL-6 and
TNF-α in bronchoalveolar lavage and a notable absence of
intrapulmonary neutrophils and macrophages.
The aim of the present study was to determine the
role of TLR4 in contributing to the identification of infection and
signaling the innate immune response against A.
baumannii.
Materials and methods
Animal
In total, 72 healthy 6-week-old Sprague-Dawley male
rats, weighing 150–180 g, were provided by the Institute of
Pharmacology, Sichuan Academy of Traditional Chinese Medicine. The
rats were randomly divided into cages at 25±0.5°C, and had access
to common feed and drinking water ad libitum. The study was
approved by the Animal Ethics Committee of Sichuan Second Hospital
of Tradition Chinese Medicine with the approval code of
2014001.
Experimental grouping
Fourteen days prior to the experiment, the rats were
randomly divided into the control group (group with normal immune
function, physiological saline was used in bronchial drip),
infection group (group with normal immune function, A.
baumannii suspension was used in bronchial drip), and
immune-suppressed infection group (group with restrained immune
function, A. baumannii suspension was used in bronchial
drip) (n=24 rats per group). For the immune-suppressed infection
group, hydrocortisone acetate (100 mg/kg) was subcutaneously
injected every other day for 14 days prior to infection. Alveolar
macrophage was collected after alveolar lavage to demonstrate
weakened phagocytic function of alveolar macrophages. Thus, the rat
model with immune-suppressed function was successfully proven. To
avoid the development of an adrenal crisis, hydrocortisone acetate
(100 mg/kg) was treated once a day for 7 days after A.
baumannii infection.
A. baumannii strains
Bacterial strains were isolated by the Department of
Microbiology Laboratory, the Second Hospital of Traditional Chinese
Medicine (Sichuan, China), from the sputum of a sepsis patient in
the ICU. The strain belonged to pan drug-resistant A.
baumannii for the conventional drug (all β-lactams, carbapenems
and sulbactam, fluoroquinolones, aminoglycoside, polymyxin,
tigecycline and colistin). Prior to the experiment, bacteria
strains were cryopreserved in the refrigerator at −70°C. On the
experiment day, A. baumannii were recovered with the
conventional method, the bacterial suspension was inoculated with 2
µl and annulus on 35°C hydrolysis of casein agar plate (MH) was
removed, placed in a constant temperature oscillation incubator
(ShanghaiZhicheng Analytical Instrument Manufacturing Corporation,
Shanghai, China), incubated for 18 h at 40 × g and 37°C, and then
standardized to 1×108 CFU/ml with sterile physiological
saline.
A. baumannii infection rat model
The rats were anesthetized using an intraperitoneal
injection with 10% chloral hydrate (4 ml/kg). The animals were
fastened on the operating table in a supine position, and neck skin
preparation and disinfection were carried out, prior to exposing
the trachea. One hundred microliters 1×108 CFU/ml
suspension of A. baumannii was dropped into the trachea.
After tracheal instillation, the animals were maintained erect for
5 min, and then laid down until they became conscious. For the
preliminary experiments, the right lower lung of rats was extracted
directly for cultivating quantitatively to confirm that the model
was successful.
Collecting of lung tissue
The mental state, breathing, eating, drinking water,
exercise, temperature, and fur of rats was observed daily and the
survival rate was recorded. Two rats were randomly sacrificed with
diethyl etheron on the 3rd and 7th day, and left lung tissue was
bronchoalveolar lavaged with Hank's solution, 10 ml each time, 4
times in total. The superior lobe of right lung was collected to
produce tissue homogenate, from which 10 µl was obtained for A.
baumannii quantitative culture. The inferior lobe of the right
lung was flushed 5 times with normal saline. Paraffin sections were
fixed, dehydrated, and dewaxed in xylene, followed by
paraffin-embedding and slicing using 4% polyformaldehyde.
Hematoxylin and eosin (H&E) staining was subsequently carried
out.
H&E staining
Changes of lung tissue were observed under an
optical microscope, and the semi-quantitative analysis of the lung
tissue was carried out, using scoring criteria mentioned in
Table I. Ten randomly selected
high-power fields (×100) were observed on each lung tissue
specimen, the score of each field was recorded, and the mean value
was obtained.
 | Table I.The schedule of pneumonia score. |
Table I.
The schedule of pneumonia score.
| Pathologic
change | 0 scores | 1 scores | 2 scores | 3 scores | 4 scores |
|---|
| Capillary blood
clot | No red blood
cells |
| Only little | More | Almost full of the
lumen |
| The alveolar cavity
fibrin exudation | No |
| Little | More | Almost full of the
lumen |
| Neutrophilic
exudate | No | Suspicious | Sporadic | Small focal
exudation | Large area of
seepage |
| Airway mucosa
epithelial cells decreased | No | Suspicious |
| Partial | Large area |
| Thickening of
alveolar interval | Relatively
normal | Slightly
broaden |
| Significant
broaden | Loss of normal
structure |
Immunohistochemistry
Integral optical density (IOD) values of positive
cells in lung tissue were detected using Image-Pro Plus 6.0 image
analysis system (Armonk, NY, USA). IOD values were used to reflect
the expression of positive material in lung tissue.
IL-6 and TNF-α were detected using
enzyme-linked immunosorbent assay (ELISA)
Bronchoalveolar lavage fluid (BALF) was collected,
and the levels of IL-6 and TNF-α were detected. The antibody
sandwich method was used, and the kit was provided by Shanghai
Jiang Lai Biotechnology Co., Ltd. (Shanghai, China).
Statistical analysis
SPSS 13.0 software (Chicago, IL, USA) was used for
statistical analysis. Lung bacterialoads were counted
logarithmically for each experiment group and compared with the
control group. Data were presented as mean ± standard deviation,
and measurement data were expressed using one-way analysis of
variance. The LSD method was used when variance was homogeneous,
and the non-parametric rank-sum test was used when the variance was
not homogeneous. P<0.05 was considered statistically
significant.
Results
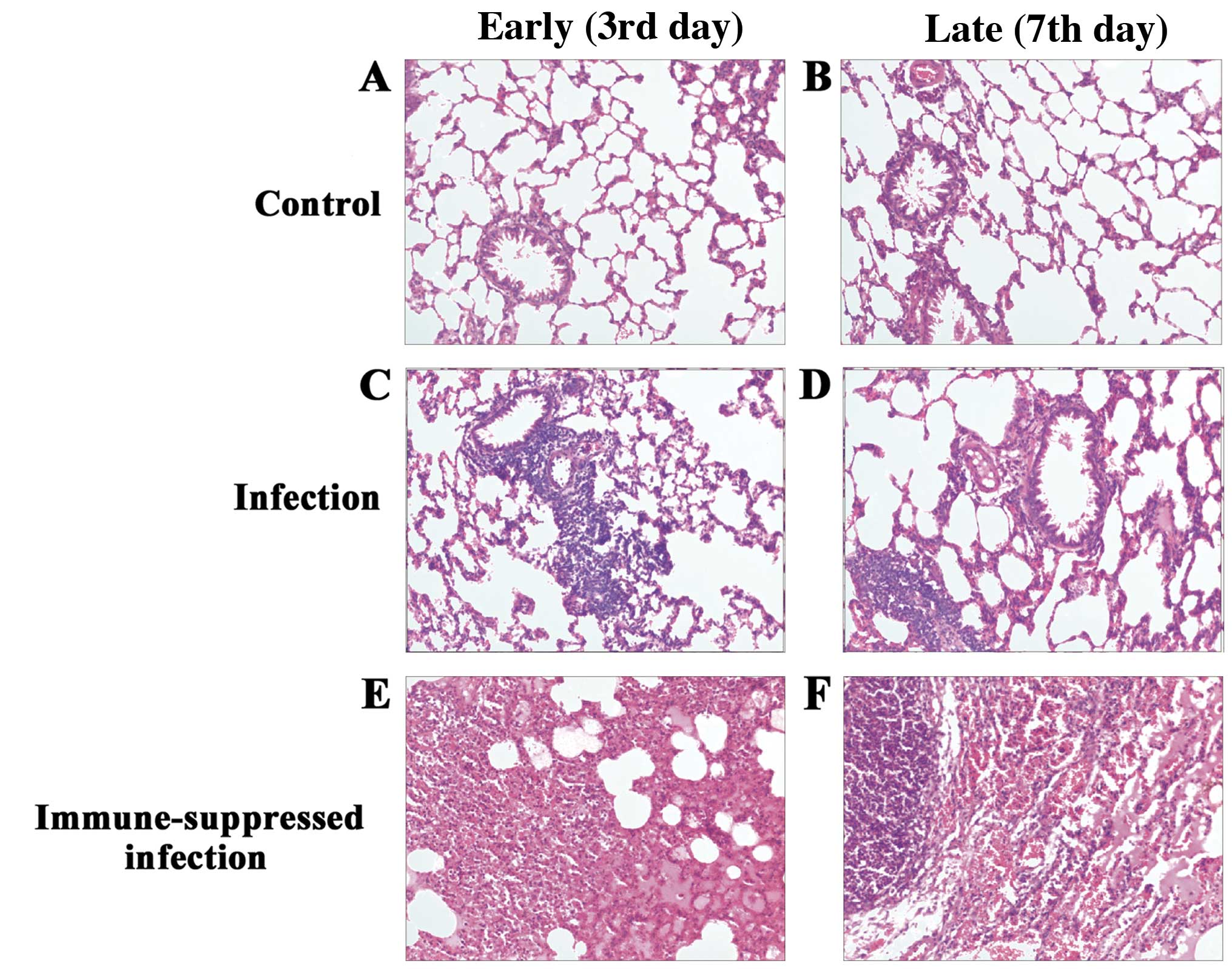
H&E staining
H&E staining for the control group showed
complete capillary bronchiole and alveolar structure, and trace
amounts of inflammatory cell infiltration were observed in the
early (3rd day) and late phase (7th day). In the A.
baumannii infection group, at the earlier time points (3rd
day), destructive capillary bronchiole and alveolar structure,
widened bronchial and alveolar septum, a large number of
inflammatory cells and lymph cell infiltration were observed. On
the late 7th day, a large number of neutrophil infiltrations,
unclear alveolar boundary, a fusion between the alveolar,
disappeared alveolar structure and a large number of fibroblasts
and extracellular matrix were observed. In the immune-suppressed
infection group, at the early phase (3rd day), destructive alveolar
structure, and pulmonary edema were observed without neutrophil or
macrophage infiltration. In the late phase (7th day), neutrophil
and macrophage infiltration, and partially restored alveolar
structure were evident in capillary bronchus and alveolar. The
pathological changes in the lung tissues of each group (H&E;
magnification, ×100) are shown in Fig.
1.
Pneumonia score
The scores of the semi-quantitative analysis of the
lung tissue at the different time points were determined (Table II). The result showed that compared
with the control group, inflammation in the A. baumannii
infection group in the early (the 3rd day) period was distinctly
aggravated, and the pathological score (5.42±0.23) was higher
(p<0.001). By contrast, inflammation in the immune-suppressed
infection group in the early (the 3rd day) period was obvious, and
the pathological score (11.67±0.63) was increased (p<0.001).
Additionally, inflammation in the immune-suppressed infection group
in the late (7th day) period was present, and the pathological
score (8.92±0.514) was higher than those for the control and
infection groups (p<0.001).
 | Table II.Pathological scores of lung
inflammation in each group (mean ± SD). |
Table II.
Pathological scores of lung
inflammation in each group (mean ± SD).
| Groups | Early stage (3rd
day) | Late stage (7th
day) |
|---|
| Control |
2.50±0.29 |
2.25±0.31 |
| Infection |
5.42±0.23a |
3.083±0.260 |
| Immune-suppressed
infection |
11.67±0.63a, b |
8.92±0.514a, b |
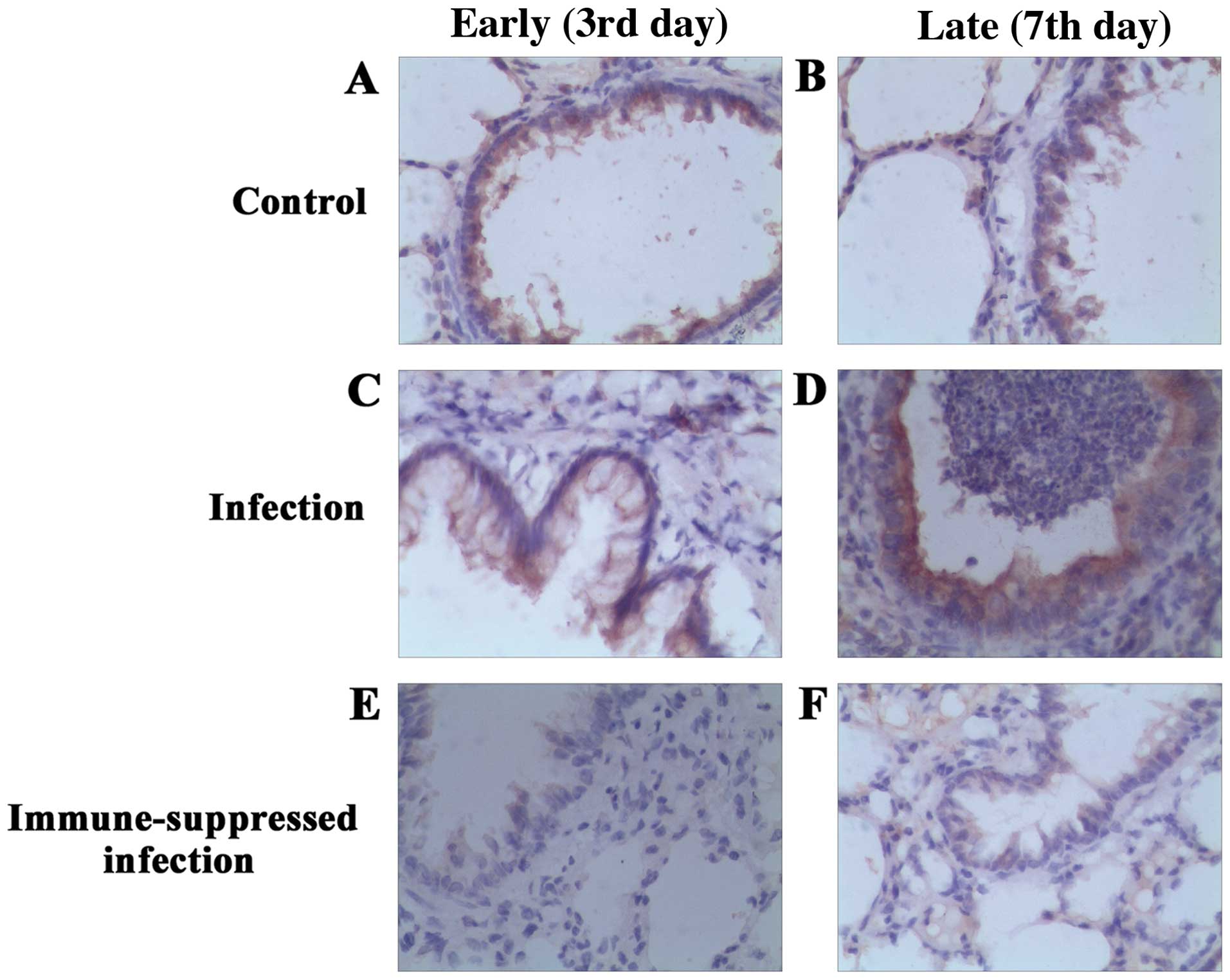
Immunohistochemical analysis of
TLR4
TLR4 protein expression in lung of each group at
different time points was examined (Fig.
2). A mild TLR4 expression was observed in the bronchial
epithelium and alveolar epithelial cell plasma of the control and
infection groups, and the plasma was brown-stained and scattered.
Compared with the control group, the number of macrophages in the
early period of pulmonary infection was increased, and brown
stained with particle aggregation. In addition, the expression of
TLR4 was upregulated. The number of macrophages was rare in the
late period of infection, and there was no significant difference
in TLR4 expression compared with the control group, with the brown
particles in the cells decreasing significantly. In the
immune-suppressed infection group, bronchial epithelial and
alveolar epithelial cells were yellow in the early period of
pulmonary infection, and no alveolar macrophages and TLR4
expression were observed in the pulmonary tissue. In the late
period, lung macrophages increased significantly, were brown
stained with particle aggregation, and the expression of TLR4
improved. The expression and comparison of TLR4 protein of
bronchial and lung tissues in each group are shown in Fig. 3.
The comparison of the IOD values of TLR4 expression
showed that in the early infection group (3rd day), the IOD value
of TLR4 expression of lung tissue (0.78±0.08) ×104
markedly increased compared with the control group, which had a
statistically significant difference (p<0.05). In the late
infection group (7th day), compared with the control group, there
was no significant improvement in the IOD value of TLR4 expression
of lung tissue (0.46±0.09) ×104, and there was no
significant difference (p>0.05). In the early immune-suppressed
infection group (3rd day), the IOD value of TLR4 expression of lung
tissue (0.23±0.08) ×104 was lower than that in the
control and infection groups, which had a statistically significant
difference (p<0.05). In the late immune-suppressed infection
group (the 7th day), there was no significant difference between
the IOD value of TLR4 expression of lung tissue (0.56±0.16)
×104 and that in the control and infection groups
(p>0.05) (Table III).
 | Table III.IOD value of TLR4 expression in each
group (×104) (mean ± SD). |
Table III.
IOD value of TLR4 expression in each
group (×104) (mean ± SD).
| Groups | Early stage (3rd
day) | Late stage (7th
day) |
|---|
| Control |
0.46±0.08 |
0.42±0.11 |
| Infection |
0.78±0.08a |
0.46±0.09 |
| Immune-suppressed
infection |
0.23±0.08a, b |
0.56±0.16 |
A. baumannii count
In the early infection group (3rd day), the A.
baumannii count after 24 h culture was 2.7±0.50 ×108
CFU. In the late infection group (7th day), the A. baumannii
count was 0.05±0.02 ×108 CFU and there was no difference
compared with that in the control group (the 7th day) (Table IV).
 | Table IV.Count of the load capacity of
Acinetobacter baumannii in lung tissue (×108 CFU,
median) (mean ± SD). |
Table IV.
Count of the load capacity of
Acinetobacter baumannii in lung tissue (×108 CFU,
median) (mean ± SD).
| Groups | Early stage (3rd
day) | Late stage (7th
day) |
|---|
| Control |
0 |
0 |
| Infection |
2.7±0.50 |
0.05±0.02 |
| Immune-suppressed
infection |
24.08±8.3a |
2.58±0.51b |
In the early immune-suppressed infection group (3rd
day), the A. baumannii count was 24.08±8.3 ×108
CFU. In the late immune-suppressed infection group (7th day), the
A. baumannii count was 2.58±0.51 ×108 CFU, and
the bacterial load capacity was significantly higher than that of
the infection group (p<0.001) (Table
IV).
Levels of IL-6 and TNF-α of alveolar
lavage fluid in each group at different time points
In the early infection group (3rd day), the content
of IL-6 of the alveolar lavage fluid (733.92±12.48) markedly
increased compared with the control group, which had a
statistically significant difference (p=0.003). In the late
infection group (7th day), the content of IL-6 of the alveolar
lavage fluid (55.17±6.72) markedly increased compared with the
control group, which had a statistically significant difference
(p<0.05) (Table V).
 | Table V.Content of IL-6 in each group at
different time points (mean ± SD). |
Table V.
Content of IL-6 in each group at
different time points (mean ± SD).
| Groups | Early stage (3rd
day) | Late stage (7th
day) |
|---|
| Control |
12.19±3.61 |
16.18±7.21 |
| Infection |
733.92±12.48a |
55.17±6.72b |
| Immune-suppressed
infection |
41.92±9.67c |
134.65±20.88d |
In the early immune-suppressed infection group (the
3rd day), the content of IL-6 of the alveolar lavage fluid
(41.92±9.67) decreased significantly compared with the infection
group, which had a statistically significant difference (p=0.003).
In the late immune-suppressed infection group (7th day), the
content of IL-6 of the alveolar lavage fluid (134.65±20.88)
markedly increased compared with the infection group, which had a
statistically significant difference (p=0.03) (Table V).
In the early infection group (3rd day), the content
of TNF-α of the alveolar lavage fluid (203.33±33.35) markedly
increased compared with the control group, which had a
statistically significant difference (p<0.001). In the late
infection group (7th day), the content of TNF-α of the alveolar
lavage fluid (39.5±11.63) markedly increased compared with the
control group, which had a statistically significant difference
(Table VI).
 | Table VI.Content of TNF-α in each group at
different time points (mean ± SD). |
Table VI.
Content of TNF-α in each group at
different time points (mean ± SD).
| Groups | Early stage (3rd
day) | Late stage (7th
day) |
|---|
| Control |
7.09±1.02 |
4.33±0.72 |
| Infection |
203.33±33.35a |
39.5±11.63b |
| Immune-suppressed
infection |
5.0±1.63c |
143.75±38.47d |
In the early immune-suppressed infection group (3rd
day), the content of TNF-α of the alveolar lavage fluid (5.0±1.63)
decreased significantly compared with the infection group, which
had a statistically significant difference (p<0.001). In the
late immune-suppressed infection group (7th day), the content of
TNF-α of the alveolar lavage fluid (143.75±38.47) markedly
increased compared with the infection group, which had a
statistically significant difference (Table VI).
Discussion
In the present study, we demonstrated under the
stimulation of A. baumannii that the expression of TLR4 was
upregulated in the bronchial epithelial cells, neutrophils and
alveolar macrophages. The secretion of IL-6 and TNF-α in BALF were
increased, which activated more neutrophils and macrophages in lung
to phagocytic bacteria. However, TLR4 expression of the lower
respiratory tract in immunosuppressed rats was downregulated. In
addition, the downregulated expression of TLR4 was accompanied with
reduced IL-6 and TNF-α secretion and the reduced elimination of
A. baumannii on the 3rd day after infection. In the
subsequent recovery period, the expression of TLR4 was upregulated
and the levels of IL-6 and TNF-α were increased.
The main modeling methods in the research of the
lung infection of A. baumannii at home and abroad were: i)
nose drip, or body was placed in a closed atomization generator,
then an amount of aerosol particles were used to cause infection.
This method cannot ensure successful inoculation. Additionally,
bacteria entered in the lungs randomly and without uniformity; and
ii) A. baumannii was dripped through endotracheal
intubation, and when the dosage was established, uniformity of
A. baumannii entering lung was also improved. However, this
method requires the anesthesia of animals, and an increase in
nursing work and mortality risk (16,17). In
the present study, A. baumannii suspension was applied
through tracheal instillation and the right lower lung of rats was
extracted directly for cultivating quantitatively to confirm that
both the infection model and immune-suppressed rats model were
successful. At the same time, the pathological results showed great
inflammatory cell infiltration, pulmonary interstitial and alveolar
edema, and hemorrhage on the third day after infection. Our results
showed the general appearance of lung tissue, or using H&E
staining, clear lung tissue inflammation, associated with an
increase in the concentration of relevant inflammatory cytokines,
such as TNF-α and IL-6, both present in BAL. With the extension of
the infection time, the degree of inflammation was reduced,
resulting in the decrease of A. baumannii.
We have shown that TLR4 protein in bronchial
epithelial cells of rats was highly expressed in the cell membrane
and cytoplasm in the infection group, and TLR4 protein in lung
tissue was only expressed in cytoplasm of alveolar macrophages and
a small amount of alveolar epithelial cells. Our
immunohistochemical analysis revealed that, on the 3rd day of
infection, TLR4 expression of alveolar macrophages was
significantly increased compared with that in the control group,
with statistical significance. On the 7th day of infection, with
the ease of inflammation degree, TLR4 expression of alveolar
macrophages was decreased, and there were no statistical
differences compared with that in the control group. The results
showed a high TLR4 expression of macrophages predicted good
prognosis of A. baumannii pulmonary infection. This result
is consistent with those of other studies which showed that the
TLR4 receptor expression of macrophage in plasma of the patients
after cardiac surgery decreased, potentially increasing the
susceptibility of the A. baumannii infection (10).
Although this experiment was not involved in the
study of macrophage TLR4 mRNA, other studies showed that A.
baumannii induced the expression of macrophage TLR4 mRNA
(5). A. baumannii with LPS
deficiency failed to stimulate macrophages to release TNF-α,
suggesting that TLR4 played an important role in the process of
LPS-activated macrophage TLR4 expression and induced the TNF-α
expression (9).
A number of common methods were employed in the
establishment of immune-suppressed infection model: i)
cyclophosphamide-treated, or diabetic rats were used; ii)
cyclophosphamide in conjunction with hydrocortisone was used; iii)
hydrocortisone was used alone (16,18–23). The
hydrocortisone model was used in this study, since single use of
hydrocortisone decreased TLR4 expression of monocyte and the level
of serum TNF-α and IL-1β, significantly delaying the elimination of
bacteria (22,24,25).
Our results have shown that hydrocortisone-treated
rats exhibit significantly increased bacterial burdens in the
lungs, as well as more severe lung pathology compared with those in
the control rats. Our findings have also shown that the expression
of TLR4 protein was significantly decreased in the
immune-suppressed rats with A. baumannii infection, together
with a decrease in the expression of IL-6 and TNF-α. It is
suggested that a decreased expression of TLR4 of alveolar
macrophages in immune-suppressed rats may downregulate the
bactericidal effect mediated by IL-6. On the other hand, when
inoculated with A. baumannii, the immune-suppressed rats had
a very small pulmonary inflammatory response with reduced
inflammatory cells, although an obvious pulmonary edema in the lung
appeared. We hypothesized that alveolar macrophage was the main
effector cell on the over-activation of alveolar macrophages
leading to the over-activation of NF-κB, which upregulated TLR4
expression, thus a positive feedback between TLR4 and NF-κB
promotes acute lung injury. This speculation remain to be
investigated in the future.
In conclusion, by studying the immunosuppressive rat
model, TLR4 expression of A. baumannii in immune-suppressed
rats decreased, alveolar macrophages were reduced, the secretion of
IL-6 and TNF-α was reduced, and the phagocytic function of
macrophage significantly was weakened, which indicated that a low
expression of TLR4 and exacerbated A. baumannii infection
mediated amplification, and resulted in aggravated A.
baumannii infection and early death. Therefore, the enhanced
expression of TLR4 protein in alveolar macrophages is a potential
treatment for A. baumannii infection.
References
|
1
|
Pierri MD, Crescenzi G2, Capestro F,
Recanatini C, Manso E, D'errico MM, Prospero E, Barbadoro P and
Torracca L: Risk factors and impact on clinical outcome of
multidrug-resistant Acinetobacter baumannii acquisition in cardiac
surgery patients. J Cardiothorac Vasc Anesth. Aug 24–2015.(Epub
ahead of print). doi: 10.1053/j.jvca.2015.08.024. PubMed/NCBI
|
|
2
|
Almomani BA, McCullough A, Gharaibeh R,
Samrah S and Mahasneh F: Incidence and predictors of 14-day
mortality in multidrug-resistant Acinetobacter baumannii in
ventilator-associated pneumonia. J Infect Dev Ctries. 9:1323–1330.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Özvatan T, Akalın H, Sınırtaş M, Ocakoğlu
G, Yılmaz E, Heper Y, Kelebek N, İşçimen R and Kahveci F:
Nosocomial Acinetobacter pneumonia: treatment and prognostic
factors in 356 cases. Respirology. 21:363–369. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chillappagari S, Venkatesan S, Garapati V,
Mahavadi P, Munder A, Seubert A, Sarode G, Guenther A, Schmeck BT,
Tümmler B, et al: Impaired TLR4 and HIF expression in cystic
fibrosis bronchial epithelial cells downregulates hemeoxygenase-1
and alters iron homeostasis in vitro. Am J Physiol Lung Cell Mol
Physiol. 307:L791–L799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren W, Wang Z, Hua F and Zhu L:
Plasminogen activator inhibitor-1 regulates LPS-induced TLR4/MD-2
pathway activation and inflammation in alveolar macrophages.
Inflammation. 38:384–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Erridge C, Moncayo-Nieto OL, Morgan R,
Young M and Poxton IR: Acinetobacter baumannii lipopolysaccharides
are potent stimulators of human monocyte activation via Toll-like
receptor 4 signalling. J Med Microbiol. 56:165–171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rivas-Santiago B and Juárez E: Toll-like
receptor in lung response to pathogens. Rev Invest Clin.
59:481–488. 2007.(In Spanish). PubMed/NCBI
|
|
8
|
Ubagai T, Nakano R, Nakano A, Kamoshida G
and Ono Y: Gene expression analysis in human polymorphonuclear
leukocytes stimulated by LPSs from nosocomial opportunistic
pathogens. Innate Immun. 21:802–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moffatt JH, Harper M, Mansell A, Crane B,
Fitzsimons TC, Nation RL, Li J, Adler B and Boyce JD:
Lipopolysaccharide-deficient Acinetobacter baumannii shows altered
signaling through host Toll-like receptors and increased
susceptibility to the host antimicrobial peptide LL-37. Infect
Immun. 81:684–689. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim CH, Jeong YJ, Lee J, Jeon SJ, Park SR,
Kang MJ and Park JH and Park JH: Essential role of toll-like
receptor 4 in Acinetobacter baumannii-induced immune responses in
immune cells. Microb Pathog. 54:20–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ji J, von Scheele I, Billing B, Dahlen B,
Lantz AS, Larsson K and Palmberg L: Effects of budesonide on
toll-like receptor expression in alveolar macrophages from smokers
with and without COPD. Int J Chron Obstruct Pulmon Dis.
11:1035–1043. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joh EH, Gu W and Kim DH: Echinocystic acid
ameliorates lung inflammation in mice and alveolar macrophages by
inhibiting the binding of LPS to TLR4 in NF-kappaB and MAPK
pathways. Biochem Pharmacol. 84:331–340. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren WY, Zhu L, Hua F, Jin JJ and Cai YY:
The effect of lipopolysaccharide on gene expression of TLR4 and
MD-2 in rat alveolar macrophage and its secretion of inflammation
cytokines. Zhonghua Jie He He Hu Xi Za Zhi. 33:367–371. 2010.(In
Chinese). PubMed/NCBI
|
|
14
|
Kim MY, Muto J and Gallo RL: Hyaluronic
acid oligosaccharides suppress TLR3-dependent cytokine expression
in a TLR4-dependent manner. PLoS One. 8:e724212013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Cowan MJ, Hasday JD, Vogel SN and
Medvedev AE: Tobacco smoking inhibits expression of proinflammatory
cytokines and activation of IL-1R-associated kinase, p38, and
NF-kappaB in alveolar macrophages stimulated with TLR2 and TLR4
agonists. J Immunol. 179:6097–6106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Manepalli S, Gandhi JA, Ekhar VV, Asplund
MB, Coelho C and Martinez LR: Characterization of a
cyclophosphamide-induced murine model of immunosuppression to study
Acinetobacter baumannii pathogenesis. J Med Microbiol.
62:1747–1754. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thompson MG, Black CC, Pavlicek RL,
Honnold CL, Wise MC, Alamneh YA, Moon JK, Kessler JL, Si Y,
Williams R, et al: Validation of a novel murine wound model of
Acinetobacter baumannii infection. Antimicrob Agents Chemother.
58:1332–1342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sugui JA, Pardo J, Chang YC, Zarember KA,
Nardone G, Galvez EM, Müllbacher A, Gallin JI, Simon MM and
Kwon-Chung KJ: Gliotoxin is a virulence factor of Aspergillus
fumigatus: gliP deletion attenuates virulence in mice
immunosuppressed with hydrocortisone. Eukaryot Cell. 6:1562–1569.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adamson TW, Diaz-Arevalo D, Gonzalez TM,
Liu X and Kalkum M: Hypothermic endpoint for an intranasal invasive
pulmonary aspergillosis mouse model. Comp Med. 63:477–481.
2013.PubMed/NCBI
|
|
20
|
Diaz-Arevalo D, Bagramyan K, Hong TB, Ito
JI and Kalkum M: CD4+ T cells mediate the protective
effect of the recombinant Asp f3-based anti-aspergillosis vaccine.
Infect Immun. 79:2257–2266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lewis RE, Liao G, Hou J, Chamilos G,
Prince RA and Kontoyiannis DP: Comparative analysis of amphotericin
B lipid complex and liposomal amphotericin B kinetics of lung
accumulation and fungal clearance in a murine model of acute
invasive pulmonary aspergillosis. Antimicrob Agents Chemother.
51:1253–1258. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heller AR, Heller SC, Borkenstein A, Stehr
SN and Koch T: Modulation of host defense by hydrocortisone in
stress doses during endotoxemia. Intensive Care Med. 29:1456–1463.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo G, Spellberg B, Gebremariam T, Bolaris
M, Lee H, Fu Y, French SW and Ibrahim AS: Diabetic murine models
for Acinetobacter baumannii infection. J Antimicrob Chemother.
67:1439–1445. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bagheri B, Sohrabi B, Movassaghpour AA,
Mashayekhi S and Garjani A, Shokri M, Pezeshkian M and Garjani A:
Hydrocortisone reduces Toll-like receptor 4 expression on
peripheral CD14+ monocytes in patients undergoing
percutaneous coronary intervention. Iran Biomed J. 18:76–81.
2014.PubMed/NCBI
|
|
25
|
Agustí C, Rañó A, Filella X, González J,
Moreno A, Xaubet A and Torres A: Pulmonary infiltrates in patients
receiving long-term glucocorticoid treatment: etiology, prognostic
factors, and associated inflammatory response. Chest. 123:488–498.
2003. View Article : Google Scholar : PubMed/NCBI
|