Introduction
Spinal cord injury (SCI) is a nerve and motor
function disorder that may be caused by a variety of damaging
factors. SCI is a serious health challenge worldwide, which has
resulted in the investigation of a range of novel technologies and
methods for the potential treatment of SCI.
A previous study observed that neural stem cells
(NSCs) exhibit a number of advantages compared with other types of
stem cells in a rat model of stroke, including enhanced
proliferation and self-renewal potential, facilitating the
induction and operation of NSCs in vitro, early embryonic
cell characteristics and the capacity to differentiate into various
types of nervous system cell (1).
NSCs are a type of stem cell with multiple differentiation
potential, which are able to proliferate and self-renew,
differentiate into astrocytes, neurons and oligodendrocytes.
Therefore, NSCs are ideal seed cells for transplantation. In the
present study, tNSCs extracted from fetal rat hippocampus were
cultured in vitro, which significantly increases the number,
activity and purity of the primary NSCs. Thus, NSCs may be a more
effective stem cell type for the treatment of SCI. However, the
simple application of neural stem cells for treatment of spinal
cord injury is not ideal. It was previously considered that EPO is
only a endocrine hormone acting on hematopoietic cells. However,
EPO has been demonstrated to be a type of tissue protection factor,
which exerts neurotrophic and neuroprotective effects (2,3). Yuan
et al (4) observed the
effects of erythropoietin on the differentiation of embryonic
cerebral cortex neural stem cells of rats cultured in vitro,
the results indicated that erythropoietin is able to promote the
differentiation of neural stem cells into neurons. Therefore, the
aim of the present study was to investigate the effectiveness of a
combination of NSC transplantation and EPO administration for the
treatment of SCI in rats, and to compare the combined treatment
with NSC transplantation alone in order to elucidate possible
underlying mechanisms.
Materials and methods
Animal sources
A study population of 40 healthy adult female Wistar
rats, weighing 220±20 g, in addition to 2 Wistar rats that were 14
days pregnant, were provided by the Laboratory Animal Center of
Inner Mongolia University (Hohhot, China). This study was approved
by the ethics committee of Inner Mongolia Medical University.
Reagents and instruments
Dulbecco's modified Eagle's medium (DMEM)/F12 media,
fetal bovine serum (FBS) and B27 supplements were purchased from
Gibco Life Technologies (Beijing, China). EPO was obtained from
Shenyang Sunshine Pharmaceutical Co., Ltd., (Shenyang, China),
while basic fibroblast growth factor (bFGF) and epidermal growth
factor (EGF) were purchased from Sigma-Aldrich (St. Louis, MO,
USA). A StreptAvidin-Biotin Complex (SABC) immunohistochemical kit,
polyclonal rabbit anti-nestin (AB5922; EMD Millipore, Billerica,
MA, USA), mouse anti-bromodeoxyuridine (BrdU; ANT-049) and
anti-neurofilament (NF)-200 (BYK-0708R; Boyanbio, Shanghai, China)
antibodies were used. Fluorescein isothiocyanate (FITC), trypsin
and 25-cm2 culture bottles were obtained from Corning
Incorporated (Corning, New York, NY, USA), a surgical instrument
and operating microscope (SXP-1C; Shanghai Medical Optical
Instrument Co., Ltd., Shanghai, China). A set of surgical
instruments and devices including a fiber operation knife,
scissors, tweezers and forceps were also obtained from this
company.
Primary culture and identification of
NSCs
Experimental methods were based on those described
in a previous study (5). The
pregnant rats were decapitated while anesthetized with a 3.5%
intraperitoneal injection of pentobarbital (1.3 ml/100 g), and the
fetal rats were immediately removed via caesarian section under
sterile conditions. An incision was made in the skull in order to
obtain the brain tissue, and tissues of the midbrain, hippocampus
and subventricular zones were separated. Isolated fetal rat brain
tissue was immersed in D-Hanks solution, then 1-mm3
tissue sections were obtained using ophthalmic scissors, digested
with 2 ml of 0.125% trypsin at 37°C for 10–15 min and subjected to
repeated mechanical pipetting. Next, 4 ml of 10% FBS in DMEM/F12
was added to terminate the digestion. The mixture was filtered
through a 200-mesh stainless steel screen and centrifuged for 10
min at 125.6 × g. The supernatant was discarded and complete medium
consisting of DMEM/F12 + B27 (2 ml/100 ml), bFGF (10 ng/ml) and EGF
(20 ng/ml) was added. A single cell suspension was administered to
flasks at a living cell concentration of 5–8 cells/ml. The cell
suspension was cultured in a CO2 incubator for 5–7 days,
centrifuged for 5 min at 125.6 × g and half the quantity of the
medium was changed every 2–3 days. The NSCs at passages 3 or 4 were
used to create a cell suspension, and the cell concentration was
adjusted to 1×105 cells/µl (6,7).
Subsequently, immunohistochemical and immunofluorescence analysis
of the nestin antigen, a neural stem cell marker, was conducted at
the Laboratory of Southern Medical University (Guangzhou,
China).
Detection of BrdU-labeled NSCs
Experimental methods are based on those described in
a previous study (8). An extracted
fraction of the single cell suspension was inoculated into a
culture flask and combined with BrdU solution at a concentration of
10 µmol/l in complete DMEM/F12 medium. The cell suspension was
prepared after incubation for 48 h, and the cell concentration was
adjusted to 1×105 cells/µl for alternation. The
expression of nestin antigen was then evaluated using
immunohistochemistry and immunofluorescence analysis.
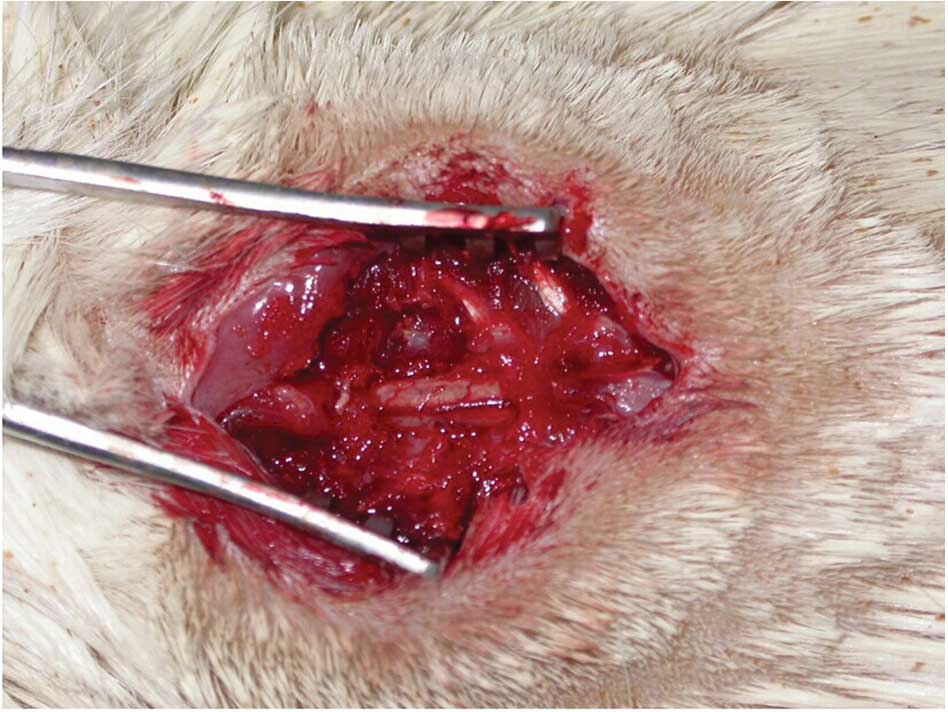
Establishing the completely transected
spinal cord model of SCI
The completely transected spinal cord model was
established in rats according to a previously described protocol
(9). Rats were anesthetized using 3.5% pentobarbital
(1.3 ml/100 g) via intraperitoneal injection, and then the T10
spinous process segments were exposed under sterile conditions
(10). Completely lifted the spinal
cord with the blunt crochet, the T10 segment was cut in addition to
the dura and spinal cord tissue using a scalpel at two positions to
isolate a 1-mm spinal segment. The transected spinal cord tissue
was then removed using bent-ended tweezers, and a gelatin sponge
was used to fill the space in the injured spinal cord (Fig. 1).
Grouping of the experimental
animals
A total of 40 adult Wistar rats were allocated at
random into four groups, namely the NSC, NSC + EPO, EPO and control
groups (n=10 per group). In the NSC group, following modeling, the
NSC suspension was centrifuged for 5 min (157 × g) using a benchtop
centrifuge (TD4A-WS; Changsha Weierkang Xiangying Centrifuge Co.,
Ltd., Changsha, China) and the cell concentration was adjusted to
1×105 cells/µl with DMEM. Next, 10 µl NSC suspension was
injected into a gelatin sponge and in the places of 1 mm at the top
and bottom of each lesion using a micro-syringe, 0.5 µl of NSC
suspension was injected at depths of 0.25, 0.5 and 0.75 mm,
respectively; in 3 of the rats, the NSCs were labeled with BrdU.
The rats of the NSC + EPO group underwent NSC transplantation and
received an intraperitoneal injection of EPO (5,000 U/kg) once per
day for 7 days; BrdU-labeled NSCs were used in 3 of the rats. The
rats in the EPO group received an intraperitoneal injection of EPO
(5,000 U/kg), once per day for 7 days and were transplanted with a
gelatin sponge injected with 10 µl DMEM/F12 medium. Control group
rats received a gelatin sponge injected with 10 µl DMEM/F12 medium
after modeling and an intraperitoneal injection of normal
saline.
Basso, Beattie and Bresnahan (BBB)
scoring of rat hindlimb motor function
BBB scoring (11) was
used to evaluate the hindlimb motor function of the rats. All rats
were subjected to the hindlimb motor function evaluation at 12 h
prior to surgery, then at 3, 7, 14, 28, 42 and 56 days following
surgery. Two experienced non-laboratory personnel conducted the
scoring under randomized, double-blinded conditions, then
calculated an average final score of hindlimb motor function for
each group.
NF-200 immunohistochemical staining
and FITC-conjugated NF-200 fluorescence-labeled spinal cord nerve
fibers
Experimental animals survived for 8 weeks after
surgery. Subsequent to anesthesia, 4% paraformaldehyde phosphate
solution was used to fix the spinal cord tissue by cardiac
perfusion (12). Injured spinal cord
tissue was incubated in 30% sucrose overnight at 4°C. Samples of
spinal cord tissue were subjected to conventional gradient
dehydration, transparentization and paraffin embedding. The slices
were then subjected to immunohistochemical staining with
anti-NF-200 antibody (1:200) for 10 min at 37°C. A portion of the
chilled spinal cord tissue was longitudinally sliced into sections
(thickness 30 µm) and one in every three was examined. NF-200 FITC
fluorescence staining was subsequently conducted. Frozen sections
were dried, then washed three times with 0.01 M
potassium-containing phosphate-buffered saline (KPBS), for 10 min
per wash. Sections were then immersed in 0.3% Triton X100 of 0.01 M
KPBS for 30 min, then washed three times with 0.01 M KPBS. Sections
were immersed in NF-200 FITC (1:400; RS-0046R; Shanghai Yan Jin
Biological Science and Technology Co., Ltd.), then rehydrated,
clarified, paraffin-embedded and observed under a fluorescence
microscope (TE2000-S; Nikon Corporation, Tokyo, Japan).
Detection of BrdU-labeled NSCs using
fluorescence microscopy
Slices of rat spinal cord tissue transplanted with
BrdU-labeled NSCs were subjected to conventional dewaxing, then
0.1% volume fraction of Triton was used to rupture the cells, which
were then washed with PBS and blocked using goat serum. Slices were
incubated with a mouse anti-BrdU antibody (1:100) overnight at 4°C
overnight. PBS replaced primary antibody as a negative control.
After washing with PBS, the slices were incubated with FITC-labeled
goat anti-mouse fluorescent secondary antibody (1:100; 6925-100;
BioVision, Inc., Milpitas, CA, USA). Subsequently, slices were
washed with PBS and mounted using a glycerol carbonate buffer
solution, and the BrdU-labeled NSCs were then observed using
immunoglobulin G fluorescence and a fluorescence microscope.
Statistical analysis
Results are expressed as the mean ± standard
deviation. One-way analysis of variance was used to conduct
statistical tests, and Fisher's least significant difference method
to conduct a pairwise comparison. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was conducted using SPSS software, version 13.0 (SPSS,
Inc., Chicago, IL, USA).
Results
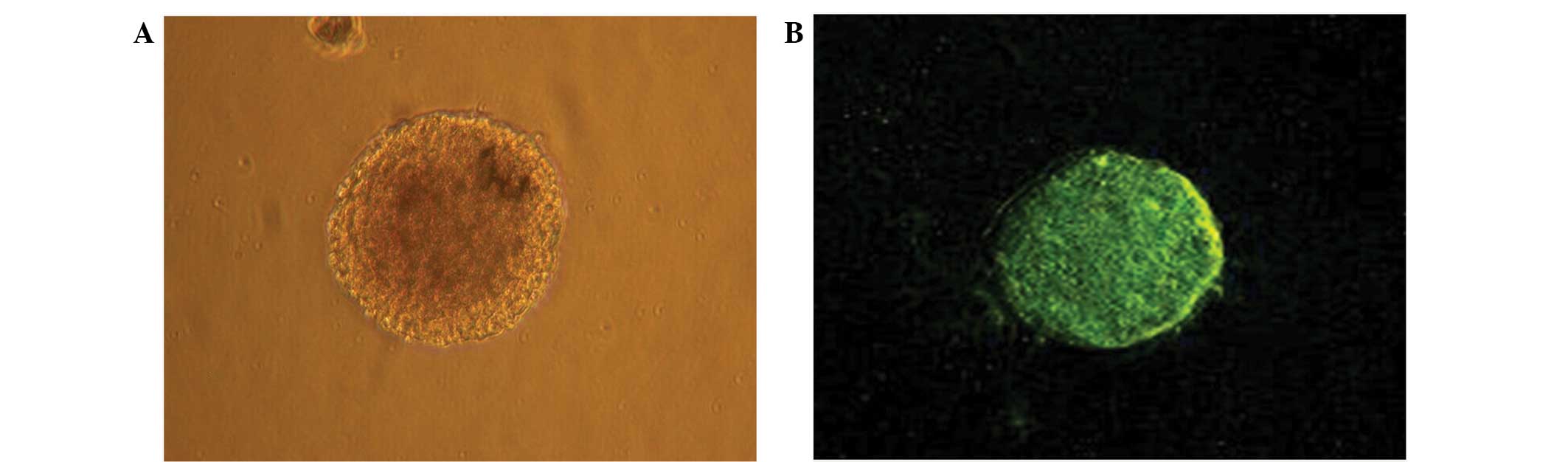
Morphological observation and
immunohistochemical identification of NSCs cultured in vitro
Single cells isolated from the hippocampal tissue of
embryonic rats appeared circular and consistently sized. The
majority of the cells cultured for 2–3 days died, while a limited
number of cells entered mitosis. The cells exhibited significant
nuclear division, with dividing cells gradually forming clusters.
After primary culture for 4–5 days, several to dozens of cells
grown in suspension were observed to have formed a spherical cell
mass in the nutrient solution. The cell clusters exhibited a
reduced volume and marked refraction. The clusters appeared
predominantly brownish and had clear boundaries with slightly
irregular edges and no evident projections. Therefore, the cells
were classed as ‘neurospheres’. After 4–5 days of primary culture,
the number of neurospheres increased significantly, the center of
the cell cluster appeared to have become darker and the coloring at
the cluster edge was comparatively light (Fig. 2A). The cells readily adhered to one
another, and if not timely passaged, the central cells of the
neurosphere typically died due to nutrient deficiency. Neurospheres
that had passaged were reprepared into single cell suspension and
cultured for 2–3 days. Single cells suspended in the culture
solution subsequently divided, forming small clusters of cells.
When these cells were cultured for 5–6 days, scattered and varied
neurospheres were observed. Larger neurospheres were detected after
7 days, with clear boundaries and strong refraction. These
observations indicate that the hippocampal cells were able to
self-proliferate. The neural stem cell marker nestin was expressed
in the primary cultured and passaged neurospheres. Furthermore,
these neurospheres presented with green fluorescence (Fig. 2B), indicating that they were
embryo-derived.
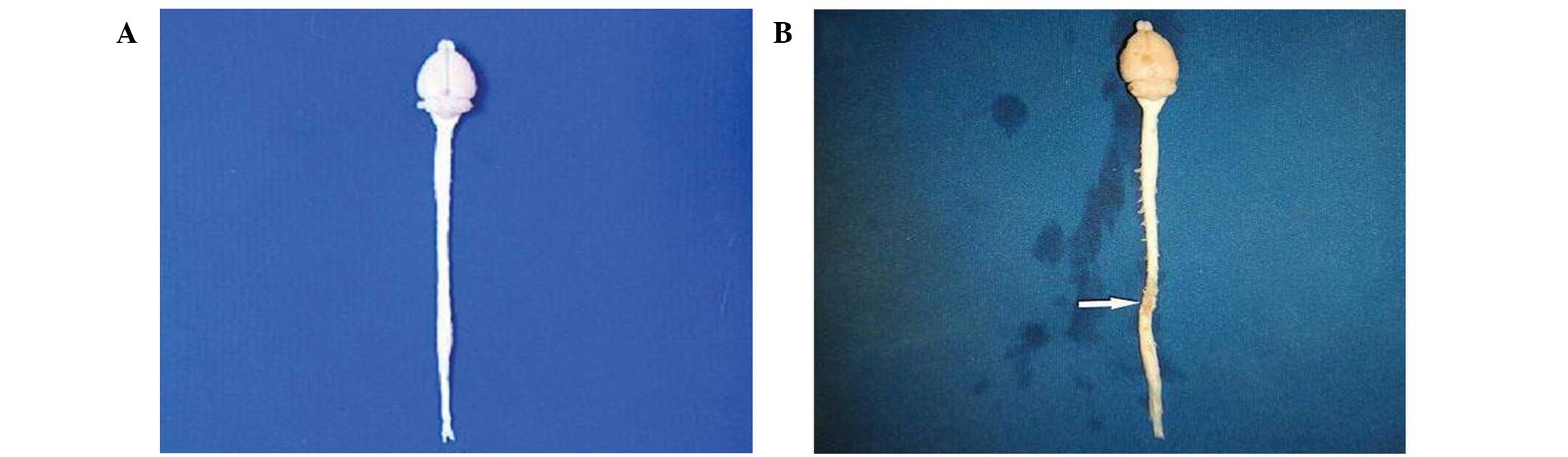
Morphological changes in SCI tissue
and detection of NSC survival in vivo
In total, 5 rats died during the experiment
including 2 control rats and 1 rat each in the EPO, NSC and NSC +
EPO groups. The causes of death included bladder rupture, hematuria
and bedsores. After 8 weeks, the injured spinal cord tissue
darkened in color due to scar tissue formation and was mildly
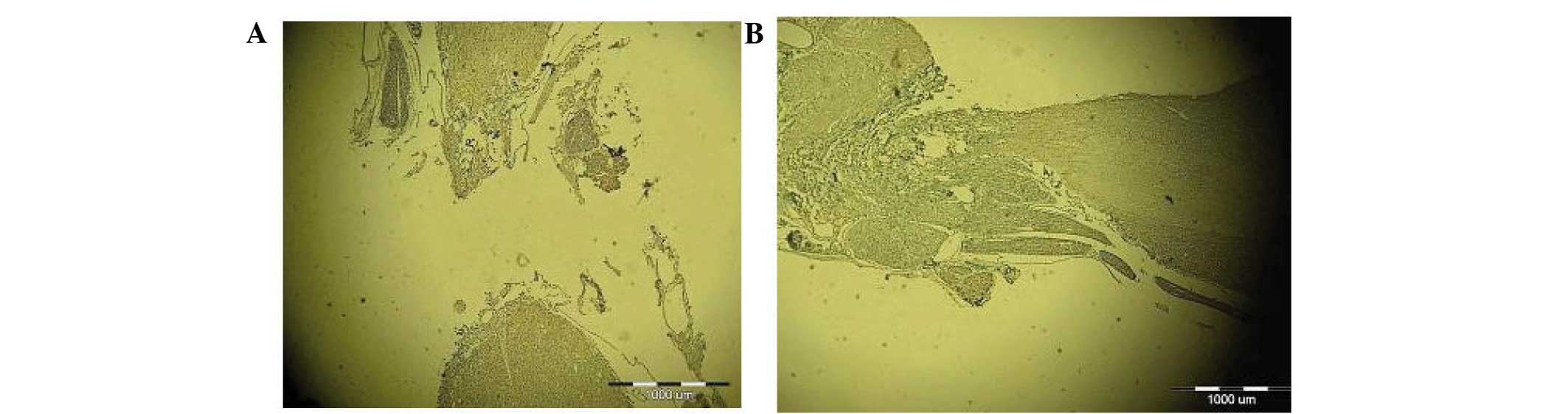
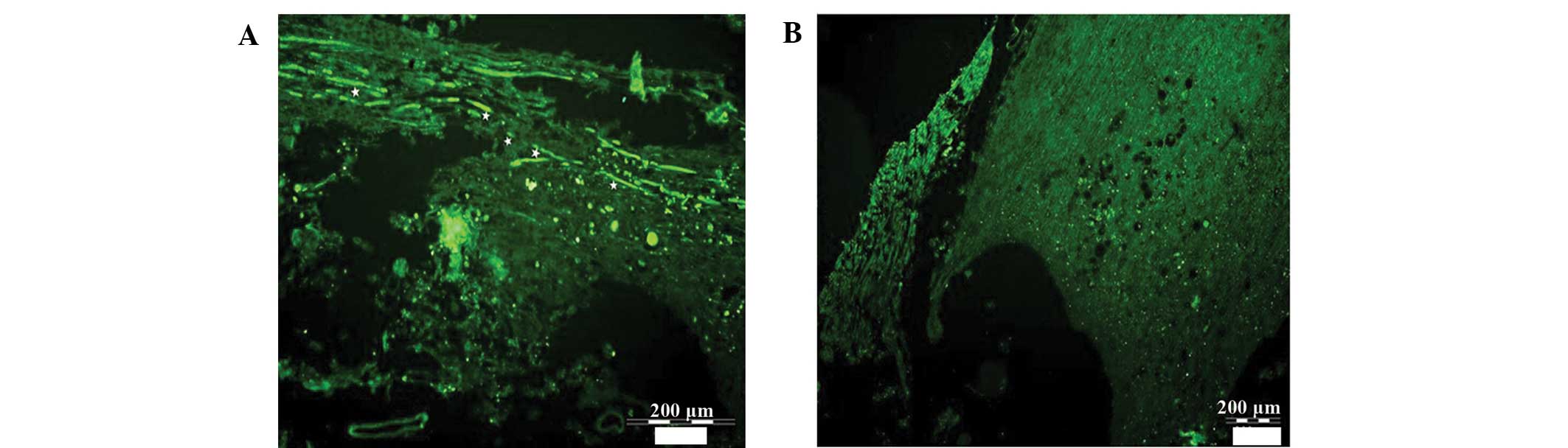
atrophied (Fig. 3). NF-200 staining
of the longitudinal slices of injured spinal cord tissue is
displayed in Fig. 4. The transected
spinal cord tissue of the control and EPO group rats was atrophied,
and transverse areas were replaced by transparent, non-colored scar
tissue. A large number of holes were observed between the white and
gray matter of the spinal cord. The transected spinal cord tissue
exhibited mild atrophy in the NSC group, and the disorderly
regeneration of nerve fibers was visible in the cross-sectional
area, with no continuous nerve fibers traversing the injured region
of the spinal cord. The transected spinal cord tissue sections from
the NSC + EPO group rats exhibited mild atrophy, with a large
quantity of regenerated nerve fibers in a disorderly state in the
cross-sectional area, and numerous continuous nerve fibers
traversing the injured region. A limited number of holes were
detected between the white and gray matter of the spinal cord. In
NSCs that succumbed to apoptosis, the BrdU-labeled nucleus
exhibited fragmentation and degradation, and was engulfed and
cleared by macrophages; therefore, these NSCs expressed no orange
fluorescence. BrdU-labeled NSCs were observed under a fluorescence
microscope (Fig. 5A); numerous
labeled NSCs were visible. Some of the stem cells migrated in the
directions of the head and tail, demonstrating that a considerable
number of NSCs survived after 8 weeks. In the experiment, numerous
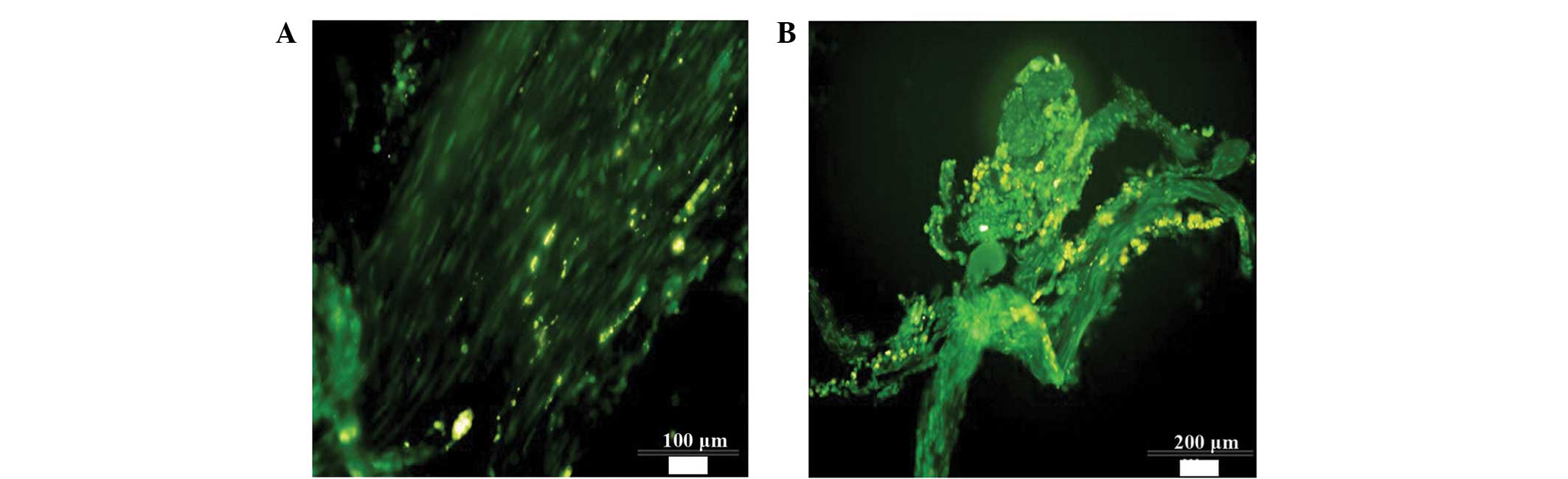
FITC-conjugated anti-NF-200 antibody-labeled nerve fibers were
detected that had regenerated at the side of the transected area
closest to the rat's head in the NSC + EPO group, which had
regenerated in a disorderly manner and penetrated the tail side of
the damaged zone. However, only a small number of regenerated nerve
fibers were detected in the control and EPO groups, which did not
traverse the damaged zone (Fig.
6).
BBB scoring of rat hindlimb motor
function
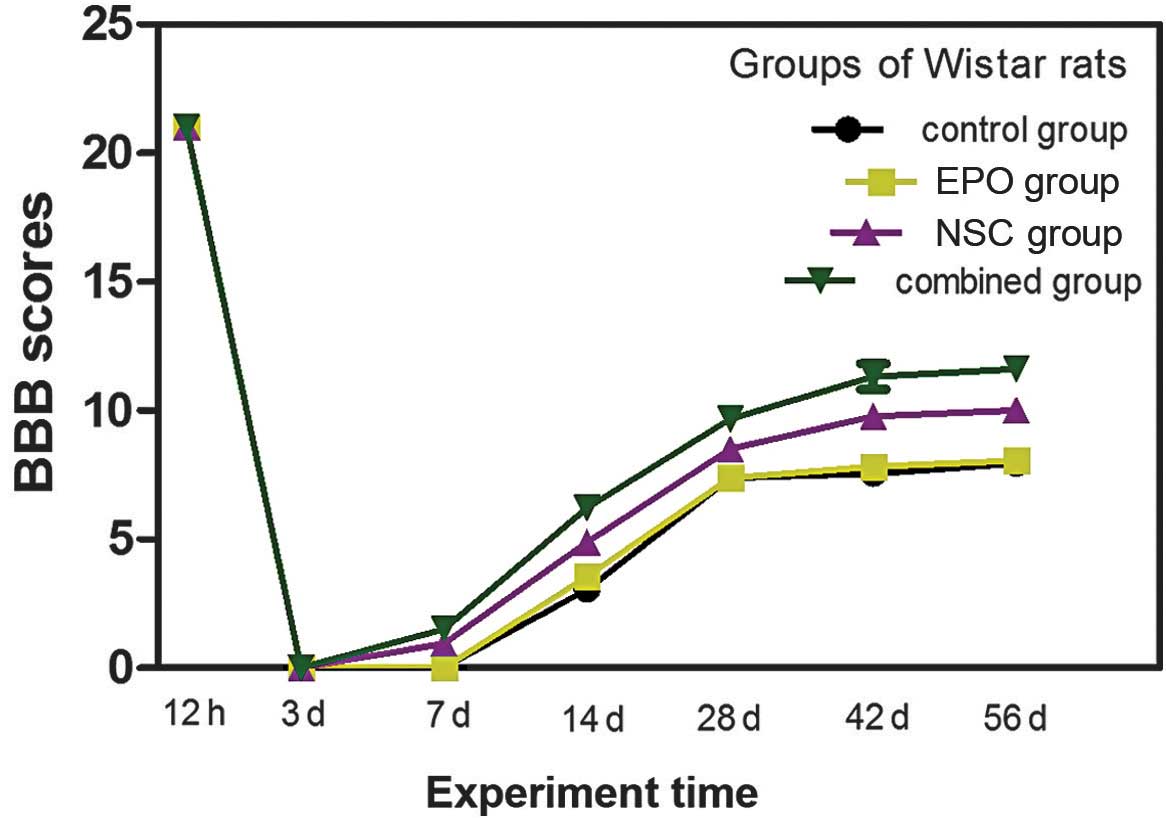
A normal rat hindlimb BBB score is 21. Following
transective SCI in rats, the BBB scores of the four groups
decreased to 0 within 3 days. The rat hindlimb motor function of
the four groups exhibited different degrees of recovery at various
time points after the injury. Recovery was most marked in the NSC +
EPO and NSC groups, which exhibited recovery of motor function
after 1 week, an increased rate of recovery between 2–5 weeks, and
significantly slower recovery between 6–8 weeks (Fig. 7). The results for the EPO, NSC, NSC +
EPO and control groups are presented and compared in Table I. At 1–4 weeks after the surgery, the
difference between the NSC and EPO groups was significant
(P>0.05). However, after 1 week a statistically significant
difference was detected between the NSC and EPO groups (P<0.05),
and the difference between the NSC + EPO and the other groups was
statistically significant (P<0.05). At 8 week after treatment,
the fore- and hindlimb motor function of the experimental rats in
the NSC + EPO group was coordinated and regular, and the hindfoot
was able to bear the rat's body weight, but was not able to lift
the body off the ground in the. The motor coordination function of
rats in the NSC group was poor, with the hindfeet being able to
bear weight in a limited number of rats while the remainder of the
rats had hindlimbs with bent toes and poor weight-bearing function.
In the control and EPO groups, the rat hindlimb motor function was
uncoordinated and exhibited irregular sliding.
 | Table I.Basso, Beattie and Bresnahan scoring
of hindlimb movements following spinal cord injury (mean ± standard
deviation). |
Table I.
Basso, Beattie and Bresnahan scoring
of hindlimb movements following spinal cord injury (mean ± standard
deviation).
| Time point | Control | EPO | NSC | NSC + EPO |
|---|
| 12 h before | 21.00 | 21.00 | 21.00 | 21.00 |
| 7 days after | 0.00±0.00 | 0.00±0.00 |
0.94±0.10a |
1.50±0.26a,b |
| 14 days after | 3.06±0.36 | 3.56±0.32 |
4.89±0.60a |
6.22±0.38a,b |
| 28 days after | 7.38±0.30 | 7.39±0.34 | 8.50±0.3a |
9.67±0.43a,b |
| 42 days after | 7.56±0.36 | 7.83±0.38 |
9.78±0.20a |
11.33±0.50a,b |
| 56 days after | 7.94±0.35 | 8.06±0.31 |
10.0±0.31a |
11.61±0.46a,b |
Discussion
In the early 1990s, Reynolds, Tetzlaff and Weiss
(13) isolated a cell population
from adult rat striatum, which was able to continually proliferate
in vitro and possessed multiple differentiation potential.
Therefore, the authors proposed the concept of ‘NSCs’, which
exhibit the following characteristics (14): i) The capacity to self-renew, and
thus maintain a stable cell population; ii) multi-directional
differentiation potential, able to divide into various cell types
associated with the nervous system, including neurons, astrocytes
and oligodendrocytes; iii) the maintenance of a primitive
undifferentiated state, without exhibiting any specific signs of
cell maturation.
Further investigation indicated a potential use for
NSCs in the treatment of SCI. NSCs transplanted into a host body
were observed to migrate toward the site of SCI, function as
chemokines, differentiate into various cell types associated with
the nervous system, replace damaged nerve cells and rebuild
synaptic connections, all of which may promote the recovery of
neurological function. Nakamura et al (15) observed that transplanted NSCs are
able to survive, differentiate and migrate in a host body, and
effectively promote the recovery of limb motor function.
Furthermore, transplanted NSCs exhibit neurotrophic effects and are
able to differentiate into neurons and glial cells in the area of
SCI. These nerve cells are able to secrete a variety of
neurotrophic factors, which improve the local internal environment
of an area of SCI, promote the regeneration of axons, repair
damaged nerve cells and activate the sequential expression of
regeneration-associated genes. Transplanted cells may produce a
variety of types of extracellular matrix, filling the cavity
produced as a result of SCI and providing support for the
regeneration of axons. In addition, transplanted NSCs are able to
differentiate into oligodendrocytes, which promote the regeneration
of nerve fibers and induce the myelinization of demyelinated nerve
fibers (16–18).
Initially, EPO was only known to function as an
endocrine hormone in hematopoietic cells. However, studies have
indicated that EPO additionally functions as a novel type of
organization protection factor, by exerting neurotrophic and
neuroprotective effects (19).
Possible mechanisms underlying this function of EPO may include its
capacity to promote the proliferation and differentiation of NSCs,
and the regeneration of nerves. Wang et al (20) used a spinal cord transection injury
model to observe the effects of EPO on the proliferation, migration
and survival of transplanted BrdU-labeled NSCs in rats with SCI.
The results suggested that EPO promotes the survival and migration
of NSCs in damaged spinal cord tissue and accelerates the recovery
of neural function; ii) EPO is able to mitigate the rate of
apoptosis of nerve cells. A previous study revealed (21) that EPO is able to protect neurons
from death due to hypoxia and other factors, including
excitotoxicity and glucose deprivation in vitro. In
addition, EPO is able to immediately prevent the cells of spinal
nerve roots from undergoing apoptosis in vivo following
crush injury. In a rat model of spinal cord compression, it was
observed that EPO exerted a protective effect on nerve cells
(22).
In the present study, NSCs extracted from fetal rat
hippocampal tissue were cultured in vitro, which
significantly increased the number, activity and purity of the
primary NSCs. The NSCs exhibited favorable cloning ability and were
stably passaged, which facilitated subsequent experiments. In the
present study, complete transection of the spinal cord was used to
establish the rat model of SCI. Although the rat model does not
completely simulate clinical SCI, the complete transection method
produces identical injury in each experimental group, thus
producing more reliable results. In rats that receive no further
treatment following modeling, BBB scores are generally <10, with
the majority of scores ~8.
No significant recovery in hindlimb motor function
was exhibited by any of the groups within the first 7 days after
the induction of SCI. The hindlimb motor function of each group
recovered to varying degrees between 7 and 56 days after SCI
induction. The hindlimb function recovery in the NSC + EPO group
was significantly improved compared with that in the other groups,
while the difference between the control and EPO groups was not
statistically significant. The rate of hindlimb function recovery
of each group was most marked between 7 and 28 days after SCI. The
BBB score of the NSC + EPO group was >10 for certain rats at 28
days after treatment, indicating that the combined treatment
significantly improved the rate of recovery of spinal cord
function. The detection of BrdU-labeled NSCs demonstrated that
transplanted NSCs were able to survive and migrate in the injured
spinal cord tissue. After 8 weeks, injured spinal cord tissue
darkened in color due to scar tissue formation, and exhibited mild
atrophy. Currently, axonal regeneration is used as a marker of
neural regeneration and repair.
NF-200 are a major component of the framework of
nerve cell bodies and axons. As a result of the degeneration and
necrosis of axons in SCI tissue, the NF-200 that constitutes the
framework of nerve axons was reduced and was in certain cases
replaced entirely by scar tissue. NF-200 staining of the
longitudinal slices of injured spinal cord tissue revealed that the
transected spinal cord tissue in the control and EPO groups
exhibited atrophy, and transverse areas were replaced by
transparent, non-colored scar tissue. Furthermore, a large quantity
of hole formation was observed between the white and gray matter of
the spinal cord. The spinal cord tissue in the NSC group exhibited
mild atrophy, and the disorderly regeneration of nerve fibers was
evident in the cross-sectional area, with no continuous nerve
fibers traversing the area. The transected spinal cord tissue was
mildly atrophied in the NSC + EPO group, with extensive disorderly
regeneration of nerve fibers and numerous continuous nerve fibers
traversing the cross-sectional area. Furthermore, a limited degree
of hole formation was observed between the white and gray matter of
the spinal cord.
In conclusion, the results of the present study
suggest that the transplantation of NSCs alone or combined with EPO
was able to promote the recovery of hindlimb motor function in a
rat model of SCI. These regenerative effects were most evident in
the NSC + EPO group. Therefore, the present study indicates that
NSC transplantation combined with EPO is an effective intervention
for treating SCI, and further studies are required to evaluate the
applicability of this treatment to clinical contexts.
Acknowledgements
This study was supported by a grant from the Inner
Mongolia Natural Science Foundation (grant no. 2011MS1139).
References
|
1
|
Wu QL, Li QG and Liu K: Stem cell
transplantation for treatment of spinal cord injury. Zhong Guo Zu
Zhi Gong Cheng Yan Jiu Yu Lin Chuang Kang Fu. 12:2343–2346.
2008.(In Chinese).
|
|
2
|
Rangarajan V and Juul SE: Erythropoietin:
Emerging role of erythropoietin in neonatal neuroprotection.
Pediatr Neurol. 51:481–488. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matis GK and Birbilis TA: Erythropoietin
in spinal cord injury. Eur Spine J. 18:314–323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuan LL, Du HM, Guan YJ and Kong YH:
Differentiation of rat embryonic cerebral cortex neural stem cells
cultured by erythropoietin in vitro. Zhong Guo Zu Zhi Gong Cheng
Yan Jiu Yu Lin Chuang Kang Fu. 15:9174–9177. 2011.(In Chinese).
|
|
5
|
Liu K, Wang HY, He Y, Zhang YZ and Wang
ZC: Experiment on cultivation and identification of neural stem
cells in embryonic rats. Zhong Guo Xian Dai Shen Jing Ji Bing Za
Zhi. 6:52–56. 2006.(In Chinese).
|
|
6
|
Zhao Z, Hu H and Shi S: The optimization
of the method of culturing neural stem cells in neonatal rat brain.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 19:544–547. 2005.(In
Chinese). PubMed/NCBI
|
|
7
|
Zhang ZS, Li JH, Zhang YZ and Wang ZC:
Effect of cell density on proliferation and differentiation of
human fetal neural stem cells. Shou Du Yi Ke Da Xue Xue. 4:180–183.
2003.(In Chinese).
|
|
8
|
Zhang XR, Guo GH, Liu DW and Peng Y:
Separation, culture and BrdU labeling of human bone marrow
mesenchymal stem cells. Zhong Guo Zu Zhi Gong Cheng Yan Jiu Yu Lin
Chuang Kang Fu. 13:3618–3622. 2009.(In Chinese).
|
|
9
|
Meng BL, Ba YC, Song SN, Chen SS, Li LY
and Wang TH: Establishment of spinal cord transection injury models
in rats. Zhong Guo Zu Zhi Gong Cheng Yan Jiu Yu Lin Chuang Kang Fu.
15:1215–1218. 2011.(In Chinese).
|
|
10
|
Zhang SX, Huang F, Gates M, White J and
Holmberg EG: Extensive scarring induced by chronic intrathecal
tubing augmented cord tissue damage and worsened functional
recovery after rat spinal cord injury. J Neurosci Methods.
191:201–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Basso DM, Beattie MS and Bresnahan JC:
Graded histological and locomotor outcomes after spinal cord
contusion using the NYU weight-drop device versus transection. Exp
Neurol. 139:244–256. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Talac R, Friedman JA, Moore MJ, Lu L,
Jabbari E, Windebank AJ, Currier BL and Yaszemski MJ: Animal spinal
cord injury for evaluation of tissue engineering treatment
strategies. Biomaterials. 25:1505–1510. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Reynolds BA, Tetzlaff W and Weiss S: A
multipotent EGF-responsive striatal embryomic progenitor cell
produces neurons and astrocytes. J Neurosci. 12:4565–4574.
1992.PubMed/NCBI
|
|
14
|
Taupin P: BrdU immunohistochemistry for
studying adult neurogenesis: Paradigms, pitfalls, limitations and
validation. Brain Res Rev. 53:198–214. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakamura M, Toyama Y and Okano H:
Transplantation of neural stem cells for spinal cord injury. Rinsho
Shinkeigaku. 45:874–876. 2005.(In Japanese). PubMed/NCBI
|
|
16
|
Tewarie RS Nandoe, Hurtado A, Bartels RH,
Grotenhuis A and Oudega M: Stem cell-based therapies for spinal
cord injury. J Spinal Cord Med. 32:105–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Foret A, Quertainmont R, Botman O, Bouhy
D, Amabili P, Brook G, Schoenen J and Franzen R: Stem cells in the
adult rat spinal cord: Plasticity after injury and treadmill
training exercise. J Neurochem. 112:762–772. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garbossa D, Boido M, Fontanella M, Fronda
C, Ducati A and Vercelli A: Recent therapeutic strategies for
spinal cord injury treatment: Possible role of stem cells.
Neurosurg Rev. 35:293–311. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subirós N, Del Barco DG and Coro-Antich
RM: Erythropoietin: Still on the neuroprotection road. Ther Adv
Neurol Disord. 5:161–173. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang ZW, Guan QK, Zhou WK, Zhang XZ and Xu
DW: Effect of erythropoietin on survival and migration of neural
stem cells transplanted into injured spinal cord of rats. Zhong Guo
Zu Zhi Gong Cheng Yan Jiu Yu Lin Chuang Kang Fu. 16:6736–6740.
2012.(In Chinese).
|
|
21
|
Lee KB, Choi JH, Byun K, Chung KH, Ahn JH,
Jeong GB, Hwang IK, Kim S, Won MH and Lee B: Recovery of CNS
pathway innervating the sciatic nerve following transplantation of
human neural stem cells in rat spinal cord injury. Cell Mol
Neurobiol. 32:149–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mofidi A, Bader A and Pavlica S: The use
of erythropoietin and its derivatives to treat spinal cord injury.
Mini Rev Med Chem. 11:763–770. 2011. View Article : Google Scholar : PubMed/NCBI
|