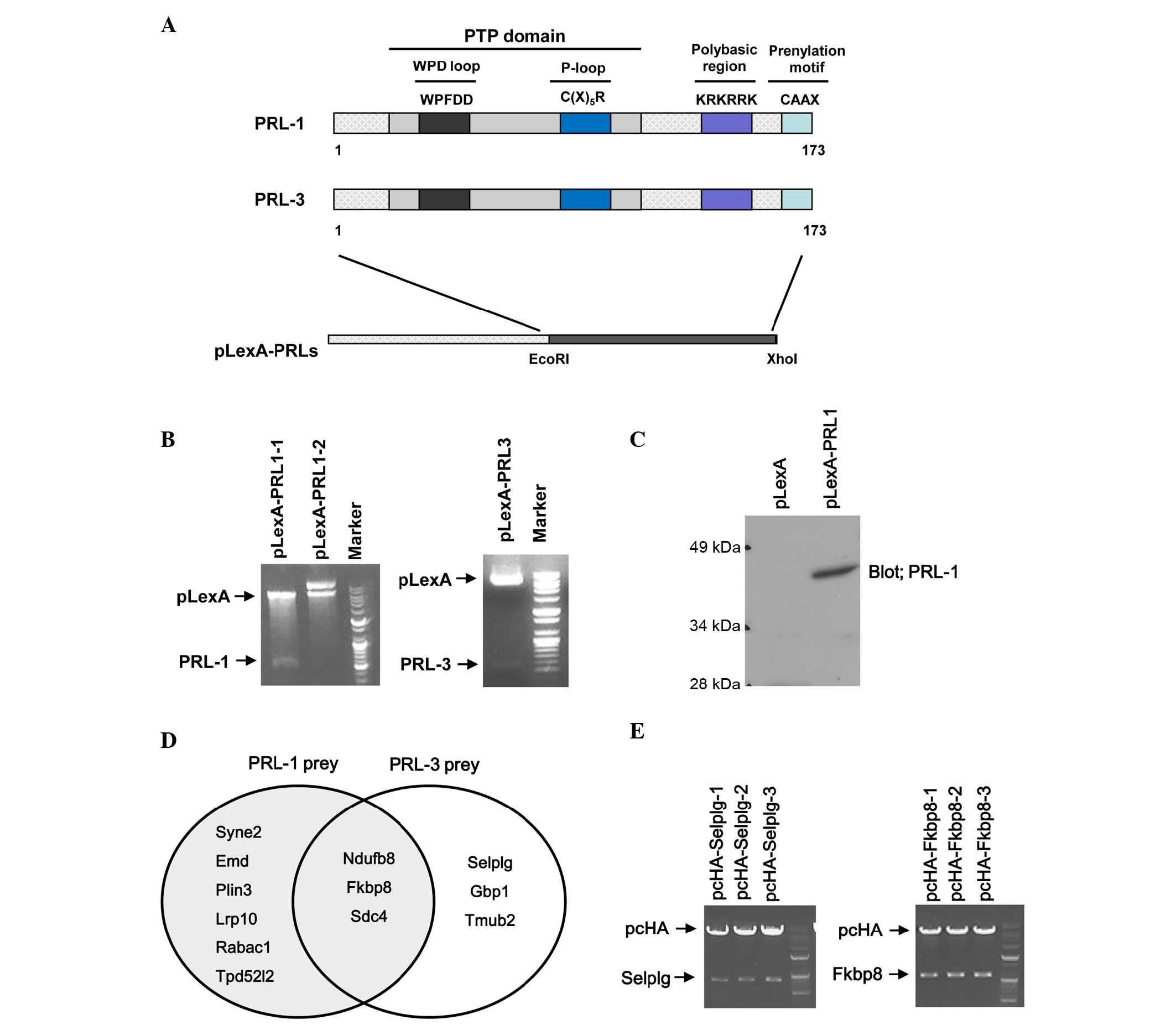
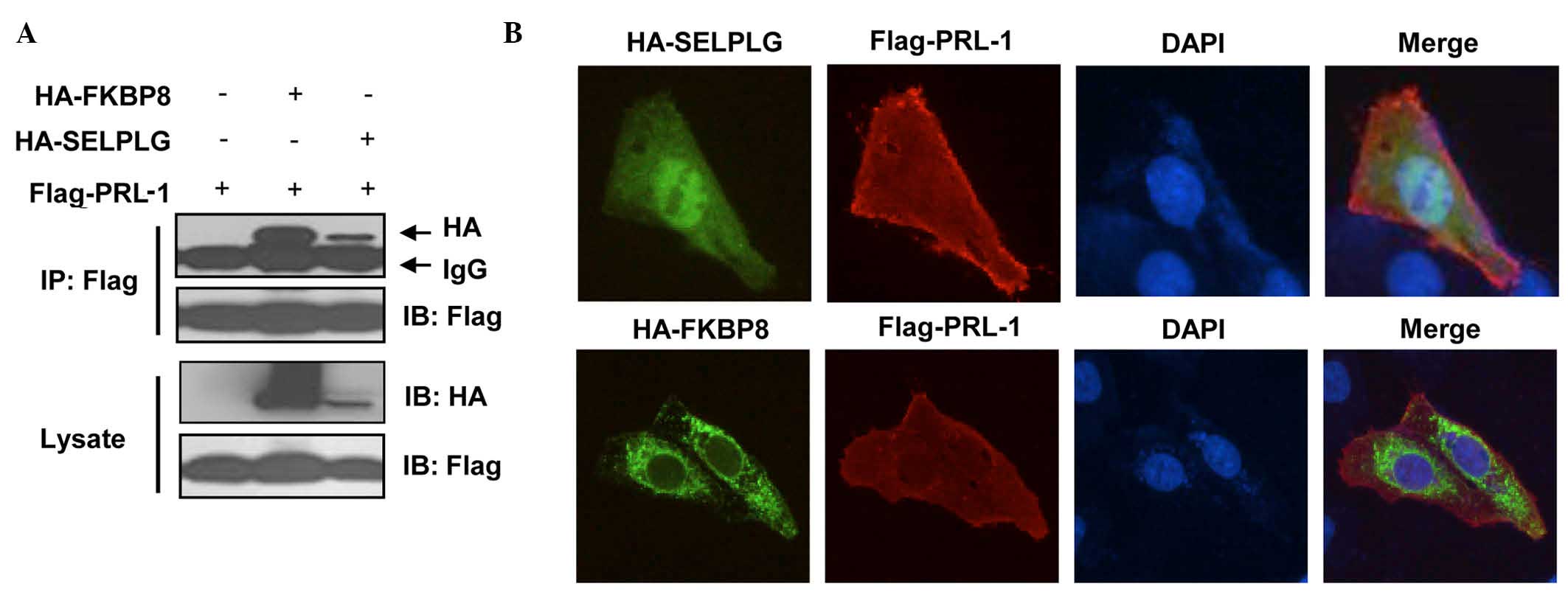
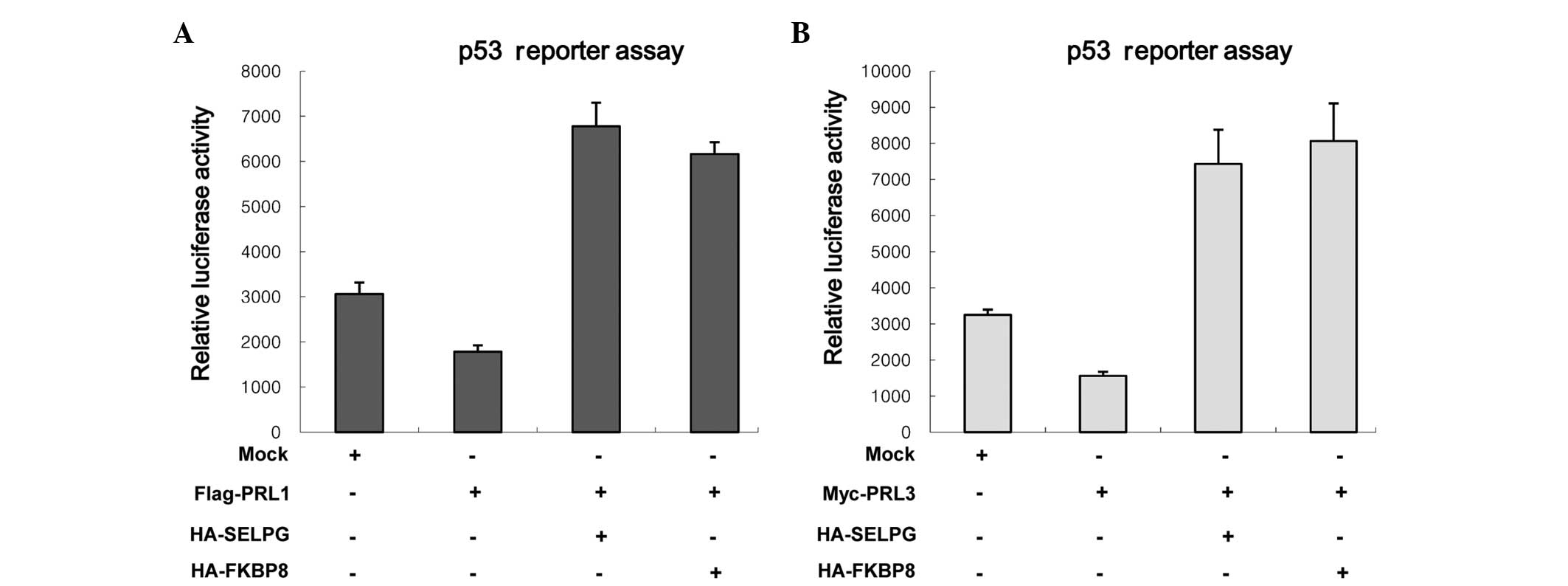
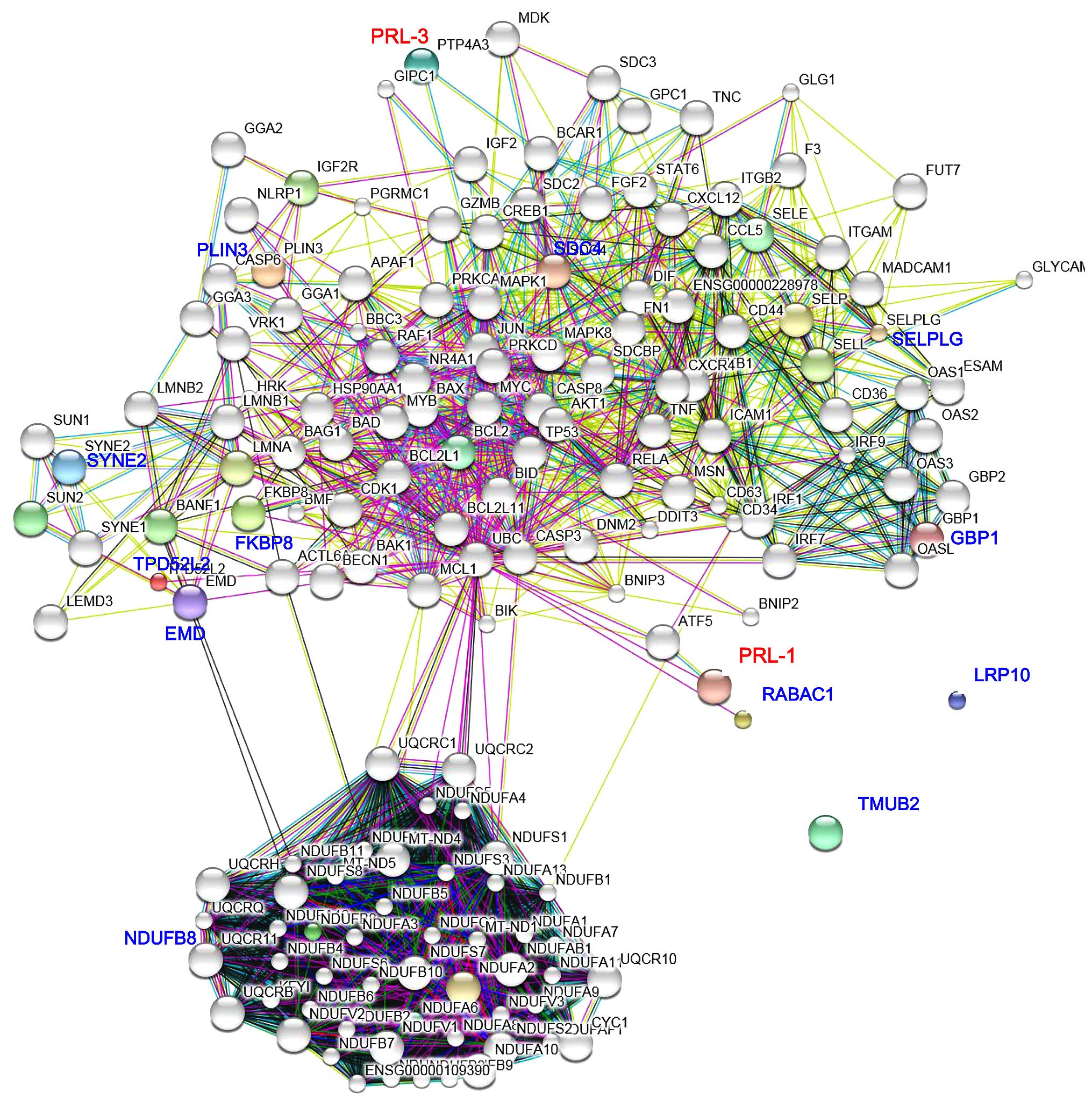
|
1
|
Al-Aidaroos AQ and Zeng Q: PRL-3
phosphatase and cancer metastasis. J Cell Biochem. 111:1087–1098.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Achiwa H and Lazo JS: PRL-1 tyrosine
phosphatase regulates c-Src levels, adherence, and invasion in
human lung cancer cells. Cancer Res. 67:643–650. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stephens BJ, Han H, Gokhale V and Von Hoff
DD: PRL phosphatases as potential molecular targets in cancer. Mol
Cancer Ther. 4:1653–1661. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saha S, Bardelli A, Buckhaults P,
Velculescu VE, Rago C, St Croix B, Romans KE, Choti MA, Lengauer C,
Kinzler KW and Vogelstein B: A phosphatase associated with
metastasis of colorectal cancer. Science. 294:1343–1346. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang Q, Holmes DI, Powell SM, Lu QL and
Waxman J: Analysis of stromal-epithelial interactions in prostate
cancer identifies PTPCAAX2 as a potential oncogene. Cancer Lett.
175:63–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Parker BS, Argani P, Cook BP, Liangfeng H,
Chartrand SD, Zhang M, Saha S, Bardelli A, Jiang Y, St Martin TB,
et al: Alterations in vascular gene expression in invasive breast
carcinoma. Cancer Res. 64:7857–7866. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miskad UA, Semba S, Kato H and Yokozaki H:
Expression of PRL-3 phosphatase in human gastric carcinomas: Close
correlation with invasion and metastasis. Pathobiology. 71:176–184.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu X, Zeng H, Zhang X, Zhao Y, Sha H, Ge
X, Zhang M, Gao X and Xu Q: Phosphatase of regenerating liver-3
promotes motility and metastasis of mouse melanoma cells. Am J
Pathol. 164:2039–2054. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Quah SY, Dong JM, Manser E, Tang
JP and Zeng Q: PRL-3 down-regulates PTEN expression and signals
through PI3K to promote epithelial-mesenchymal transition. Cancer
Res. 67:2922–2926. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kato H, Semba S, Miskad UA, Seo Y, Kasuga
M and Yokozaki H: High expression of PRL-3 promotes cancer cell
motility and liver metastasis in human colorectal cancer: A
predictive molecular marker of metachronous liver and lung
metastases. Clin Cancer Res. 10:7318–7328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mizuuchi E, Semba S, Kodama Y and Yokozaki
H: Down-modulation of keratin 8 phosphorylation levels by PRL-3
contributes to colorectal carcinoma progression. Int J Cancer.
124:1802–1810. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Min SH, Kim DM, Heo YS, Kim YI, Kim HM,
Kim J, Han YM, Kim IC and Yoo OJ: New p53 target, phosphatase of
regenerating liver 1 (PRL-1) downregulates p53. Oncogene.
28:545–554. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiordalisi JJ, Keller PJ and Cox AD: PRL
tyrosine phosphatases regulate rho family GTPases to promote
invasion and motility. Cancer Res. 66:3153–3161. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Werner SR, Lee PA, DeCamp MW, Crowell DN,
Randall SK and Crowell PL: Enhanced cell cycle progression and down
regulation of p21 (Cip1/Waf1) by PRL tyrosine phosphatases. Cancer
Lett. 202:201–211. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liang F, Liang J, Wang WQ, Sun JP, Udho E
and Zhang ZY: PRL3 promotes cell invasion and proliferation by
down-regulation of Csk leading to Src activation. J Biol Chem.
282:5413–5419. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng L, Jin G, Wang L, Guo J, Meng L and
Shou C: Identification of integrin alpha1 as an interacting protein
of protein tyrosine phosphatase PRL-3. Biochem Biophys Res Commun.
342:179–183. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hinds PW: Too much of a good thing: The
Prl-3 in p53's oyster. Mol Cell. 30:260–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Basak S, Jacobs SB, Krieg AJ, Pathak N,
Zeng Q, Kaldis P, Giaccia AJ and Attardi LD: The
metastasis-associated gene Prl-3 is a p53 target involved in
cell-cycle regulation. Mol Cell. 30:303–314. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Min SH, Kim DM, Heo YS, Kim HM, Kim IC and
Yoo OJ: Downregulation of p53 by phosphatase of regenerating liver
3 is mediated by MDM2 and PIRH2. Life Sci. 86:66–72. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Forte E, Orsatti L, Talamo F, Barbato G,
De Francesco R and Tomei L: Ezrin is a specific and direct target
of protein tyrosine phosphatase PRL-3. Biochim Biophys Acta.
1783:334–344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jeong DG, Kim SJ, Kim JH, Son JH, Park MR,
Lim SM, Yoon TS and Ryu SE: Trimeric structure of PRL-1 phosphatase
reveals an active enzyme conformation and regulation mechanisms. J
Mol Biol. 345:401–413. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li M, Brooks CL, Wu-Baer F, Chen D, Baer R
and Gu W: Mono-versus polyubiquitination: Differential control of
p53 fate by Mdm2. Science. 302:1972–1975. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pascaru M, Tanase C, Vacaru AM, Boeti P,
Neagu E, Popescu I and Szedlacsek SE: Analysis of molecular
determinants of PRL-3. J Cell Mol Med. 13:3141–3150. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peters CS, Liang X, Li S, Kannan S, Peng
Y, Taub R and Diamond RH: ATF-7, a novel bZIP protein, interacts
with the PRL-1 protein-tyrosine phosphatase. J Biol Chem.
276:13718–13726. 2001.PubMed/NCBI
|
|
25
|
Si X, Zeng Q, Ng CH, Hong W and Pallen CJ:
Interaction of farnesylated PRL-2, a protein-tyrosine phosphatase,
with the beta-subunit of geranylgeranyltransferase II. J Biol Chem.
276:32875–32882. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun JP, Wang WQ, Yang H, Liu S, Liang F,
Fedorov AA, Almo SC and Zhang ZY: Structure and biochemical
properties of PRL-1, a phosphatase implicated in cell growth,
differentiation, and tumor invasion. Biochemistry. 44:12009–12021.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bessette DC, Qiu D and Pallen CJ: PRL
PTPs: Mediators and markers of cancer progression. Cancer
Metastasis Rev. 27:231–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choi MS, Min SH, Jung H, Lee JD, Lee TH,
Lee HK and Yoo OJ: The essential role of FKBP38 in regulating
phosphatase of regenerating liver 3 (PRL-3) protein stability.
Biochem Biophys Res Commun. 406:305–309. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Min SH, Lau AW, Lee TH, Inuzuka H, Wei S,
Huang P, Shaik S, Lee DY, Finn G, Balastik M, et al: Negative
regulation of the stability and tumor suppressor function of Fbw7
by the Pin1 prolyl isomerase. Mol Cell. 46:771–783. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peng L, Xing X, Li W, Qu L, Meng L, Lian
S, Jiang B, Wu J and Shou C: PRL-3 promotes the motility, invasion,
and metastasis of LoVo colon cancer cells through PRL-3-integrin
beta1-ERK1/2 and-MMP2 signaling. Mol Cancer. 8:1102009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang CB, Feng L, Chia J and Yoon HS:
Molecular characterization of FK-506 binding protein 38 and its
potential regulatory role on the anti-apoptotic protein Bcl-2.
Biochem Biophys Res Commun. 337:30–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bulgakov OV, Eggenschwiler JT, Hong DH,
Anderson KV and Li T: FKBP8 is a negative regulator of mouse sonic
hedgehog signaling in neural tissues. Development. 131:2149–2159.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fong S, Mounkes L, Liu Y, Maibaum M,
Alonzo E, Desprez PY, Thor AD, Kashani-Sabet M and Debs RJ:
Functional identification of distinct sets of antitumor activities
mediated by the FKBP gene family. Proc Natl Acad Sci USA.
100:14253–14258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rosner M, Hofer K, Kubista M and
Hengstschläger M: Cell size regulation by the human TSC tumor
suppressor proteins depends on PI3K and FKBP38. Oncogene.
22:4786–4798. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bai X, Ma D, Liu A, Shen X, Wang QJ, Liu Y
and Jiang Y: Rheb activates mTOR by antagonizing its endogenous
inhibitor, FKBP38. Science. 318:977–980. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luan SL, Boulanger E, Ye H, Chanudet E,
Johnson N, Hamoudi RA, Bacon CM, Liu H, Huang Y, Said J, et al:
Primary effusion lymphoma: Genomic profiling revealed amplification
of SELPLG and CORO1C encoding for proteins important for cell
migration. J Pathol. 222:166–179. 2010. View Article : Google Scholar : PubMed/NCBI
|