Introduction
The incidence of pyemic secondary acute kidney
injury (AKI) is extremely high at 50–80% (1). It is mostly believed that pathogenesis
is correlated with: i) specific tissue structure of kidney; ii)
ischemia reperfusion; iii) inflammation caused by endotoxin; and
iv) gene expression theory (2).
Several RCT studies have confirmed that early continuous renal
replacement therapy (CRRT) is able to improve clinical symptoms and
reverse disease course (3,4). Based on the RENAL study conducted in
2009 and CRRT study performed on 1,349 cases of comprehensive
JSEPTIC and BEST databases in 2013, the use of high dose of CRRT
did not produce better results compared to a low dose of CCRT
(5,6). It was also suggested that AKI patients
receive CRRT as early as possible before any obvious complications
appear. Indexes such as blood urea nitrogen, creatinine and urine
volume serve only as references for CRRT, and uniform and ideal
clinical criteria and reference index are lacking.
Pyemia and AKI were shown to be linked to
inflammatory response and imbalance (6,7). Ronco
et al found that hs-CRP was an acute phase reactive protein
and CRRT was capable of reducing the hs-CRP level, which was
closely associated with prognosis (8). Nevertheless, no final conclusion has
been drawn regarding wehther hs-CRP is able to guide CRRT.
Cartilage glycoprotein 39 (YKL-40) and Annexin A1 are two newly
identified inflammatory factors, with possible links to the
incidence and development of pyemia and AKI (9). To the best of our knowledge, few
studies have demonstrated whether these proteins can be used as
sensitive and specific indexes for CRRT.
The aim of the present study was to examine the
value of applying YKL-40 and Annexin A1 in guiding CRRT based on
monocentric and small sample clinical controlled trials.
Materials and methods
Object data
From October, 2013 to October, 2015, 45 pyemic
secondary AKI cases as well as 40 pyemic non-secondary AKI cases
were selected. There were also 35 cases of physical examination
volunteers. Pyemia was diagnosed in accordance with diagnostic
criteria jointly prepared by the USA SCCM, ACCP, ATS, ESICM and
SIS. AKI was based on diagnostic criteria recommended by KDIGO
(10).
Inclusion criteria for the study were: i) patients
were ≥18 years but <75 years; ii) they were all diagnosed in
accordance with CRRT indexes and features suggested by KDIGO; and
iii) they all had complete clinical data.
Exclusion criteria for the study were: i) none of
our cases had primary renal diseases such as nephrotic syndrome;
history of renal transplantation, solitary kidney and congenital
nephrarctia; ii) none of them had septic shock, multiple resistant
bacteria, severe underlying diseases such as heart failure, liver
failure, malignant tumor and coagulation disorders; iii) patients
with <12 months to live were excluded; and iv) pregnant women,
patients with autoimmune disease and non-compliant patients were
also excluded. Signed written informed consent was obtained from
all the participants prior to the study. The study was approved by
the Ethics Committee of the First People's Hospital of Xuzhou.
For pyemic secondary AKI patients, there were 28
males and 17 females; aged between 29 and 68 years, with an average
age of 45.7±15.6 years. Of these there were 22 cases of
drug-resistant bacteremia, 8 cases of infection after injury, 5
cases of infection after burn, 4 cases of infection after severe
acute pancreatitis and 6 cases of infection after autoimmune
disease drugs. For pyemic non-secondary AKI patients, there were 25
males and 15 females; aged between 28 and 69 years, with an average
age of 46.3±13.8 years. Of these, there were 20 cases of
drug-resistant bacteremia, 7 cases of infection after injury, 3
cases of infection after burn, 5 cases of infection after severe
acute pancreatitis and 5 cases of infection after autoimmune
disease drugs. The physical examination volunteers group comprised
23 males and 12 females; aged 24–67 years, with an average age of
43.8±14.4 years. Differences of age and gender in the three groups
were not statistically significant. Differences between types of
infection in the pyemic secondary and non-secondary AKI group were
not statistically significant.
Study method
Treatment was started based on the ‘CRRT application
indexes and features suggested by KDIGO’ (10). Reasonable sepsis bundle was also
applied. The bundle included: i) initial fluid resuscitation; ii)
antibiotic; iii) vasoactive agent; and iv) active correction on
primary diseases. Additionally, glucocorticoid, blood transfusion
and mechanical ventilation adjuvant therapy were also applied when
necessary. CVVH was used in CRRT and blood flow volume was 180–200
ml/min and the flow was 35–40 ml/kg·h. Substitution fluid was 0.9%
sodium chloride (3,000 ml) + water (1,000 ml) + 5% glucose (250 ml)
+ 10% calcium chloride (10 ml) + 50% magnesium sulfate (1.8 ml) +
5% sodium bicarbonate (250 ml) + 10% potassium chloride (15 ml).
Prior to initiation of the experiment, unfractionated heparin
(25,000 units) was used to prime blood filter and blood filter line
and low molecular heparin 6,000 units to maintain anticoagulation
and monitor coagulation function. APTT equal to 1.5- to 2-fold over
the normal value was considered appropriate and this was filtered
for 8–12 h each time. The main instruments included in the study
were: i) Baxter Aquarius CRRT machine and ii) Aqu-line disposable
blood purification tube (both from Baxter, Deerfield, IL, USA);
iii) 12 Fr double channel deep vein catheter (Arrow Medical,
Kington, Herefordshire, UK); and iv) Baxter blood filtration
apparatus HF 1200 (Baxter).
Observation index and testing
method
Serum YKL-40 and Annexin A1 levels in the three
groups were compared. CRRT was used for pyemic secondary AKI
patients, who were divided into the success and failure groups
based on results. YKL-40, Annexin A1, hs-CRP, creatinine and urea
nitrogen levels were compared after 1, 6, 12, 24, 48 and 72 h of
AKI.
Separation of mononuclear cells from peripheral
serum: Anticoagulant blood (5 ml) was centrifuged for 20 min at
1,500 × g and the supernatant was discarded. Hank solution (2.5 ml)
was added to the pellet based on a volume ratio of 1:1 provided by
Sangon Biotech Co., Ltd. (Shanghai, China). Subsequently 5 ml of
LTS1077 lymphocytes separating solution (Sigma, Darmstadt, Germany)
was added and centrifuged for 20 min at 1,500 × g. Plasma was
removed and Hank solution was added to the PBMC. The mix was
subsequently centrifuged for 10 min at 1,000 × g. RPMI-1640 medium
(Sigma) was used to rinse the pellets to remove impurities such as
blood platelet fully.
YKL-40 concentration was tested using ELISA. PBMC
separating medium (1 ml) was incubated for 30 min at 37°C. Serum
was centrifuged for 10 min at 2,500 × g and the supernatant was
removed. The YKL-40 ELISA kit (no. 8020; Quidel Corp., San Diego,
CA, USA) was used.
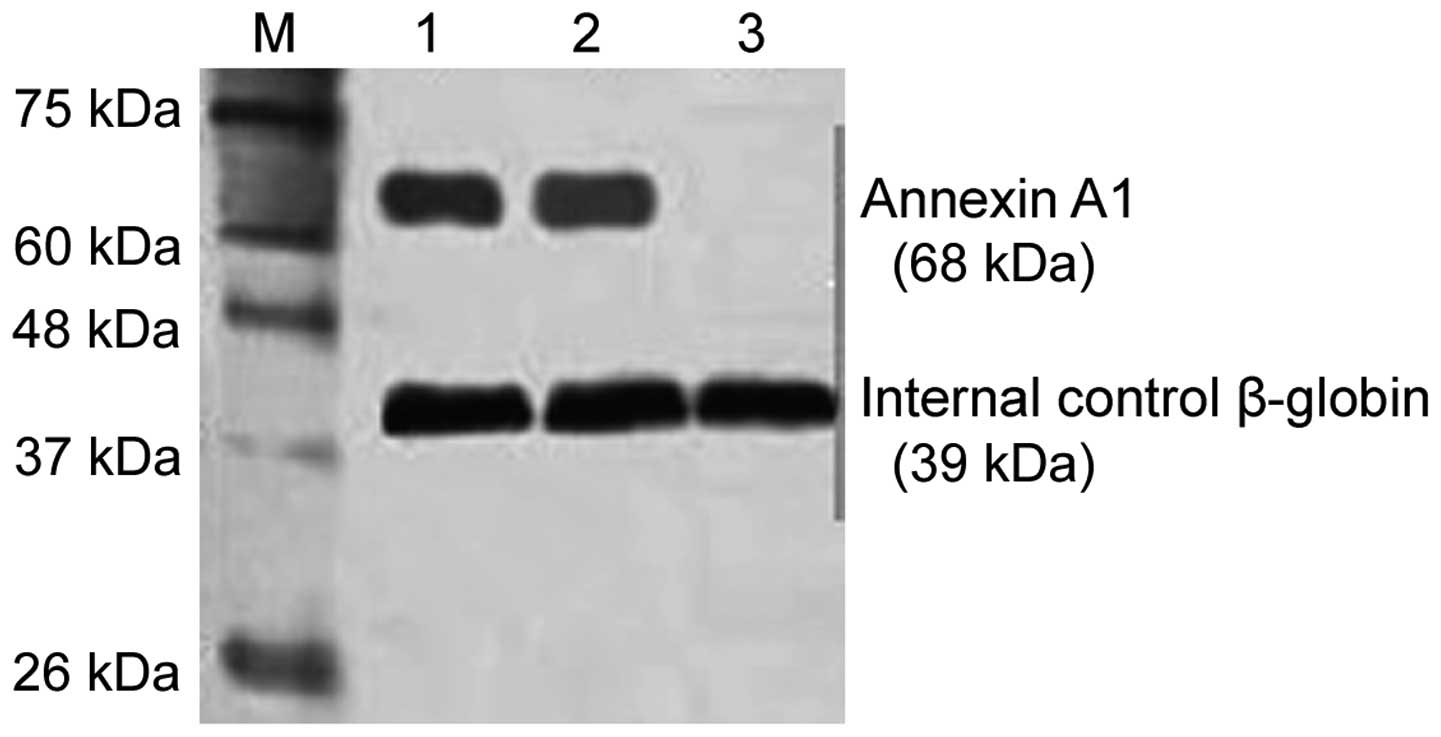
Western blot analysis was used to test the Annexin
A1 expression level. Total protein in PBMC was extracted using the
conventional protein extraction method. After SDS-PAGE, the
proteins were transferred to a nitrocellulose membrane. The
membrane was blocked for 2 h (5% skim milk), and primary rabbit
polyclonal Annexin A1 antibody (Abcam, Cambridge, MA, USA; catalog
no. ab33061; dilution, 1:1,000) were added and incubated at 4°C
overnight. The membrane was then rinsed and secondary horseradish
peroxidase coupled with goat anti-rabbit antibody was added (Abcam;
catalog no. ab6721; dilution, 1:5,000). After 1-h incubation at
25°C, DAB development was used and the gel image processing system
(Denver Company, CO, USA) was employed for quantitative analysis
(β-globin expression level as internal control). The protein
expression level was shown as the density ratio between the
targeted gene and β-globin.
Statistical method
SPSS 19.0 statistical software (Chicago, IL, USA)
was used for data analysis. Measurement data were presented as mean
value ± standard deviation, analysis of variance (ANOVA) was
applied for comparisons among groups. The independent sample t-test
was used for comparisons between two groups. Comparison of
different points within one group were carried out using repeated
measurement data. Enumeration data were shown as case or (%), and
the χ2 test was used for comparisons between groups.
P<0.05 was considered statistically significant.
Results
Comparison of YKL-40 and Annexin A1
level in the three groups
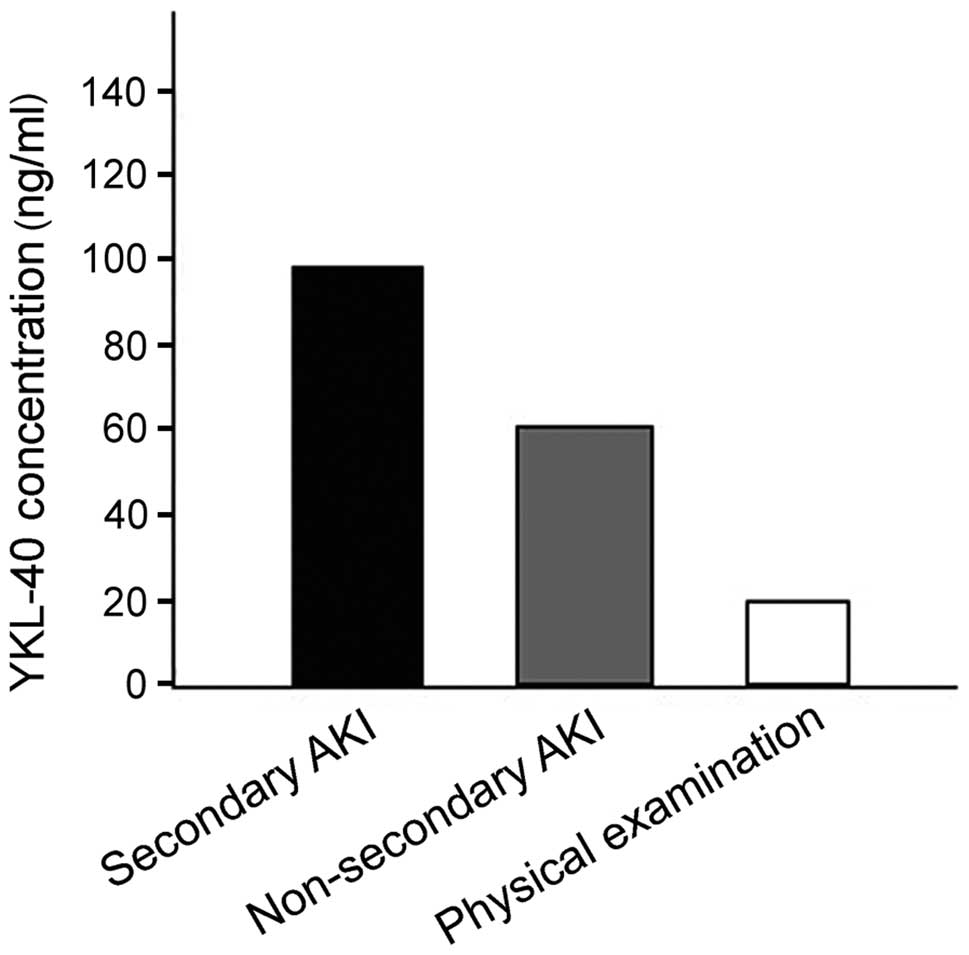
YKL-40 level in the pyemic secondary AKI group was
obviously higher than those in the other two groups and differences
were statistically significant (P<0.001). The YKL-40 level was
105.7±26.3 ng/ml in the pyemic secondary AKI group and 62.4±22.9
and 18.9±6.6 ng/ml in the other two groups.
The Annexin A1 level was also significantly higher
in the pyemic secondary AKI group compared with the other two
groups (0.853±0.076 ng/ml vs. 0.582±0.055 ng/ml and 0.043±0.022
ng/ml). Differences were statistically significant (P<0.001)
(Figs. 1 and 2).
Comparison of YKL-40 and Annexin A1
levels between the success and failure secondary AKI group
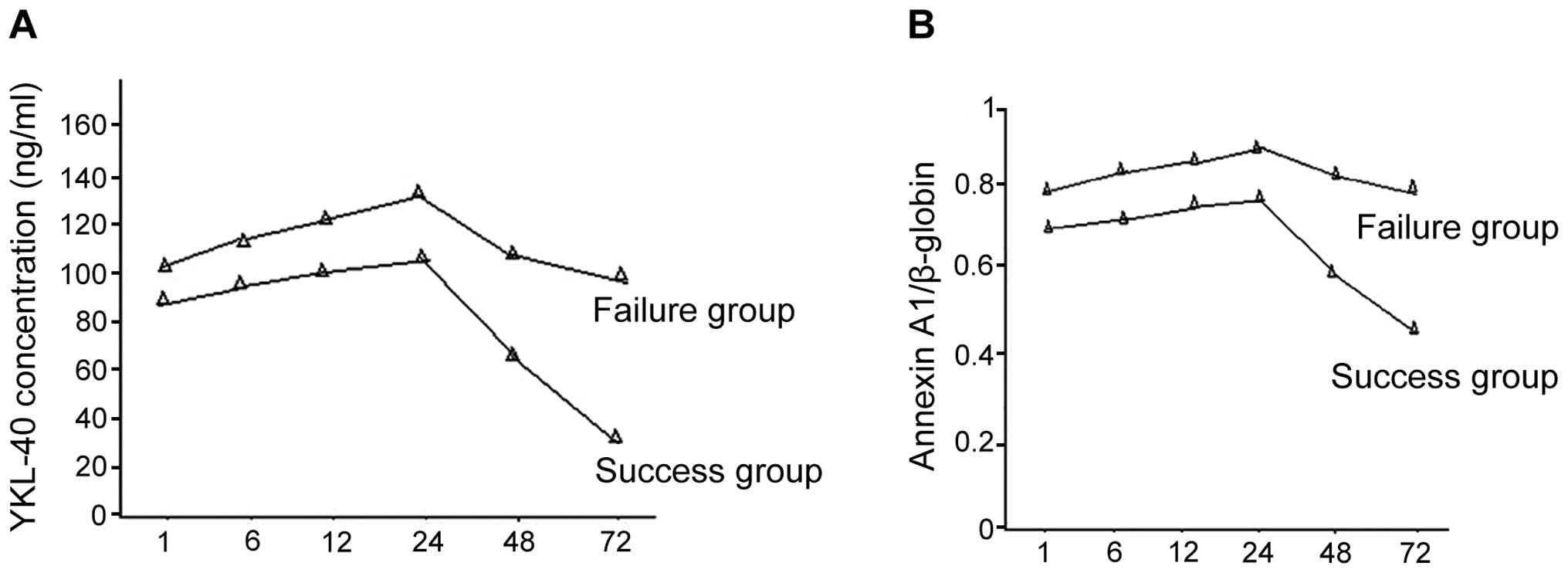
There were 30 success cases together with 15 failure
cases in the secondary AKI group. Of these cases, 5 individuals in
the failure group succumbed, and there were 8 cases of acute renal
failure and 2 cases of respiratory failure. Differences in the time
period of using CRRT in the success and failure groups showed no
statistical significance. YKL-40 and Annexin A1 expression levels
in the success group were decreased more significantly and the
difference was statistically significant (P<0.05) (Fig. 3).
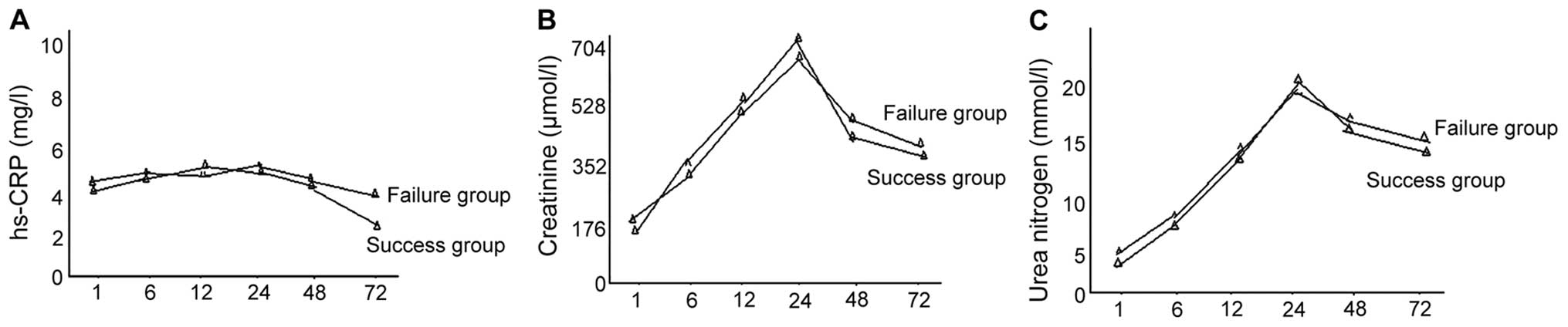
Comparisons of the hs-CRP, creatinine
and urea nitrogen level
Differences between the creatinine and urea nitrogen
levels in the success and failure groups, at all time points, were
not statistically significant (P>0.05). The levels of creatinine
and urea nitrogen in the success group were significantly decreased
as compared to the failure group at 72 h, while comparisons at
other time points showed no statistical significance (P>0.05)
(Fig. 4).
Discussion
YKL-40 is a carbohydrate-binding protein belonging
to the chitinase family, and has a molecular weight of 40 kDa.
YKL-40 is present in very low quantities in normal human
mononuclear cells (18.9 ng/ml) but may increase significantly after
infection (in pyemic non-secondary AKI it reached 62.4 ng/ml and in
the secondary AKI group it reached 105.7 ng/ml). Prior studies have
shown that for child patients suffering from pyemia, the YKL-40
concentration was reduced by 40% after 12 h of CRRT and returned to
normal at 24 h (11). The results of
the current study have shown that YKL-40 concentration in pyemic
secondary AKI patients increased with time and reached its peak at
24 h. YKL-40 concentration in the failure group was higher than
that of the success group at all time-points. The YKL-40
concentration was significantly decreased by CRRT and in some cases
the YKL-40 concentration decreased more significantly in the
success group. Thus, changes in the YKL-40 concentration may be
used as a sensitive index for using CRRT and to predict its
treatment effects. YKL-40 secretion may be closely associated with
a group of inflammatory cytokines. Findings have shown that
excessive release of interleukin (IL)-6 during pyemia was the key
factor in enhancing the YKL-40 expression level (12). Activation of the toll-like
receptor/NF-κB signaling pathway and excessive release of
pro-inflammatory factors such as IL-1, Il-6 and TNF-α is an
important mechanism for YKL-40 secretion.
Annexin A1 is a structure-dependent,
calcium-dependent phospholipid-binding protein superfamily. Annexin
A1 is a multifunctional protein, involved in the regulation of
inflammation, phagocytosis, signal transmission, cell
differentiation and apoptosis (13).
Annexin A1 is an important regulatory molecule for glucocorticoid
in the anti-inflammatory process by: i) preventing the formation of
arachidonic acid and inflammatory precursor formation; ii) inducing
the production of anti-inflammatory factors by inhibiting
phospholipase A2 activity; iii) inhibiting the formation of
cyclooxygenase and nitric oxide synthase; iv) activation and
emigration of neutrophile granulocytes; and v) promoting the
removal of apoptotic cells to avoid necrosis that triggers an
inflammatory response (14). The
anti-inflammatory action of Annexin A1 is mainly regulated through
the FPRs signaling pathway (15).
The Annexin A1 expression level in normal human body
is normally low, but is increased in pyemia significantly and is
higher in secondary AKI. Annexin A1 is associated with inflammatory
response and immune regulation. We observed that the Annexin A1
expression level in pyemic secondary AKI patients increased with
time without any obvious peak value. In the failure group, the
values of the time-points were significantly higher than those in
the success group. Annexin A1 expression was greatly reduced by
CRRT and there were more cases of reduced expression in the success
group than in the failure group. Thus, changes of Annexin A1
expression are an important index to predict treatment effects,
although it was not as sensitive as YKL-40 in terms of guiding the
start of CRRT.
There were also no obvious changes in hs-CRP in all
the time-points in the pyemic secondary AKI success and failure
groups. Creatinine and urea nitrogen levels increased with time but
differences in all the time-points were not statistically
significant. Following treatment, the hs-CRP level in the success
group was significantly reduced while creatinine and urea nitrogen
level in the two groups decreased, albeit differences were not
statistically significant. Thus, hs-CRP may be used as an important
index to predict CRRT effects. It was not ideal to use the
creatinine and urea nitrogen level to start CRRT and predict
clinical effects. Notably, changes in serum cartilage glycoprotein
39 constitute a sensitive index for using CRRT, which may predict
treatment effects together with Annexin A1.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 31571187).
References
|
1
|
Zhang P, Yang Y, Lv R, Zhang Y, Xie W and
Chen J: Effect of the intensity of continuous renal replacement
therapy in patients with sepsis and acute kidney injury: a
single-center randomized clinical trial. Nephrol Dial Transplant.
27:967–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang N, Jiang L, Zhu B, Wen Y and Xi X-M:
Beijing Acute Kidney Injury Trial (BAKIT) Workgroup: Fluid balance
and mortality in critically ill patients with acute kidney injury:
a multicenter prospective epidemiological study. Crit Care.
19:3712015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith OM, Wald R, Adhikari NKJ, Pope K,
Weir MA and Bagshaw SM: Canadian Critical Care Trials Group:
Standard versus accelerated initiation of renal replacement therapy
in acute kidney injury (STARRT-AKI): study protocol for a
randomized controlled trial. Trials. 14:3202013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chou YH, Huang TM, Wu VC, Wang CY, Shiao
CC, Lai CF, Tsai HB, Chao CT, Young GH, Wang WJ, et al: NSARF Study
Group: Impact of timing of renal replacement therapy initiation on
outcome of septic acute kidney injury. Crit Care. 15:R1342011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vesconi S, Cruz DN, Fumagalli R,
Kindgen-Milles D, Monti G, Marinho A, Mariano F, Formica M,
Marchesi M, René R, et al: DOse REsponse Multicentre International
collaborative Initiative (DO-RE-MI Study Group): Delivered dose of
renal replacement therapy and mortality in critically ill patients
with acute kidney injury. Crit Care. 13:R572009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yasuda H, Uchino S, Uji M, Ohnuma T, Namba
Y, Katayama S, Kawarazaki H, Toki N, Takeda K, Izawa J, et al:
Japanese Society for Physicians and Trainees in Intensive Care
Clinical Trial Group: The lower limit of intensity to control
uremia during continuous renal replacement therapy. Crit Care.
18:5392014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Limaye AP, Kirby KA, Rubenfeld GD,
Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Hayes TK
Santo, Corey L, et al: Cytomegalovirus reactivation in critically
ill immunocompetent patients. JAMA. 300:413–422. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ronco C, Tetta C, Mariano F, Wratten ML,
Bonello M, Bordoni V, Cardona X, Inguaggiato P, Pilotto L, d'Intini
V, et al: Interpreting the mechanisms of continuous renal
replacement therapy in sepsis: the peak concentration hypothesis.
Artif Organs. 27:792–801. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hattori N, Oda S, Sadahiro T, Nakamura M,
Abe R, Shinozaki K, Nomura F, Tomonaga T, Matsushita K, Kodera Y,
et al: YKL-40 identified by proteomic analysis as a biomarker of
sepsis. Shock. 32:393–400. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kliger AS, Foley RN, Goldfarb DS,
Goldstein SL, Johansen K, Singh A and Szczech L: KDOQI US
commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia
in CKD. Am J Kidney Dis. 62:849–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schmidt IM, Hall IE, Kale S, Lee S, He CH,
Lee Y, Chupp GL, Moeckel GW, Lee CG, Elias JA, et al:
Chitinase-like protein Brp-39/YKL-40 modulates the renal response
to ischemic injury and predicts delayed allograft function. J Am
Soc Nephrol. 24:309–319. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, He H, Wang J and Sheng D:
Expression of cartilage glycoprotein 39 in peripheral blood
monocytes of septic rat and its role in TLR 4-NF-κB signaling
pathways. Int J Clin Exp Med. 8:2459–2464. 2015.PubMed/NCBI
|
|
13
|
Horlacher T, Noti C, de Paz JL,
Bindschädler P, Hecht ML, Smith DF, Fukuda MN and Seeberger PH:
Characterization of annexin A1 glycan binding reveals binding to
highly sulfated glycans with preference for highly sulfated heparan
sulfate and heparin. Biochemistry. 50:2650–2659. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McArthur S, Cristante E, Paterno M,
Christian H, Roncaroli F, Gillies GE and Solito E: Annexin A1: A
central player in the anti-inflammatory and neuroprotective role of
microglia. J Immunol. 185:6317–6328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brooks AC, Rickards KJ and Cunningham FM:
Modulation of equine neutrophil adherence and migration by the
annexin-1 derived N-terminal peptide, Ac2-26. Vet Immunol
Immunopathol. 145:214–222. 2012. View Article : Google Scholar : PubMed/NCBI
|