Introduction
Atrial fibrillation (AF) is a condition involving an
irregular heart rhythm, known as arrhythmia. It is the most common
type of arrhythmia (1). The most
common conditions leading to AF are: i) rheumatic valvular disease,
ii) coronary heart disease, and iii) hypertensive heart disease. AF
is the main risk factor inducing cardiogenic strokes (2). Increased pressure and blood stagnation
occurred in AF, due to the particular anatomical structure of the
left atrial appendage, which is the narrowest part in the left
atrium with the roughest endocardial surface. Therefore thrombus
easily occurs in the left atrial appendage, and in previous
studies, length, width, emptying rate and other indicators were
employed to evaluate its function (3).
In the present study, we measured the left atrial
appendage volume (LAA-V) through real-time three-dimensional
transesophageal echocardiography (RT3D-TEE) to calculate LAA
ejection fraction (LAA-EF). Subsequently, the association between
the changes in LAA-V, LAA-EF and thrombosis when LAA occurred was
analyzed. These analyses provided valuable information pertaining
to patients with a high risk of thrombosis and guided our clinical
treatment and reduced the occurrence of LAA and cardiogenic
stroke.
Materials and methods
Research object
From September, 2014 to December, 2014, 74 patients
diagnosed with AF at the Xiangyang Hospital Affiliated to Hubei
University of Medicine (Hubei, China), were selected. Patients with
valvular heart diseases were excluded. There were 32 men and 42
women, with an age range of 34–76 years (average, 58.86±9.85
years). Of the 74 cases, 26 suffered from persistent AF while 48
had paroxysmal AF. According to the results obtained through
ultrasonic cardiogram and clinical examinations all 74 cases were
diagnosed with AF. There were 3 groups: i) the LAA thrombosis group
(TH group) with 22 cases, ii) the non-LAA thrombosis group (NTH
group) with 52 cases, and iii) the control group with 20 randomly
chosen patients from our hospital. Patients in the control group
comprised 10 men and 10 women (average age, 44.21±12.29 years), who
did not have AF.
Apparatus and method
Philips iE33 color Doppler ultrasonic diagnostic
apparatus was used (Philips, Eindhoven, The Netherlands). Patients
were detected using TTE, connected to the electrocardiogram, to
have the information related to their heart size, morphology,
structure and function. Informed consent was obtained from the
patients. Approval of the study was obtained by the ethics
committee of the Xiangyang Hospital Affliated to Hubei University
of Medicine. TEE (X7-2t probe, 2–7 MHz) was used. After TEE,
patients fasted for >8 h with no water for >4 h. In cases
with dentures they were removed at the same time. On the
examination of TEE, the patients with local anesthesia of lidocaine
hydrochloride were examined in the left lateral decubitus position,
biting mouthparts were placed in their oral cavity, and probe X7-2t
was sent into the esophagus to scan each section of left atrial
appendage in the middle of the esophagus, to observe thrombosis and
measure LAA peak empty velocity (LAA-PEV). Then full-volume was
initiated, five cardiac cycles were stored, and QLAB 9.0 software
(Philips Medical System, Andover, MA, USA) was applied in the
analysis.
Measurement index
LAA-PEV
In the middle of the TEE esophagus, approximately
45°, a long axis of the left atrial appendage was displayed, a
pulse-waved (PW) Doppler was applied in placing the sample volume
at the central position with <1 cm the distance from the left
atrial appendage entrance to obtain a spectrogram, and forward wave
in the beginning of P-wave in patients with sinus rhythm, the peak
velocity of which was LAA-PEV. In the AF patients, the average
value of maximum forward velocity in each cardiac cycle of five
cardiac cycles was LAA-PEV.
LAA end-diastolic volume (LAA-EDV), LAA
end-systolic volume (LAA-ESV) and LAA-EF
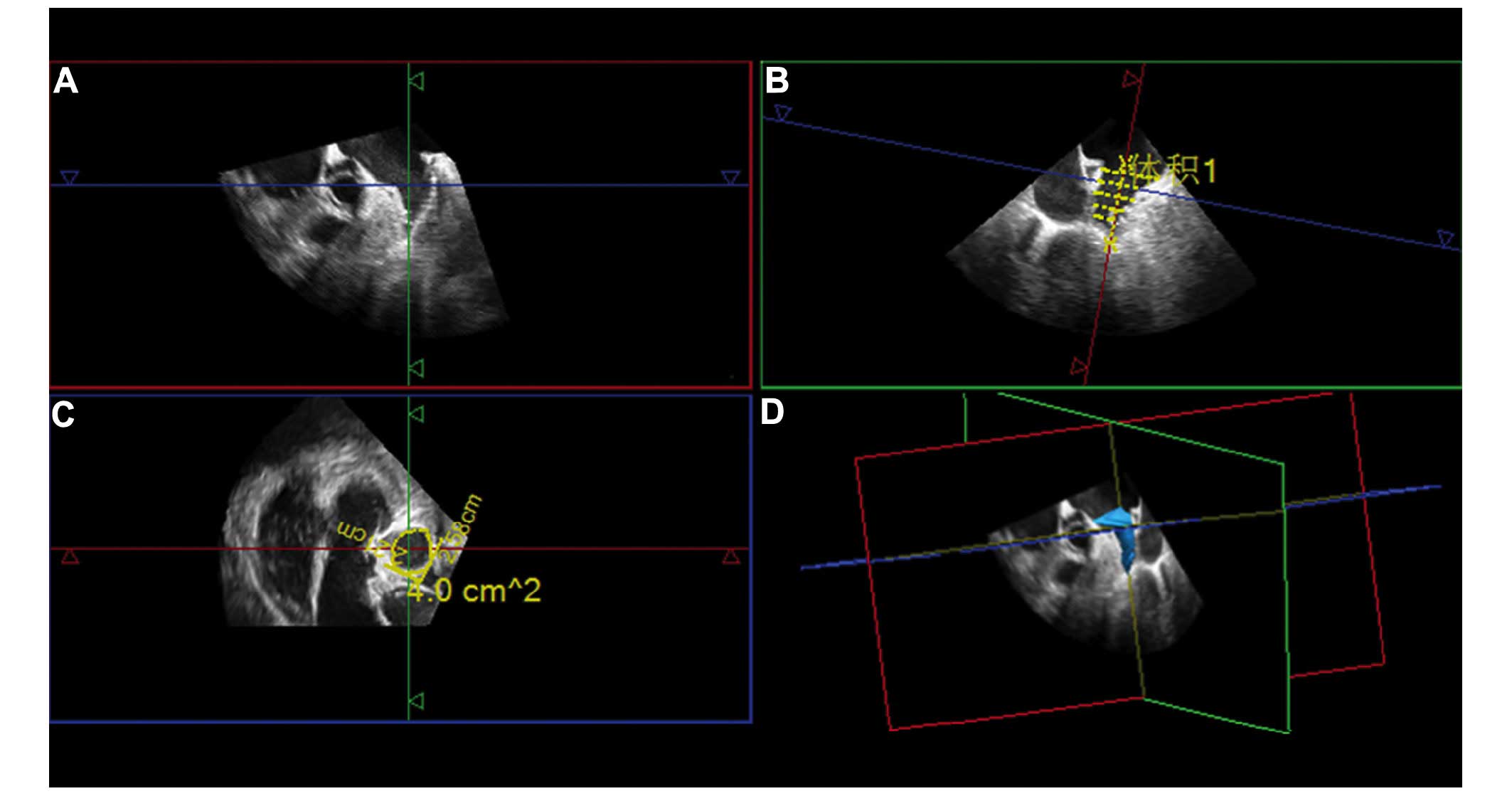
The full-volume image was opened by GI3DQ plugin and
the target area was adjusted, as there were three perpendicular
sections of LAA, two long axis section and a transverse section
(Fig. 1A-C). One of the long axis
sections was selected, stacked contours button was clicked, and the
software automatically measured the vertical distance from the
entrance to the top of LAA. A long axis section was cut into
several layers of equidistant cross sections (adjustable 3–16
layers, Fig. 1B), and the contour of
each depicting cross section was shown. The instrument then
automatically generated the 3D image of LAA and computed the LAA-V
(Fig. 1D).
The measurement of LAA-EDV and LAA-ESV
Patients with sinus rhythm were detected,
respectively, in the beginning of P-wave and in the end of QRS
wave. The mean values of the maximum volume and the minimum volume
in each cardiac cycle of 5 consecutive cardiac cycles were measured
in patients with AF. Subsequently, LAA-EF was calculated using the
following formula: (LAA-EDV-LAA-ESV)/(LAA-EDV).
Statistical analysis
SPSS 18.0 software package (Chicago, IL, USA) was
used for data processing. Data were expressed as mean ± standard
deviation (SD). One-way analysis of variance (ANOVA) was employed
in the comparison of multiple sets of measurement data. LSD methods
were used to compare between two mean values of multiple sets of
mean values. Pearson's method was applied to compare LAA-PEV, LAA-V
and LAA-EF. Receiver operating characteristic (ROC) curve was used
in the prediction of cut-off values of the left atrial appendage
thrombus through LAA-EDV and LAA-ESV. P<0.05 was considered
statistically significant.
Results
Left atrial thrombosis
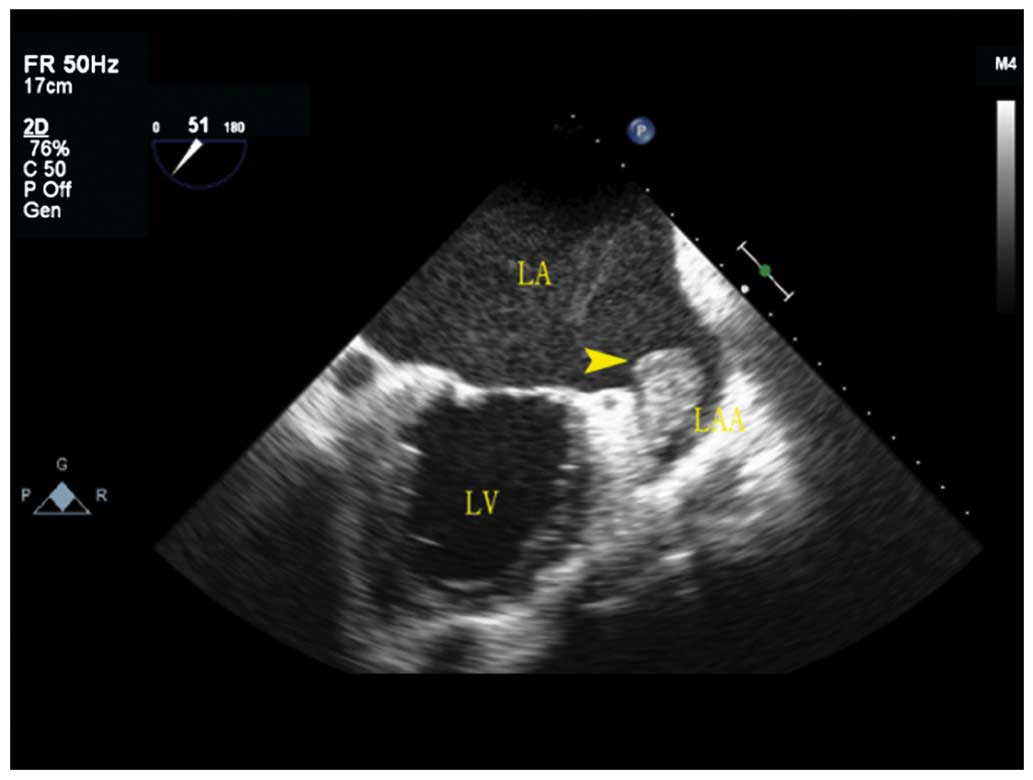
Of the 74 patients with AF, 22 cases had LAA
thrombus by TEE, flat or papillary, 2.0×3.3 mm to 8.9×5.7 mm. Of
the 22 cases, 14 cases were fresh thrombus with weak and low echo,
and 8 cases had organized thrombus with strong echo (Fig. 2), 10 cases were located in the
pectinate muscles at the top of LAA, 7 cases were located on the
lateral wall of LAA, and 5 cases were located on the medial wall of
left atrial appendage. After thrombolytic therapy for 3–5 months,
LAA thrombus of 14 patients disappeared through TEE review, and
left atrial appendage thrombus of 8 patients was significantly
shrunk.
Comparison of ultrasonic measurement
values of LAA
The comparison of parameters in the three groups is
shown in Table I. Differences
between the LAA-PEV and LAA-EF, NTH group and TH group were
statistically significant (P<0.05). Differences between LAA-EDV
and LAA-ESV in all 3 groups were statistically significant
(P<0.05).
 | Table I.Measurement value of the left atrial
appendage in the three groups. |
Table I.
Measurement value of the left atrial
appendage in the three groups.
| Groups | Cases | LAA-PEV (cm/s) | LAA-EDV (ml) | LAA-ESV (ml) | LAA-EF (%) |
|---|
| Control group | 20 | 77.08±21.02 | 9.18±2.54 | 2.41±1.41 | 74.50±12.03 |
| NTH group | 52 | 70.28±32.58 |
13.56±3.69a |
5.58±2.24a |
59.53±9.55a |
| TH group | 22 |
43.90±22.09a,b |
20.61±5.74a,b |
10.72±3.93a,b |
47.82±15.44a,b |
| F-value |
| 5.933 | 20.467 | 26.069 | 12.712 |
| P-value |
| 0.005 | <0.001 | <0.001 | <0.001 |
Association between LAA-PEV and other
measurement values
LAA-EDV and LAA-PEV showed a moderate negative
correlation (r=−0.531, P<0.001). LAA-PEV and LAA-ESV showed a
strong negative correlation (r=−0.741, P<0.001), while LAA-EF
and LAA-PEV had a strong positive correlation (r=0.693,
P<0.001).
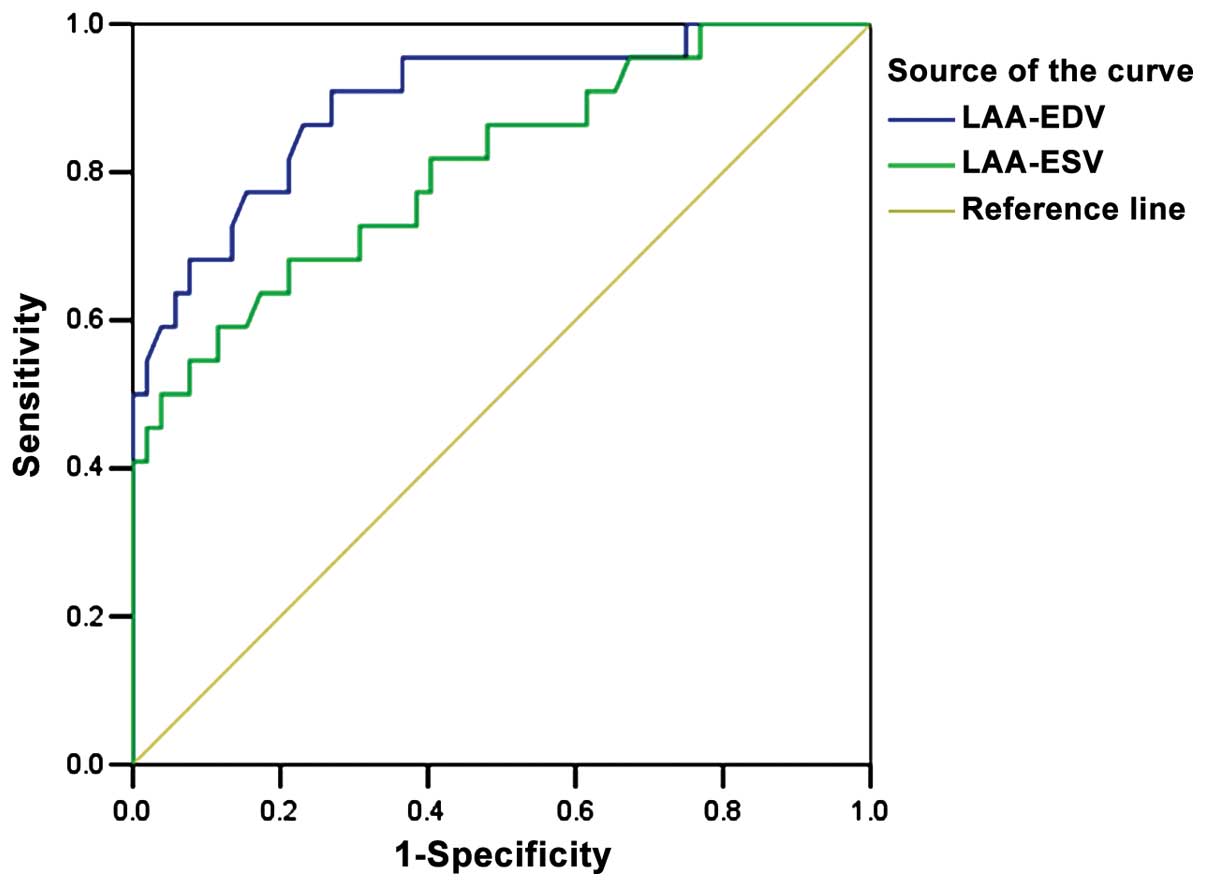
ROC curve analysis of LAA-V
The cut-off values for the forecast of thrombus by
LAA-EDV and LAA-ESV were 18.45 and 9.69 ml, area under the curve
was 0.896 and 0.807, 95% confidence interval was 0.814–0.978 and
0.693–0.920, respectively (P<0.05) (Fig. 3).
Discussion
LAA is the remnants of primitive atrium in embryonic
period, a narrow and curved pipe, the spire is a narrow blind side,
and the body is rich in myocardial cells. These special anatomical
features lead to thrombus formed in the left atrial appendage
rather than in atrium sinistrum and other structures in left atrial
blood flow deposition (4,5). The most common causes of inducing left
atrial blood flow deposition are AF. In AF, the coordination
between atrial and ventricular is broken. This is combined with the
non-coordinated atrial contraction, which induces the atrial blood
ejection deficiency and blood flow deposition, and eventually leads
to higher atrial volume and pressure (6).
In the case of LAA-V, it is believed that ‘t’ can be
used as a predictive value of thromboembolic stroke for patients
with AF (7), and studies have found
that increased LAA-V and decreased LAA-EF were significantly
correlated with paroxysmal AF. The variability of LAA-V observed
through the detection of R3D-TEE was small (8). The present study used GI3DQ of Q-lab
software to respectively calculate LAA-EDV and LAA-ESV, then
calculate LAA-EF. A comparison of LAA-V in all three groups, showed
that the control group had the lowest LAA-V and the TH had the
highest. Differences were statistically significant. These results
indicated that LAA-V increased in AF, and with the increased LAA-V,
the risk of suffering from LAA thrombus was also increased. The
increase of LAA-V in patients with AF may be due to an increase in
left auricle cardiomyocytes in AF and loss of muscle fiber in the
cells, which decreased left atrial systolic function, reduced LAA
blood ejection (9) and shortened
left atrial diastolic and systolic time. The two factors lead to
blood stagnation of the left atrial appendage and increased LAA-V.
LAA-V was usually only approximately 10% of the left atrium, but it
played an important role in the left atrium function. Compared with
the intrinsic atrial, the compliance of LAA was improved; thus,
when the pressure and volume of the left atrial increased, LAA
acted as a reservoir. This was the reason for the intrinsic atrial
becoming only slightly enlarged in patients with AF, while LAA
became significantly expanded (10).
In the group of patients with AF combined with left atrial
thrombus, conventional transthoracic echocardiography did not show
the thrombus. Therefore, conventional TEE examinations in patients
with AF were very important.
Previous findings confirmed that formation of LAA
thrombus, significantly decreased LAA-PEV (11). A low LAA-PEV value has been proven to
increase the risk of LAA thrombus (12). In clinical examinations, we often
evaluated the function of the left atrial appendage in patients
with AF by measuring LAA-PEV. It is believed that LAA-PEV are
independent risk factors in the formation of LAA thrombus (13). The present study has shown that LAA-V
and LAA-PEV were negatively correlated and changes in LAA function
reflected by LAA-V and hemodynamics changes reflected by LAA-PEV
were similar. Additionally, according to the ROC curve, and area
under the curve (AUC), the cut-off values by LAA-ESV and LAA-EDV
were 0.807 and 0.896, respectively, which indicated that use of
LAA-V in the assessment of risk of LAA thrombosis had a higher
accuracy. Results obtained in the present study were similar with
results reported by Tanaka et al on the relationship between
LAA-VI and the occurrence of paroxysmal AF in patients with
cerebral infarction (8). Tanaka
et al evaluated the cut-off point of paroxysmal AF with
LAA-VI and obtained higher AUC (8).
Previous findings showed that, compared with the
control group, the LAA-EF of patients with non-valvular AF was
significantly lower (14). In the
present study, the comparison of LAA-EF in three groups through
LAA-V showed that, the control group was the highest and the TH
group was the lowest. This indicated that the left fibrillation
systolic function of patients with fibrillation decreased, and with
the reduced systolic function, the risk of thrombus increased. Left
fibrillation systolic function was an important factor in affecting
left atrial ejection (15); thus,
the study on the relationship of LAA-EF and LAA-PEV was
significant. This study found that LAA-EF had a strong positive
correlation with LAA-PEV, which showed that the measurement of
LAA-EF with RT3D-TEE in assessment of left fibrillation function
was feasible. Through measurement of the left fibrillation function
in patients in acute stage of cerebral infarction, it was found
that LAA-EF and LAA-PEV in the paroxysmal AF groups were
significantly lower than those of non-AF groups. Through the ROC
curve, it was hypothesized that, LAA-EF <49.1% was able to
predict the occurrence of paroxysmal AF (16), which provided more information for
clinical prognosis and active treatment.
In conclusion, application of RT3D-TEE identified
the structure of LAA comprehensively in real-time and evaluated the
function of LAA through the measurement of LAA-PEV, LAA-EDV,
LAA-ESV and LAA-EF. This method can be used to evaluate the
prognosis of patients with non-valvular AF, and reduce the
occurrence of cardiac stroke.
References
|
1
|
Nattel S, Li D and Yue L: Basic mechanisms
of atrial fibrillation - very new insights into very old ideas.
Annu Rev Physiol. 62:51–77. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwahana H, Ishikawa S, Ishikawa J,
Kabutoya T, Kayaba K, Gotoh T and Kajii E: Atrial fibrillation is a
major risk factor for stroke, especially in women: the Jichi
Medical School cohort study. J Epidemiol. 21:95–101. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wysokinski WE, Ammash N, Sobande F, Kalsi
H, Hodge D and McBane RD: Predicting left atrial thrombi in atrial
fibrillation. Am Heart J. 159:665–671. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burrell LD, Horne BD, Anderson JL,
Muhlestein JB and Whisenant BK: Usefulness of left atrial appendage
volume as a predictor of embolic stroke in patients with atrial
fibrillation. Am J Cardiol. 112:1148–1152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Habara S, Dote K, Kato M, Sasaki S, Goto
K, Takemoto H, Hasegawa D and Matsuda O: Prediction of left atrial
appendage thrombi in non-valvular atrial fibrillation. Eur Heart J.
28:2217–2222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stoddard MF, Dawkins PR, Prince CR and
Ammash NM: Left atrial appendage thrombus is not uncommon in
patients with acute atrial fibrillation and a recent embolic event:
A transesophageal echocardiographic study. J Am Coll Cardiol.
25:452–459. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Santiago D, Warshofsky M, Li MG, Di Tullio
M, Coromilas J, Reiffel J and Homma S: Left atrial appendage
function and thrombus formation in atrial fibrillation-flutter: A
transesophageal echocardiographic study. J Am Coll Cardiol.
24:159–164. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanaka K, Koga M, Sato K, Suzuki R,
Minematsu K and Toyoda K: Three-dimensional analysis of the left
atrial appendage for detecting paroxysmal atrial fibrillation in
acute ischemic stroke. Int J Stroke. 9:1045–1051. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li AL, Li ZA, Wang Y, Zeng YJ and Sun CL:
Assessment of left atrial appendage function by real-time
three-dimensional transesophageal echocardiography. Chinese J
Ultrasonography. 19:737–740. 2010.
|
|
10
|
Nakajima H, Seo Y, Ishizu T, Yamamoto M,
Machino T, Harimura Y, Kawamura R, Sekiguchi Y, Tada H and Aonuma
K: Analysis of the left atrial appendage by three-dimensional
transesophageal echocardiography. Am J Cardiol. 106:885–892. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Demirçelik MB, Çetin M, Çiçekcioğlu H,
Uçar Ö and Duran M: Effect of left ventricular diastolic
dysfunction on left atrial appendage function and thrombotic
potential in nonvalvular atrial fibrillation. Anadolu Kardiyol
Derg. 14:256–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zabalgoitia M, Halperin JL, Pearce LA,
Blackshear JL, Asinger RW and Hart RG: Transesophageal
echocardiographic correlates of clinical risk of thromboembolism in
nonvalvular atrial fibrillation. Stroke prevention in atrial
fibrillation III investigators. J Am Coll Cardiol. 31:1622–1626.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zateyshchikov DA, Brovkin AN, Chistiakov
DA and Nosikov VV: Advanced age, low left atrial appendage
velocity, and factor V promoter sequence variation as predictors of
left atrial thrombosis in patients with nonvalvular atrial
fibrillation. J Thromb Thrombolysis. 30:192–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bi WJ, Sun FF, Ren WD, Pan FZ, Hu Q and Xu
M: Real-time three-dimensional transesphageal echocardiography in
evaluaying on morphology and mechanical function of left atrial
appendage in patients with non-valvular atrial fibrillation.
Chinese Journal of Medical Imaging Technology. 10:1616–1620.
2013.
|
|
15
|
Meng FX, Chen M, Sun JP and Dong Y: The
assessment of left atrial appendage function in patients at high
risk of thrombosis through transesophageal echocardiography.
Chinese J Medical Imaging Technol. 6:473–479. 2014.
|
|
16
|
Shimizu T, Takada T, Shimode A, Fujita Y,
Usuki N, Kato B, Takaishi S, Hirayama T, Hanzawa K and Hasegawa Y:
Association between paroxysmal atrial fibrillation and the left
atrial appendage ejection fraction during sinus rhythm in the acute
stage of stroke: a transesophageal echocardiographic study. J
Stroke Cerebrovasc Dis. 22:1370–1376. 2013. View Article : Google Scholar : PubMed/NCBI
|