Introduction
Cardiovascular diseases and depression are very
prevalent among the general population and often occur
simultaneously in the same patient (1). The association between depression and
heart disease is two directional. Depression is regarded as a risk
factor for coronary heart disease and acute cardiovascular
sequelae, including myocardial infarction and heart failure
(2). Conversely, patients with heart
disease exhibit increased episodes of depression (3,4).
Moreover, morbidity and mortality rates in patients with depression
and cardiovascular disease have been found to be significantly
higher than those in patients with cardiovascular disease without
depression (4). It has been observed
that patients who experienced a myocardial infarction and became
depressed had a 5-fold increase in mortality rate during the 6
months after the infarction (5).
Therefore, the effective treatment of depression in patients with
cardiovascular disease is vital and requires the simultaneous use
of multiple medications.
Clinical trials have indicated that there is an
association between cardiovascular improvement and the degree by
which low-density lipoprotein cholesterol (LDL-C) levels are
reduced (6). The reduction of high
blood cholesterol levels is a crucial purpose of pharmacotherapy.
The most effective lipid-lowering drugs are statins, also known as
3-hydroxy-3-methylglutaryl-CoA reductase inhibitors. A drug of the
statin class, atorvastatin, reduces the risk of cardiovascular
events by reducing the total cholesterol, LDL-C and B
apolipoprotein levels in serum (7).
Atorvastatin is also effective in treating and preventing
atherosclerosis, and affects all stages of atherosclerotic plaque
formation (8).
Selective serotonin reuptake inhibitors (SSRIs) are
the most frequently prescribed class of antidepressants. SSRIs are
generally recommended for use in patients with cardiac disease and
may also have a protective effect against myocardial infarction.
Several studies have documented the cardiovascular effects of
fluoxetine treatment (9–11). The SSRI fluoxetine appears to be safe
for use in the treatment of depressed patients with cardiac disease
and does not cause orthostatic hypotension or slow conduction
(10). Another SSRI, paroxetine, is
one of the most well-known and effective SSRIs (12,13).
According to Nelson et al, depressed patients with ischemic
heart disease can be treated with paroxetine effectively and safely
as paroxetine has no consistent significant effects on the pulse or
blood pressure, and the authors suggest that paroxetine is as
effective as nortriptyline but less likely to produce serious side
effects (5).
Atorvastatin undergoes extensive microsomal
metabolism by the cytochrome P450, CYP3A4 isoenzyme (14). Co-administration of drugs that
interact with the CYP450 system may significantly affect the plasma
concentrations and potential toxicity of atorvastatin. Fluoxetine
and its circulating metabolite norfluoxetine constitute a complex
multiple inhibitor system, causing reversible or time-dependent
inhibition of CYP3A4, and also CYP2D6 and CYP2C19 in vitro
(15). Paroxetine is extensively
metabolized and is a mechanism-based inhibitor of CYP2D6 and CYP3A.
This inhibition most likely involves the irreversible binding of a
paroxetine-reactive metabolite to the heme complex in the P450
enzyme (16). Long-term
polypharmacotherapy can lead to increased side effects of drugs and
may cause an oxidation-reduction imbalance followed by the
generation of reactive oxygen species (ROS) (17–19). ROS
are produced intracellularly through multiple mechanisms, and the
sources of ROS in cells include CYP450 CYP and NADPH oxidase, with
NADPH oxidase complexes being the major producers of ROS in cells.
Biochemical and pharmacokinetic studies have revealed that during
the biotransformation of drugs, reactive metabolites capable of
directly reducing molecular oxygen to generate ROS are often
produced. Cytochrome P450 is a superfamily of heme-thiolate
proteins that are, in general, the terminal oxidase enzymes in an
electron transfer chain that delivers an electron to an oxygen
molecule (17,18,20).
During phase I metabolism, atorvastatin is extensively metabolized
by the monooxygenase CYP3A4, with the insertion of an oxygen atom
into the atorvastatin molecule to form o- and
p-hydroxyatorvastatin, while the other oxygen atom of
molecular oxygen is reduced to water with the concomitant oxidation
of NADPH; however, premature and incomplete reduction may occur, to
generate the superoxide radical (O2·-)
(20,21). SSRIs cause reversible or
time-dependent inhibition of CYP3A4 and also CYP2D6 which could
affect the metabolism of atorvastatin (22).
Drug metabolism generates ROS and reactive
metabolites as by-products; however, cells have a defense mechanism
for the removal of free radicals to attenuate intracellular damage
and ameliorate the harmful effects of ROS (18,23).
Drug interactions may alter cell metabolism causing the cellular
antioxidant capacity to be exceeded, possibly resulting in
increased oxidative stress. Drug-induced oxidative stress has been
implicated as a mechanism of toxicity in numerous tissues (18). Therefore, any potential drug
interactions and adverse effects require close monitoring.
To the best of our knowledge, there are no published
data concerning the effects of concomitant treatment with
atorvastatin and SSRIs on redox imbalance. The present study aimed
to assess antioxidant defense parameters in the blood of rats after
28 days treatment with atorvastatin in combination with fluoxetine
or paroxetine. The activity of glutathione peroxidase (GPX) was
determined in whole blood and the glutathione reductase (GR)
activity and total antioxidant status (TAS) were determined in
serum.
Materials and methods
Drugs and chemicals
The following substances were used in the study:
Atorvastatin (atorvastatin calcium salt trihydrate, Atorvastatin
Ranbaxy; Ranbaxy, Warsaw, Poland), fluoxetine (fluoxetine
hydrochloride, Fluoksetyna Egis; Egis Pharmaceuticals PLC,
Keresztúri, Hungary), paroxetine (paroxetine hydrochloride
semihydrate, Seroxat; GlaxoSmithKline, Brentford, UK) and aqua
pro injectione (Baxter, Warsaw, Poland). Ready-made diagnostic
kits (Randox Laboratories Ltd., Crumlin, UK) were used to determine
the GPX (RS505) and GR (GR2368) activities and the TAS
(NX2332).
Animals
The present study was carried out on 48 male Wistar
rats (age, 6-weeks-old; initial weight, 200–250 g) that were
obtained from a licensed breeder (Breeding of Laboratory Animals,
Zbigniew Lipiec, Brwinów, Poland). The animals were kept at room
temperature (20±2°C) under a 12 h day/12 h night cycle under
constant environmental conditions (55±10% humidity and noise). They
had access to food and water ad libitum. The study was
approved by the Ethics Committee on Animal Experimentation of the
Medical University of Lublin (Lublin, Poland).
Experimental procedures
Aqueous solutions of atorvastatin (10 mg/kg),
fluoxetine (10 mg/kg) and paroxetine (10 mg/kg) were prepared ex
tempore and injected intraperitoneally once a day for 28 days,
individually or combined, in a constant volume of 0.5 ml/100 g of
body weight. The doses were selected based on those reported in the
literature and in our previous experiments, which demonstrated that
the aforementioned doses of statin and antidepressant drugs are
effective (24–26). The rats in the control group received
the same amounts of aqua pro injectione at the same time
points. Six groups of rats, each consisting of 8 animals, were
treated in the following order: i) Control group, treated with
aqua pro injection; ii) atorvastatin group; iii) fluoxetine
group; iv) paroxetine group; v) atorvastatin plus fluoxetine group
(treated with atorvastatin followed by fluoxetine 5 min later); and
vi) atorvastatin plus paroxetine group (treated with atorvastatin
followed by paroxetine 5 min later). The studied drugs were
administered to rats in the aforementioned effective doses. At 24 h
after the last injection, the animals were decapitated and blood
samples were taken and divided as follows: One portion was stored
in a heparin tube (whole blood) and the other was left to clot.
Animals decapitation was performed in accordance with European
standards related to the experimental studies on animal models.
According to the guidelines, handling of animals and injection of
sedatives or anaesthetics prior to decapitation may increase stress
prior to euthanasia and is therefore not considered good for the
welfare of the animal. In the present study, the animals were not
anesthetized prior to decapitation.
The whole heparinized blood (for quantitative in
vitro determination of GPX in whole blood) was tested to
estimate the GPX activity by kinetic methods, using the
aforementioned kit. The method of determination was based on a
method previously described by Paglia and Valentine (23). In this method, GPX catalyzes the
oxidation of glutathione by cumene hydroperoxide, and in the
presence of GR and NADPH the oxidized glutathione (GSSG) is
immediately converted to the reduced form with a concomitant
oxidation of NADPH to NADP+. The reduction in absorbance
at 340 nm was measured.
In the portion of the blood that was allowed to
clot, the serum fraction was removed and the GR activity and TAS in
the serum fraction were determined by kinetic methods using the
aforementioned kits. The GR activity assay is based on the
reduction of GSSG catalyzed by GR in the presence of NADPH, which
is oxidized to NADP+. The reduction in absorbance at 340
nm was measured. The TAS determination method consists of
incubating 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate (ABTS)
with a peroxidase (metmyoglobin) and H2O2 to
produce ABTS+. This radical cation has a relatively
stable blue-green color, which is measured at 600 nm.
All procedures were performed according to the
instructions supplied with the respective kits.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Groups in which single drugs were administered were
compared with the control group and one-way analysis of variance
(ANOVA) was used (followed by Dunnett's test). Double-drug groups
were compared with the control and single-drug groups and two-way
ANOVA with Tukey's post hoc test was used to determine
statistical significance. P<0.05 was considered to indicate a
statistically significant difference.
Results
GPX activity
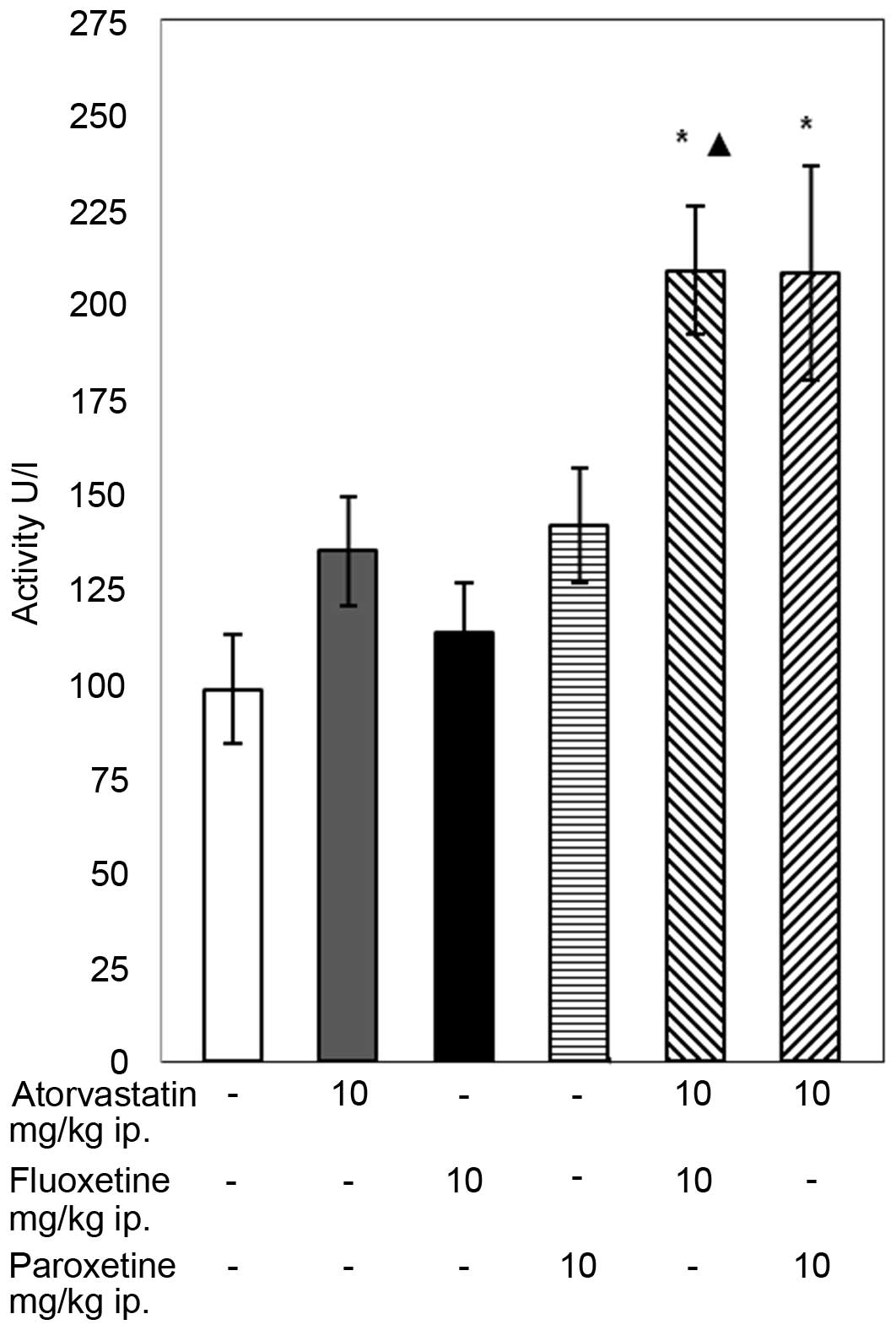
The conducted experiments demonstrated that the
28-day concomitant administration of atorvastatin (10 mg/kg) with
fluoxetine (10 mg/kg) caused an increase in the GPX activity in the
blood of the rats compared with that in the control group and with
the group of animals receiving only fluoxetine (P<0.05; Fig. 1). The combined administration of
atorvastatin with paroxetine caused a significant increase in the
activity of GPX compared with the control group. In animal groups
receiving atorvastatin or an SSRI separately, the activity of GPX
remained unaffected compared with the control.
GR activity
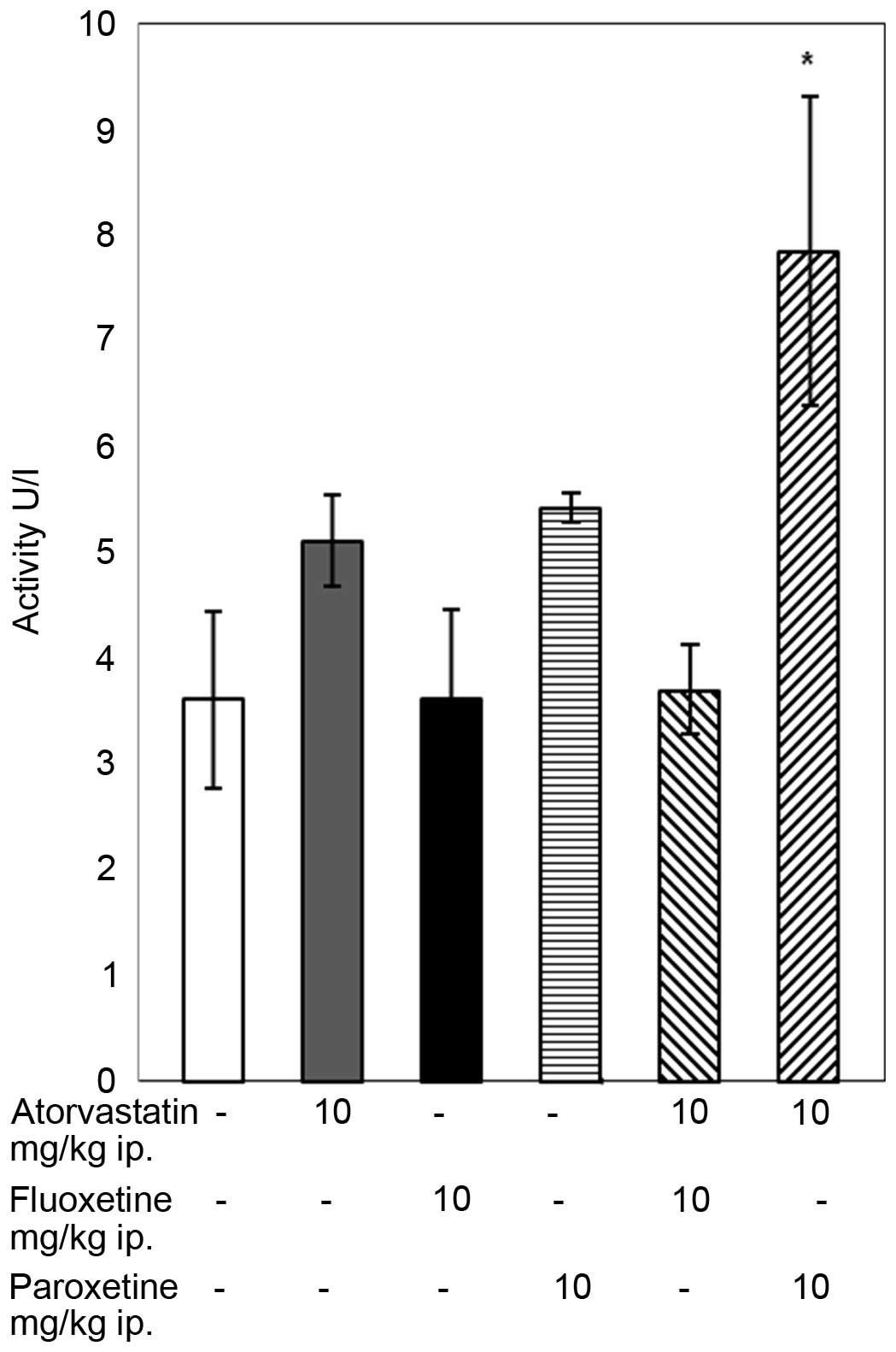
Atorvastatin (10 mg/kg) administered to rats with
paroxetine (10 mg/kg) increased the activity of GR compared with
that of the control group (P<0.05; Fig. 2). In the groups of rats receiving
atorvastatin or an SSRI separately and in the group treated with
atorvastatin and fluoxetine simultaneously the GR relevant activity
did not change from that in the control group.
TAS of blood
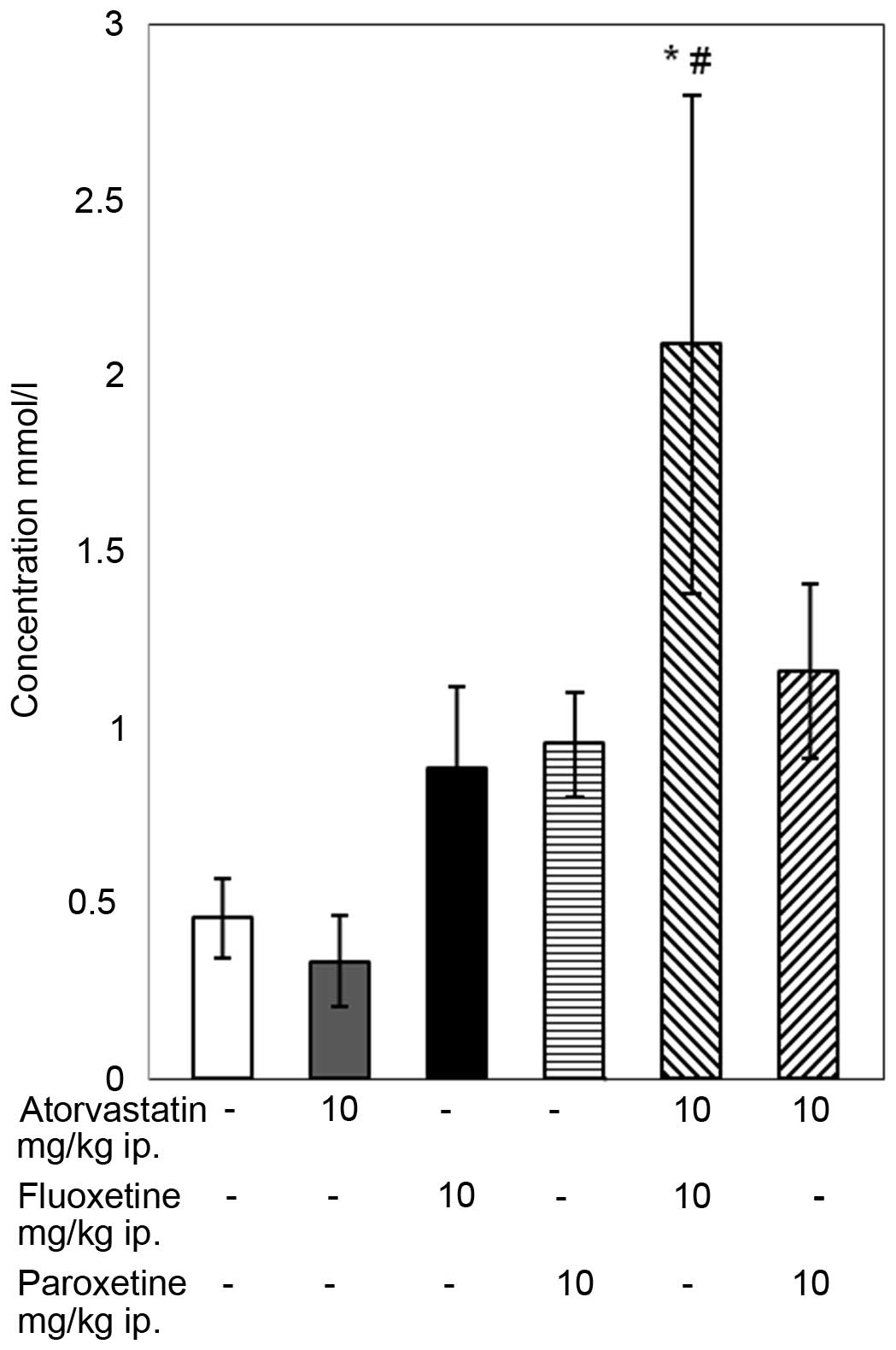
The results of the TAS assay indicate that the
4-week combined treatment with atorvastatin and fluoxetine caused
an increase in the TAS compared with that of the control group and
the group receiving atorvastatin alone (P<0.05; Fig. 3), although the standard error of the
mean was high. In the remaining groups, no statistically
significant changes in TAS level were observed.
Discussion
The formation of chemically reactive metabolites of
drugs is associated with numerous adverse drug reactions (18,19).
Reactive metabolites may react with cellular proteins, lipids and
nucleic acids and thus cause oxidative stress. Atorvastatin is
extensively metabolized in the gut and liver by oxidation,
lactonization and glucuronidation. Metabolism in the liver produces
two active hydroxy metabolites, o-hydroxyatorvastatin and
p-hydroxyatorvastatin (21).
During the bioactivation of atorvastatin, the reduction of
molecular oxygen by reactive metabolites may generate ROS (17,8,20). The
risk of ROS being generated increases during simultaneous treatment
with several drugs metabolized by cytochrome P450 and this can lead
to redox imbalance (17). Oxidative
stress causes cell damage through a number of mechanisms including
lipid peroxidation, protein oxidation, DNA oxidation and
mitochondrial damage. This impairment of cellular function can
result in cell death and possible organ failure (27,28).
However, cells contain antioxidant enzymes that function to
maintain the redox status.
In the present study, the combined administration of
atorvastatin with fluoxetine caused an increase of GPX activity and
did not influence GR activity. GPX is one of the body's most potent
antioxidant defense agents. Its purpose is to balance pro-oxidative
and antioxidant actions and to prevent the excessive accumulation
of ROS in the body. A change in the activity of this enzyme may
reflect the impairment of antioxidant activity in the blood and can
be useful in monitoring the effects of treatment (23). In the present study, the increase of
GPX activity indicated that endogenous antioxidant levels were
increased. This might indicate that the production of free radicals
was increased following the concomitant application of atorvastatin
with fluoxetine. Changes in GPX activity have been observed in
animals exposed to pro-oxidative drug action (18). In our previous study, increases in
the activity of both GPX and GR were observed after the combined
14-day administration of rosuvastatin with fluoxetine to rats
(24). In the present study, the
simultaneous application of atorvastatin with fluoxetine caused an
increase in the TAS, although with a high error of the mean. In the
body, the total antioxidant system consists of multiple components
providing protection against the molecular damage of cell
structures. The TAS is defined as the ability of the serum to
quench free radical production, and is determined in order to
indicate the efficiency of cellular antioxidant mechanisms. A
reduction in the TAS suggests an increase in the number of oxygen
free radicals and a reduction of the activity of the antioxidant
defense system. The TAS also indicates the antioxidant potential of
drugs and can indicate whether a new treatment is detrimental to
the body's anti-oxidation system. In the present study, an increase
of the TAS following the co-administration of atorvastatin with
fluoxetine is probably associated with metabolic mobilization and
may indicate that the amounts of endogenous antioxidants were
increased. Our previous study carried out on rats treated
simultaneously with rosuvastatin and fluoxetine for 14 days
revealed a reduction in the TAS (24). This difference may result from the
shared metabolism of rosuvastatin and fluoxetine; these drugs are
biotransformed by cytochrome P450 isoenzyme CYP2C9, while
atorvastatin is metabolized primarily by isoenzyme CYP3A4 (15,21,28). It
is also important to note that different durations of treatment
were applied (14 days in the previous study, and 28 days in the
current study). The present study was a preliminary study, and did
not include the monitoring of transcription factors, such as Nrf2,
that are responsible for activating the antioxidant capacity of
cells.
Combined administration of atorvastatin with
paroxetine caused an increase in the activity of both GPX and GR
(only in comparison with the control group) and did not affect the
TAS. GR is closely associated with GPX and plays an important role
in protecting cells against oxidative damage by increasing the
level of reduced glutathione in the process of aerobic glycolysis
(29). The increased activity of GR
may be influenced by the reduced form of glutathione, and
potentially indicates that there is an increased activity of the
enzyme system that prevents oxidation. Similar results were
obtained in our previous study, in which rats were treated with
rosuvastatin and paroxetine simultaneously for 14 days (25).
The present study revealed no significant changes in
the activity of GPX and GR enzymes or in the TAS in rats treated
with only atorvastatin. However, a number of studies support the
role of atorvastatin in the maintenance of the antioxidant status
(30–32). The possible antioxidant mechanisms of
atorvastatin include reducing the generation of ROS by the
inhibition of vascular NAD(P)H oxidase, modifying redox homeostasis
in LDL particles, modulating RNA expression, increasing the
synthesis of nitric oxide in the vasculature and binding to the
surface phospholipids of lipoproteins (30,33). In
the current study, no statistically significant changes concerning
the oxidative stress parameters in the groups of rats receiving
antidepressants individually were noted. Previous studies suggest
that SSRIs have a beneficial effect in maintaining the
oxidative-reductive balance (34,35). The
potentially favorable antioxidant effect of fluoxetine may be
mediated by three mechanisms: Inhibition of lipid peroxidation,
increase of glutaminergic transmission and reduction of immune and
inflammatory components that favor the generation of ROS (36). Similar results, which did not reveal
any crucial changes in oxidative stress parameters in rats
receiving fluoxetine for 14 days, were obtained in a previous study
(24). Other research indicates that
the antidepressive effect of paroxetine is at least partially
associated with the attenuation of oxidative stress imbalance
(37).
An increase in GPX activity in the rats receiving
atorvastatin with fluoxetine may be associated with the defense
mechanism against the increased ROS load. An increase in the TAS is
probably connected with the metabolic mobilization of the rats
treated with atorvastatin plus fluoxetine, and may indicate an
increasing amount of endogenous antioxidants. Concurrent increases
in the activity of GPX and GR are likely to be due to the
augmentation of free oxygen radical production during the
simultaneous administration of atorvastatin with paroxetine. The
present study was a preliminary study, aiming to assess antioxidant
defense parameters in the blood of rats by assaying the activity of
antioxidant enzymes and TAS level. In the future, this research may
be expanded, for example, to determine the level of
glutathione.
References
|
1
|
Frasure-Smith N, Lespérance F, Irwin MR,
Talajic M and Pollock BG: The relationships among heart rate
variability, inflammatory markers and depression in coronary heart
disease patients. Brain Behav Immun. 23:1140–1147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thombs BD, Bass EB, Ford DE, Stewart KJ,
Tsilidis KK, Patel U, Fauerbach JA, Bush DE and Ziegelstein RC:
Prevalence of depression in survivors of acute myocardial
infarction. J Gen Intern Med. 21:30–38. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mathews MJ, Mathews EH and Liebenberg L:
The mechanisms by which antidepressants may reduce coronary heart
disease risk. BMC Cardiovasc Disord. 15:822015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nemeroff CB and Goldschmidt-Clermont PJ:
Heartache and heartbreak-the link between depression and
cardiovascular disease. Nat Rev Cardiol. 9:526–539. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nelson JC, Kennedy JS, Pollock BG,
Laghrissi-Thode F, Narayan M, Nobler MS, Robin DW, Gergel I,
McCafferty J and Roose S: Treatment of major depression with
nortriptyline and paroxetine in patients with ischemic heart
disease. Am J Psychiatry. 156:1024–1028. 1999.PubMed/NCBI
|
|
6
|
Sirtori CR and Fumagalli R:
LDL-cholesterol lowering or HDL-cholesterol raising for
cardiovascular prevention. A lesson from cholesterol turnover
studies and others. Atherosclerosis. 186:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kobayashi J, Maruyama T, Masuda M and
Shinomiya M: Effect of atorvastatin treatment on lipoprotein lipase
mass in the pre-heparin plasma in Japanese hyperlipidemic subjects.
Clin Chim Acta. 314:261–264. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eshtehardi P, McDaniel MC, Dhawan SS,
Binongo JN, Krishnan SK, Golub L, Corban MT, Raggi P, Quyyumi AA
and Samady H: Effect of intensive atorvastatin therapy on coronary
atherosclerosis progression, composition, arterial remodeling, and
microvascular function. J Invasive Cardiol. 24:522–529.
2012.PubMed/NCBI
|
|
9
|
Baumeister H, Hutter N and Bengel J:
Psychological and pharmacological interventions for depression in
patients with coronary artery disease. Cochrane Database Syst Rev.
CD0080122011.PubMed/NCBI
|
|
10
|
Roose SP: Treatment of depression in
patients with heart disease. Biol Psychiatry. 54:262–268. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roose SP and Miyazaki M: Pharmacological
treatment of depression in patients with heart disease. Psychosom
Med. 67(Suppl 1): S54–S57. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanchez C, Reines EH and Montgomery SA: A
comparative review of escitalopram, paroxetine, and sertaline: Are
they all alike? Int Clin Psychopharmacol. 29:185–196. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vaswani M, Linda FK and Ramesh S: Role of
selective serotonin reuptake inhibitors in psychiatric disorders: A
comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry.
27:85–102. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bolego C, Baetta R, Bellosta S, Corsini A
and Paoletti R: Safety considerations for statins. Curr Opin
Lipidol. 13:637–644. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andrade C: Selective serotonin reuptake
inhibitor drug interactions in patients receiving statins. J Clin
Psychiatry. 75:e95–e99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jornil J, Jensen KG, Larsen F and Linnet
K: Identification of cytochrome P450 isoforms involved in the
metabolism of paroxetine and estimation of their importance for
human paroxetine metabolism using a population-based simulator.
Drug Metab Dispos. 38:376–385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bhattacharyya S, Sinha K and Sil PC:
Cytochrome P450s: Mechanisms and biological implications in drug
metabolism and its interaction with oxidative stress. Curr Drug
Metab. 15:719–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deavall DG, Martin EA, Horner JM and
Roberts R: Drug-induced oxidative stress and toxicity. J Toxicol.
2012:6454602012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cunha-Oliveira T, Rego AC and Oliveira CR:
Oxidative stress and drugs of abuse: An update. Mini-Rev Org Chem.
10:000. 2013. View Article : Google Scholar
|
|
20
|
Guengerich FP: Cytochrome P450s and other
enzymes in drug metabolism and toxicity. AAPS J. 8:E101–E111. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lins RL, Matthys KE, Verpooten GA, Peeters
PC, Dratwa M, Stolear JC and Lameire NH: Pharmacokinetics of
atorvastatin and its metabolites after single and multiple dosing
in hypercholesterolaemic haemodialysis patients. Nephrol Dial
Transplant. 18:967–976. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li X, Fang P, Mai J, Choi ET, Wang H and
Yang XF: Targeting mitochondrial reactive oxygen species as novel
therapy for inflammatory diseases and cancers. J Hematol Oncol.
6:192013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paglia DE and Valentine WN: Studies on the
quantitative and qualitative characterization of erythrocyte
glutathione peroxidase. J Lab Clin Med. 70:158–169. 1967.PubMed/NCBI
|
|
24
|
Herbet M, Gawrońska-Grzywacz M and
Jagiełło-Wójtowicz E: Evaluation of selected biochemical parameters
of oxidative stress in rats pretreated with rosuvastatin and
fluoxetine. Acta Pol Pharm. 72:261–265. 2015.PubMed/NCBI
|
|
25
|
Herbet M, Izdebska M, Piątkowska-Chmiel I,
Poleszak E and Jagiełło-Wójtowicz E: Estimation of oxidative stress
parameters in rats after simultaneous administration of
rosuvastatin with antidepressants. Pharmacol Rep. 68:172–176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Almeida J, Duarte JO, Oliveira LA and
Crestani CC: Effects of nitric oxide synthesis inhibitor or
fluoxetine treatment on depression-like state and cardiovascular
changes induced by chronic variable stress in rats. Stress.
18:462–474. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park BK, Laverty H, Srivastava A, Antoine
DJ, Naisbitt D and Williams DP: Drug bioactivation and protein
adduct formation in the pathogenesis of drug-induced toxicity. Chem
Biol Interact. 192:30–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Webb HM, Regan S, Antoine DJ, Lane N,
Walsh RJ, Srivastava A, Starkey-Lewis P, Benson C, Williams DP,
Laverty H, et al: Drug Bioactivation and Oxidative
StressEncyclopedia of Drug Metabolism and Interactions. Lyubimov
AV: John Wiley & Sons; New York: pp. 1–31. 2012
|
|
29
|
Chang JC, van der Hoeven LH and Haddox CH:
Glutathione reductase in the red blood cells. Ann Clin Lab Sci.
8:23–29. 1978.PubMed/NCBI
|
|
30
|
Koksal M, Eren MA, Turan MN and Sabuncu T:
The effects of atorvastatin and rosuvastatin on oxidative stress in
diabetic patients. Eur J Intern Med. 22:249–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bloom HL, Shukrullah I, Veledar E, Gutmann
R, London B and Dudley SC: Statins decrease oxidative stress and
ICD therapies. Cardiol Res Pract. 2010:2538032010.PubMed/NCBI
|
|
32
|
Nagila A, Permpongpaiboon T, Tantrarongroj
S, Porapakkham P, Chinwattana K, Deakin S and Porntadavity S:
Effect of atorvastatin on paraoxonase1 (PON1) and oxidative status.
Pharmacol Rep. 61:892–898. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cangemi R, Loffredo L, Carnevale R, Perri
L, Patrizi MP, Sanguigni V, Pignatelli P and Violi F: Early
decrease of oxidative stress by atorvastatin in
hypercholesterolaemic patients: Effect on circulating vitamin E.
Eur Heart J. 29:54–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abdel-Salam OME, Morsy SM Youssef and
Sleem AA: The effect of different antidepressant drugs on oxidative
stress after lipopolysaccharide administration in mice. EXCLI J.
10:290–302. 2011.
|
|
35
|
Zafir A and Banu N: Antioxidant potential
of fluoxetine in comparison to Curcuma longa in restraint-stressed
rats. Eur J Pharmacol. 572:23–31. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Novío S, Núñez MJ, Amigo G and
Freire-Garabal M: Effects of fluoxetine on the oxidative status of
peripheral blood leucocytes of restraint-stressed mice. Basic Clin
Pharmacol Toxicol. 109:365–371. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qiu HM, Yang JX, Wu XH, Li N, Liu D, Wang
LJ, Qin LJ and Zhou QX: Antidepressive effect of paroxetine in a
rat model: Upregulating expression of serotonin and norepinephrine
transporter. Neuroreport. 24:520–525. 2013. View Article : Google Scholar : PubMed/NCBI
|