Introduction
Portal hypertension (PH) is the primary factor
resulting in serious complications and mortality in patients with
liver cirrhosis (1–3). Early and consistent intervention of PH
has been recommended in the clinic to reduce morbidity and
mortality of patients with cirrhosis; however, currently, there are
no ideal oral medicines for limiting PH due to side effects and
poor efficacy (4).
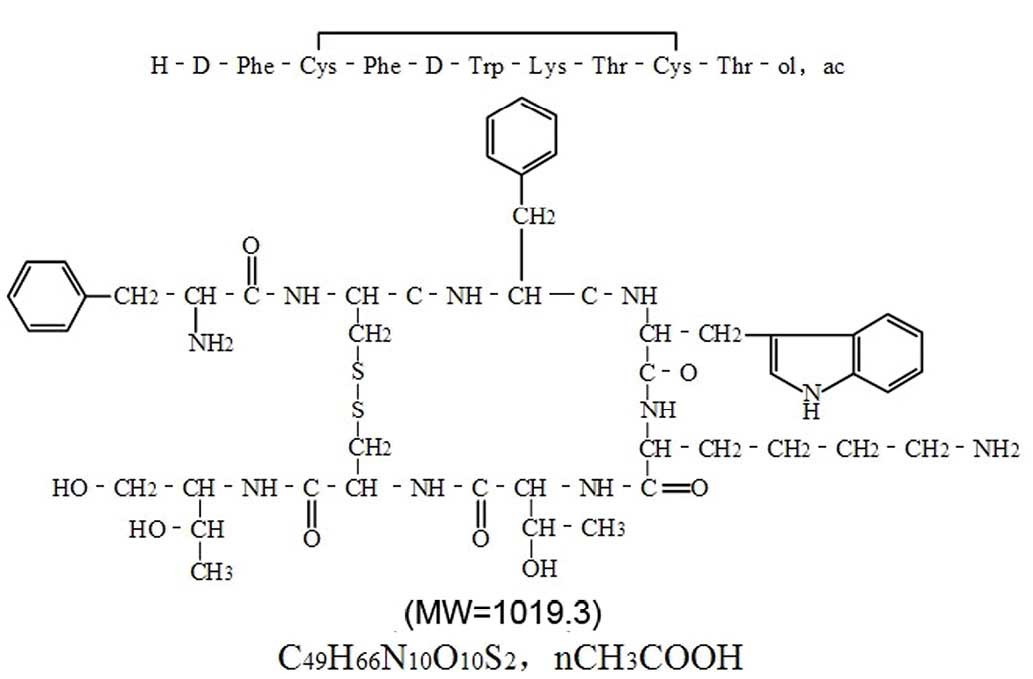
Octreotide (OCT; Fig.
1), an octapeptide that pharmacologically mimics natural
somatostatin, can alleviate PH through intravenous (i.v.) or
intramuscular infusion, with limited side effects (1). As OCT is stable against enzymatic
degradation, it may partially overcome the problems associated with
therapeutically active peptides, which are often limited by their
short biological half-lives (5). OCT
may be a potential oral medicine for long-time use that can
persistently decrease portal vein pressure (PVP). A research study
by Tuvia et al suggested that orally administered OCT may be
an alternative to parenteral OCT treatment for patients with
acromegaly (6). However, the
extensive intestinal and hepatic first-pass elimination of OCT
limits its clinical application via oral administration (2,7).
Previous studies have attempted to improve the
intestinal absorption of OCT by changing its chemical structure or
adding an absorption enhancer to increase paracellular absorption,
but with little effect (2,8,9). The
elucidation of the factors that affect the oral absorption of OCT
would facilitate the development of strategies to improve its
absorption. In addition, it is unclear whether orally administrated
OCT can decrease PVP effectively. In a previous study, we have
indicated that the effect of hepatic first-pass is minor on the PH
state (unpublished data). Furthermore, the intestinal transporters
of drugs, the ATP-driven drug efflux pumps, P-glycoprotein (P-gp;
MDR1-gene product, mdr1a and mdr1b subtypes) (10,11),
multidrug resistance-associated protein 2 (MRP2; mrp2-gene product)
and cytochrome P450 3A4 (CYP3A4; cyp3A1-gene product in rats) are
among the most important CYP enzymes in the small intestine,
functioning as a barrier against enteral absorption of OCT under
the conditions of PH. P-gp, MRP2 and CYP3A4 are crucial factors in
the intestinal first-pass effects of OCT, thus affecting its oral
absorption (data to be published elsewhere). However, the
inhibition of the enteral absorption of OCT by P-gp, MRP2 and
CYP3A4 has not been evaluated, particularly with respect to the
stage of cirrhosis with PH. The aim of the current study was to
determine whether inhibitors of intestinal first-pass elimination
can be used to effectively increase OCT absorption, thus decreasing
PVP.
Materials and methods
Animal care
Male Sprague Dawley rats (n=52; age, 4–6 months;
weight, 200–230 g) were obtained from the Experimental Animal
Centre at Dalian Medical University (Liaoning, China). This study
was performed in strict accordance with the recommendations of the
Guide for the Care and Use of Laboratory Animals of the Canadian
Council on Animal Care. The protocol was approved by the Committee
on the Ethics of Animal Experiments of Dalian Medical University
(permit no. L2008102001) in accordance with the Code of Ethics of
the World Medical Association. Ether anaesthesia was persistently
used for all surgeries, and all efforts were made to mitigate
animal suffering. Under ether anaesthesia, the animals were
euthanised via cervical dislocation.
Animal model
The rats were fed in a specific pathogen-free centre
at 24–26°C with a relative humidity of 60–65%. The rats were
allowed free access to water and fed a chow diet for 3 days prior
to any experimental protocols. Prior to the pharmacokinetic
experiments, the animals were fasted for 12 h with water available
ad libitum. Biliary cirrhosis with PH was induced via bile
duct ligation (BDL) as previously described (12). The surgical procedures were approved
by the Animal Care and Use Committee of Dalian Medical University.
The laparotomy was performed under anaesthesia with ether. The bile
duct was isolated and double-ligated using a 3-0 silk suture. The
abdominal wall and skin were closed with a 4-0 silk suture, and the
antibiotic gentamicin (0.3 ml; Sigma-Aldrich) was injected
intramuscularly. The rats were continuously fed and housed for a
four-week period after surgery. The administration methods and
measurements for each group will be further specified when
mentioned. The jejunum was selected in the present study as it is
the main site of OCT absorption in the intestine (9). For the experiment of intrajejunal
(i.j.) injection, a mid-line laparotomy was made and the caecum was
removed to create a more open operating field. A 15-cm loop of
mid-jejunum was located ~30 cm distally from the pylorus. Then the
portex tubing was cannulated through stab incisions in the left
side of the abdominal wall.
In vivo absorption evaluation
Normal rats were randomly assigned to two groups: i)
I.j. injection of 2 ml OCT (0.1 mg/kg; purity, 99%; Chengdu
Xinlinbang Bio-pharmaceutical Co., Ltd., Chengdu, China); and ii)
i.v. injection (bolus method) of 0.2 ml OCT (0.01 mg/kg) through
the internal jugular vein (n=4 per group). The PH rats were
randomly assigned to three groups (n=4 per group): i) I.j.
injection of 2 ml OCT (0.1 mg/kg); ii) i.v. infusion of 0.2 ml OCT
(0.01 mg/kg); and iii) i.j. injection of 4 ml OCT (0.1 mg/kg) and
P-gp/MRP2/CYP3A4 mixed inhibitors (4.9 mg/kg verapamil
hydrochloride, 300 mg/kg probenecid and 5.3 mg/kg ketoconazole;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The beginning of
the jejunum was localised 5 cm distal to the Treitz ligament. A
0.3-ml blood sample was collected from the external jugular vein at
1, 5, 10, 15, 30, 45, 60, 120, 240, 360, 480 and 720 min for OCT
determination via liquid chromatography-tandem mass spectrometry
(LC-MS/MS). The main pharmacokinetic parameters were calculated
according to the method described by Thanou et al (13), using the Bioavailability Program
Package. Absolute bioavailability values (F) and absorption
enhancement ratios (ER) were obtained according to the
following formulae:
F(%)=AUCi.j.xDi.v./AUCi.v.xDi.j.x100.ER=F(OCT+inhibitors)/F(OCTalone).
in which D is the administered dose and AUC (area
under the curve) is the individual concentration-time curve.
Effects of OCT and P-gp/MRP2/CYP3A4
mixed inhibitors on the expression of P-gp/MRP2/CYP3A4 in the
intestinal mucosa of rats with or without PH
Rats were randomly assigned to the following groups
(n=4 per group): i) Normal rats (N group); ii) normal rats with
oral administration of OCT (N + OCT group); iii) PH rats (PH
Group); iv) PH rats with oral administration of OCT (PH + OCT
Group); and v) PH rats with oral administration of OCT and
P-gp/MRP2/CYP3A4 mixed inhibitors (verapamil hydrochloride,
probenecid, and ketoconazole) (PH + OCT + I group). The
administered drug doses were identical to those used in the in
vivo absorption experiment and were administered for 3 days;
untreated rats were fed ad libitum for 3 days. The tissue
samples collected from the upper jejunum of each group were used
for reverse transcription-polymerase chain reaction (RT-PCR),
western blot and immunohistochemistry analyses.
RT-PCR
RT-PCR was performed as previously described
(14). The jejunum tissue samples
collected from the upper jejunum of each group (normal rats as a
control group) were stored in an RNA stabiliser (Dalian Pauley
Shield Bio-Engineering Co., Ltd., Dalian, China) and were rapidly
frozen to prevent possible RNA degradation. Total RNA was extracted
from each perfused sample using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Carlsbad, CA, USA) according to the
manufacturer's recommendation and analysed by ultraviolet
spectrophotometry. RT-PCR was performed using the PrimeScript™
RT-PCR kit (cat. no. RR014A) as recommended by the manufacturer
(Takara Biotechnology Co., Ltd., Dalian, China). After treatment
with DNAse (D7076; Beyotime Institute of Biotechnology, Shanghai,
China), cDNA was subsequently amplified using a TC512 thermal
cycler (Bibby Scientific Ltd., Stone, UK). RNA samples (500 ng)
were reverse transcribed and immediately amplified by PCR. Reverse
transcription was performed for 10 min at 30°C, followed by 30 min
at 42°C, and the samples were subsequently heated for 5 min at 95°C
to terminate the reverse transcription reaction. The primers for
β-actin were 5′-GGGACCTGACAGACTACCTC-3′ (forward) and
5′-GACAGCACTGTGTTGGCATAG-3′ (reverse), which yielded a PCR product
of 351 bp. The primers of mdr1, mdr2, mrp2 and cyp3A1 (Takara
Biotechnology Co., Ltd.) and PCR cycling conditions are summarised
in Table I. Quantity One software
(version 4.40; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to analyse the densities of the bands on the gel. β-actin
served as a housekeeping gene constitutively expressed at a
constant amount, and the level of each mRNA in each group was
normalised against the corresponding β-actin mRNA level. All
samples were amplified in triplicate.
 | Table I.Nucleotide sequences and cycling
conditions of rat reverse transcription-polymerase chain reaction
primers. |
Table I.
Nucleotide sequences and cycling
conditions of rat reverse transcription-polymerase chain reaction
primers.
| Gene | Primer sequence
(5′-3′) | Denaturation | Annealing | Elongation | Product size
(bp) |
|---|
| mdr1a |
|
|
|
|
|
| F |
GATGGAATTGATAATGTGGACA | 94°C (30 sec) | 48°C (30 sec) | 72°C (45 sec) | 352 |
| R |
AAGGATCAGGAACAATAAA |
|
|
|
|
| mdr1b |
|
|
|
|
|
| F |
GAAATAATGCTTATGAATCCCAAAG | 94°C (30 sec) | 54°C (30 sec) | 72°C (45 sec) | 327 |
| R |
GGTTTCATGGTCGTCGTCTCTTGA |
|
|
|
|
| mrp2 |
|
|
|
|
|
| F |
ACCTTCCACGTAGTGATCCT | 94°C (30 sec) | 56°C (30 sec) | 72°C (45 sec) | 449 |
| R |
ACCTGCTAAGATGGACGGTC |
|
|
|
|
| cyp3A1 |
|
|
|
|
|
| F |
GAGGAGTAATTTGCTGACAGAACCTGC | 95°C (15 sec) | 57°C (30 sec) | 72°C (30 sec) | 149 |
| R |
CCAGGAATCCCCTGTTTCTTGAA |
|
|
|
|
Western blot analysis
Tissue samples of the upper jejunums were frozen and
stored in rapid immunoprecipitation assay buffer (Beyotime
Institute of Biotechnology). Protein concentration was determined
according to the Lowry method using bovine serum albumin as a
standard. The western blot assay was performed as previously
described (12). Total protein (20
µg/ml) was separated by 10% sodium dodecyl sulphate-polyacrylamide
gel electrophoresis and transferred to a polyvinylidene difluoride
membrane (EMD Millipore, Bedford, MA, USA). The membrane was
blocked with 5% skimmed milk powder with Tris-buffered saline-Tween
20 (TBST) for 2 h at 37°C and then incubated with primary
antibodies for for 2 h at 37°C. The primary antibodies were mouse
P-gp monoclonal antibody (1:75; MA1-26528; Pierce Biotechnology;
Thermo Fisher Scientific, Inc., Waltham, MA, USA),
rabbit-anti-mouse MRP2 monoclonal antibody (1:100; ab203397),
rabbit-anti-mouse CYP3A4 polyclonal antibody (1:1,000; ab3572;
Abcam, Cambridge, MA, USA) and monoclonal antibody for β-actin
(1:1,000; TA-09; Beijing Zhongshan Golden Bridge Biological
Technology Co., Ltd., Beijing, China). After incubation, the
membrane was washed three times with TBST and then incubated with
horseradish peroxidase (HRP)-linked secondary antibody, anti-rabbit
(1:800; A0208) or anti-mouse (1:1,000; A0208) IgG (Beyotime
Institute of Biotechnology) at room temperature for 2 h. The
membrane was immersed for 1 min in enhanced chemiluminescence
detection reagent (Amersham; GE Healthcare Life Sciences, Chalfont,
UK). Protein bands were visualised and photographed under
transmitted ultraviolet light for semiquantitative measurement
based on band densitometry. Quantity One software was used to
analyse the densities of the bands.
Immunohistochemistry
Tissue samples of the upper jejunums were prepared
for immunohistochemical analysis as previously described (12). The primary antibodies for P-gp, MRP2
and CYP3A4 were the same as those used in the western blot
analysis, and were used at dilutions of 1:58, 1:100 and 1:70,
respectively, with incubation at room temperature for 1–2 h. The
primary antibody was replaced with phosphate-buffered saline
(Beyotime Institute of Biotechnology) to serve as a negative
control. Goat anti-mouse HRP (IgG, H&L) (1:1,000; ab6789;
Abcam, Cambridge, MA, USA) was used as the secondary antibody, with
incubation at room temperature for 15 min. DAB was used as the
chromogen. Yellow staining in the membranes and cytoplasm of cells
indicated P-gp and MRP2 positivity, while yellow material in the
membrane only indicated CYP3A4-positivity. A total of five
high-power microscopic fields were randomly chosen per slide. Cell
staining was assigned via four potential scores, and cell staining
intensity was scored based on its colour. The final score was
defined as the staining intensity × percentage of positive cells
(15). The mean score of five fields
was used to compare the five groups.
Effects of P-gp/MRP2/CYP3A4 mixed
inhibitors on PVP
The PH rats were randomly assigned to three groups
(n=4 per group): i) Without OCT (PH group); ii) with oral
administration of OCT (PH + OCT group); and iii) with oral
administration of OCT and P-gp/MRP2/CYP3A4 mixed inhibitors (PH +
OCT + I group). The oral doses were the same as those used in the
previously described in vivo absorption experiment and were
administered for 3 days. The rats were anaesthetised, and a
catheter connected to a pressure transducer (BL-420F; Chengdu
Technology and Market Co., Ltd., Chengdu, China) was placed in the
portal vein to measure the PVP.
Determination of OCT by LC-MS/MS
An Agilent LC system (HP1200; Agilent Technology,
Inc., Palo Alto, CA, USA) was used to analyse the samples.
Isocratic chromatographic separation was performed using a Hypersil
BDS-C18 column (150 × 4.6; i.d., 5 µm; Dalian Elite Analytical
Instruments Co. Ltd., Dalian, China), which was maintained at room
temperature. A mixture of methanol and water (60:40, v/v) with 0.1%
formic acid was used as the mobile phase at a flow rate of 0.5
ml/min. An API 3200 triple-quadrupole mass spectrometer (Applied
Biosystems; Thermo Fisher Scientific, Inc., Foster City, CA, USA)
was operated using a turbo ion spray interface in positive ion
mode. The instrument was operated using an ion spray voltage at +4
psi, heater gas temperature of 500°C, nebuliser gas (Gas 1) at 40
psi, heater gas (Gas 2) at 40 psi, curtain gas at 10 psi and
collision gas at 16 psi.
The declustering potential was set at 30 V for the
analyses and internal standard (1 µg/ml Gly-Sar, IS). Multiple
reaction monitoring (MRM) was employed for data acquisition. The
optimised MRM fragmentation transition was 510.2–120.1 m/z with a
collision energy of 50 eV for OCT. The dwell time for each
transition was 200 msec. Data processing was performed using the
Analyst 1.4.1 software package (Applied Biosystems).
Sample preparation for LC-MS/MS
analysis
Frozen samples were thawed at room temperature prior
to preparation. Cell lysate (200-µl aliquot) contained 1 ml Tris
(Beyotime Institute of Biotechnology), 99 ml D-Hanks and 200 µl
methyl cyanides (both Sigma-Aldrich). The mixture was vortexed for
1 min and centrifuged at 12,000 × g for 10 min, and the
supernatants were transferred to another glass tube. The
supernatants were then evaporated to dryness at 40°C under
nitrogen. A 10-µl aliquot was injected for LC-MS/MS analysis. A
total of 200 µl methanol was added to 50-µl samples of blood
plasma, which were vortexed and centrifuged as described above to
remove the protein precipitate. A total of 200 µl of the
supernatants was transferred and evaporated. The residues were then
reconstituted with 100 µl mobile phase. An aliquot of 10 µl was
used for LC-MS/MS analysis.
Statistical analysis
SPSS version 11.5 (SPSS, Inc., Chicago, IL, USA) was
used for data analysis. All measurements are expressed as the mean
± standard deviation. A one-way analysis of variance was performed
to test for significant differences between multiple treatments for
a given parameter. The non-paired t-test was used to assess
significant differences between mean values. Values of P<0.05 or
P<0.01 were considered to indicate a statistically significant
difference.
Results
Influence of PH and P-gp/MRP2/CYP3A4
mixed inhibitors on OCT absorption in rats
In vivo experiments were performed to
evaluate the effects of P-gp/MRP2/CYP3A4 mixed inhibitors on the
rate and efficiency of OCT absorption in rats and to compare the
absorption differences between normal and PH rats. As shown in
Tables II and III, OCT was rapidly dispelled from plasma
following i.v. infusion, and OCT underwent slower elimination or
more rapid absorption in normal rats compared with PH rats. The
rapid elimination in the PH state may be due to increased
expression or activities of transporters and metabolic enzymes in
the liver. Longer Tmax and lower
Cmax and AUC values were observed in PH rats
compared with normal rats upon i.j. administration of OCT,
indicating that increased expression levels or activities of
transporters and metabolic enzymes may occur in the intestines of
PH rats, thereby inhibiting OCT absorption. Shorter
Tmax and higher Cmax and AUC
values were observed in the group administered OCT with
P-gp/MRP2/CYP3A4 mixed inhibitors compared with normal or PH rats
without inhibitors. In addition, F and ER increased
~4-fold in PH rats when administered mixed inhibitors. These
results indicate that mixed inhibitors may markedly improve OCT
absorption and that P-gp, MRP2 and CYP3A4 may function as key
factors in the transportation and metabolism of OCT in the
intestine.
 | Table II.Intravenous administration of
octreotide in rats. |
Table II.
Intravenous administration of
octreotide in rats.
| Parameter | Normal rats | PH rats |
|---|
|
t1/2 (min) |
81.47±6.51 | 82.34±4.91 |
|
Vd (ml/kg) |
2.95±0.18 |
1.53±0.03a |
| AUC (ng/ml ×
min) |
88,283.17±7,062.72 |
628,615±3,756.92b |
 | Table III.Intestinal absorption of octreotide
in rats by intra-jejunal injection. |
Table III.
Intestinal absorption of octreotide
in rats by intra-jejunal injection.
| Group |
Tmax (min) |
Cmax (ng/ml) | AUC0-12h
(ng/ml × min) | F (%) | ER |
|---|
| Normal rats | 30 |
414±20.71 |
74,442.75±8,188.7 |
1.18±0.06 |
|
| PH rats | 45 | 264.37±31.75 |
34,893.75±2,442.6a |
3.95±0.24 | 3.35 |
| PH rats with
inhibitors | 15 | 726.33±58.14 |
127,777.9±11,500.4b,c |
14.47±1.01b | 12.26 |
Effects of OCT and P-gp/MRP2/CYP3A4
mixed inhibitors on mRNA and protein expression levels of
P-gp/MRP2/CYP3A4 in the intestinal mucosa of rats with or without
PH
RT-PCR analysis revealed that the mRNA expression
levels of P-gp/MRP2/CYP3A4 was significantly higher in group PH
than in group N (P<0.05). The mRNA expression of these genes was
also increased by the use of OCT (group N + OCT > group N, group
PH + OCT > group PH; P<0.05) and was the lowest in group PH +
OCT + I due to inhibitor usage (P<0.01). No significant
difference in mRNA expression was observed between group N + OCT
and group PH (P>0.05; Fig. 2A).
The results of the western blot analysis of protein expression were
consistent with the RT-PCR results (Fig.
2B). Immunohistochemical analysis showed that the
P-gp/MRP2/CYP3A4 scores for group PH + OCT were significantly
higher than for the other groups (P<0.01; Fig. 2C) and were significantly higher in
group PH compared with group N (P<0.05). Among all subgroups,
the P-gp/MRP2/CYP3A4 scores in group PH + OCT + I were the lowest
(P<0.01), and there was no significant difference between group
N + OCT and group PH (P>0.05). These results indicate that the
extended use of OCT could increase the mRNA and protein expression
levels of P-gp/MRP2/CYP3A4 and that P-gp/MRP2/CYP3A4 are
upregulated in PH rats.
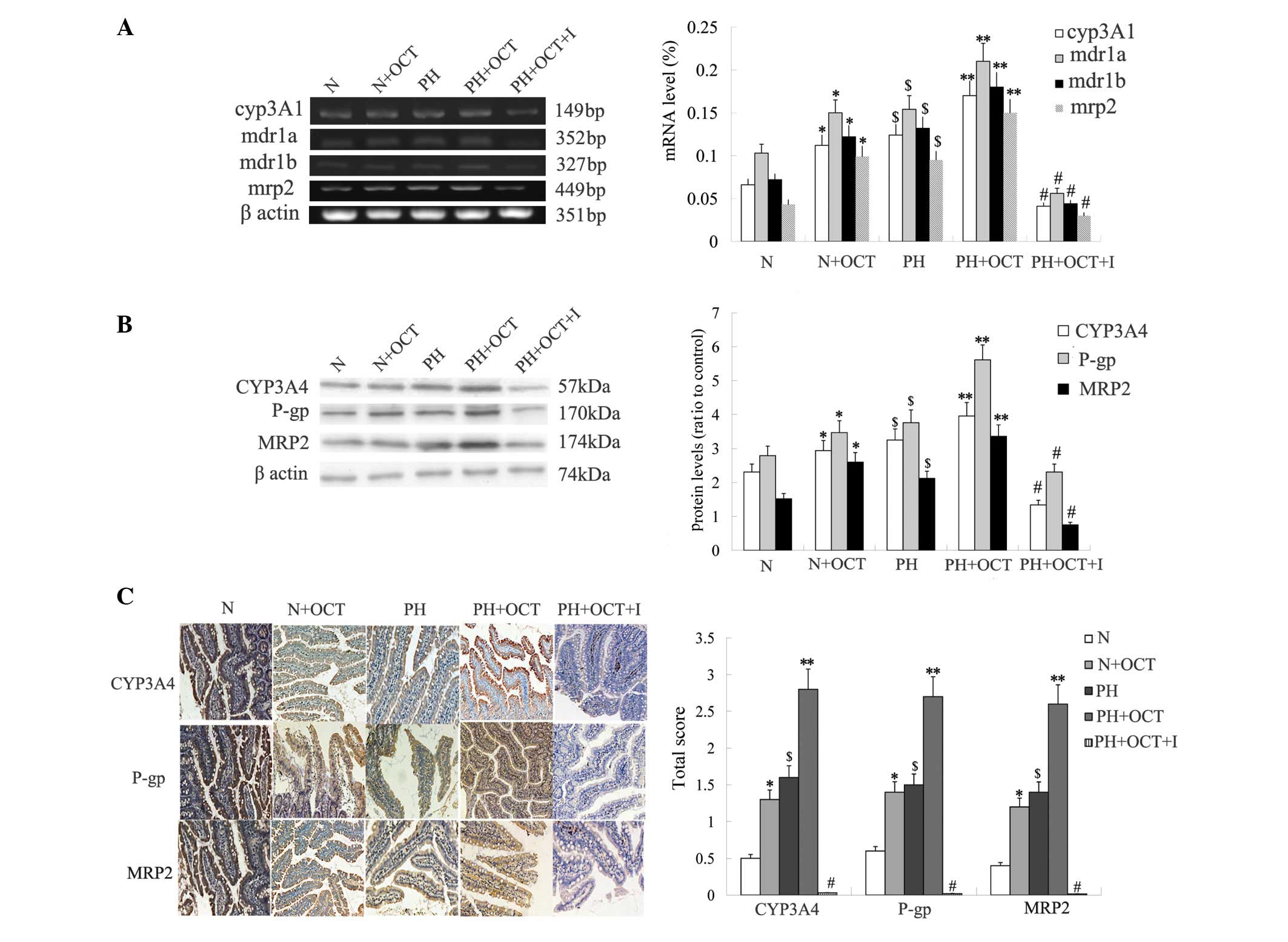 | Figure 2.mRNA and protein expression levels of
P-gp/MRP2/CYP3A4 in the intestinal mucosa of rats with or without
PH. Data are expressed as the means ± standard deviation
(n=4). A two-tailed unpaired t-test was used to
assess significant differences. (A) Reverse
transcription-polymerase chain reaction data showing the CYP3A1,
MDR1 (mdr1a, mdr1b) and MRP2 expression levels in the rat intestine
in each group. (B) Western blot data showing the CYP3A4, P-gp and
MRP2 protein expression levels in the rat intestine in each group.
(C) Immunohistochemistry showing the location and extent of CYP3A4,
P-gp and MRP2 protein expression in the rat intestine in each group
(magnification, ×100). *P<0.05, Group N + OCT vs. Group N.
**P<0.05, Group PH + OCT vs. Group PH; $P<0.05,
Group PH vs. Group N; #P<0.01, Group PH + OCT + I vs.
each other group. N, normal control; OCT, octreotide; PH, portal
hypertension; I, inhibitors; cyp3a1, cytochrome P450 3A1; MRP2,
multidrug-resistence associated protein 2; P-gp,
P-glycoprotein. |
Effect on PVP of co-administration of
OCT and mixed P-gp/MRP2/CYP3A4 inhibitors
The mean PVP level in group PH (15.56±2.36 mmHg) was
higher than that in normal (9.24±0.76 mmHg; P<0.01) and group PH
+ OCT rats (12.51±1.50 mmHg; P<0.05). The PVP level in group PH
+ OCT + I (6.95±1.12 mmHg) was significantly lower than that in
group PH or group PH + OCT (P<0.01) and was within normal
levels. Oral administration of OCT with P-gp/MRP2/CYP3A4 mixed
inhibitors thus effectively decreased PVP.
Discussion
In the present study, a rat model of biliary
cirrhosis with PH was induced via BDL. This method was selected to
reduce cholestasis in the intestine, easily induce pathological
conditions of PH and diminish rat suffering.
To decrease the influence of gastric acid and
enzymes, the i.j. injection method was used in the in vivo
experiment. Verapamil hydrochloride and probenecid are specific
inhibitors of P-gp and MRP2, respectively. Ketoconazole inhibits a
wide range of CYP enzymes. However, the results of our previous
study, involving experiments of intestinal microsome and
recombinant human P450 CYP3A4, indicate that OCT is a substrate of
CYP3A4 and that ketoconazole can decrease CYP3A4 content
effectively (unpublished data).
In the present study it was observed that the
gastrointestinal absorption of OCT markedly decreased under PH but
was significantly increased upon administration of P-gp/MRP2/CYP3A4
mixed inhibitors. In addition, an approximately four-fold increase
in absorption was observed when mixed inhibitors were administered
to PH rats. Previous studies have indicated that OCT was a
substrate for P-gp, MRP2 and CYP3A4 (4,16–18).
Increased expression or activities of these were observed under a
PH state to decrease the intestinal absorption of OCT. To
investigate the mechanism underlying the effect of the mixed
inhibitors on the first pass effects, we also evaluated the
expression levels of P-gp/MRP2/CYP3A4 using RT-PCR, western blot
and immunohistochemistry analyses. The results found that the mRNA
and protein expression levels of P-gp/MRP2/CYP3A4 increased in PH
model rats, and that the extended use of OCT further increased this
expression under some conditions of liver damage due to
physiological environment changes (such as PH, PH enteropathy, PH
gastropathy and rich collateral circulation), as well as
alterations in the intestinal flora. Moreover, mixed inhibitors
were able to markedly decrease these expression levels. As OCT is
clinically used to reduce PVP, the co-administration of OCT with
mixed inhibitors to PH rats was evaluated in this study to examine
its capacity to increase oral OCT bioavailability in a PH state.
The study found that the oral administration of OCT with
P-gp/MRP2/CYP3A4 mixed inhibitors effectively decreased PVP.
Previous research has demonstrated that the decreased oral
bioavailability of OCT was potentially associated with the
transporters P-gp and MRP2 and the metabolic enzyme CYP3A4
(16–18). The upregulated expression or
activities of P-gp/MRP2/CYP3A4 further decreased OCT
bioavailability in PH model rats; the present study further
indicated the extended use of OCT may affect this process. In
addition, mixed inhibitors of P-gp, MRP2 and CYP3A4 markedly
decreased the PVP.
Certain changes of efflux transporters and metabolic
enzymes have been identified in the intestine and liver in liver
diseases by previous studies (18–20).
Wang et al found that P-gp expression and CYP isoenzymatic
activities of the small intestine were enhanced in liver fibrosis,
thus inducing decreased bioavailability and increased elimination
of orally administered ofloxacin (21). A study has also indicated that the
canalicular export pumps MRP2 are preserved and that MDR P-gp
(MDR1, MDR3) is enhanced in patients with advanced-stage primary
biliary cholangitis (22). Various
adaptive responses, including the induction of intestinal, hepatic
and renal bile acid transport proteins, could be triggered under
conditions of cholestatic liver disease and increased
concentrations of serum bile acid (20). The results of previous studies were
consistent with those of the present study, indicating that the
increased expression or activities of export pumps (P-gp and MRP2)
and CYP enzymes (CYP3A4) may occur in cirrhosis complicated by PH
in the intestine (21–23). P-gp, CYP3A4 and MRP2 may have been
involved in intestinal first-pass effects. In addition, P-gp, MRP2
and CYP3A4 may affect the intestinal absorption of OCT.
Previous studies have primarily focused on
investigating the desirable effects of P-gp and CYP3A4 on mediating
drug transport through their inhibition (18,24,25). The
results of a prior study suggested that the intestinal absorption
of OCT may be increased and its bioavailability increased 23-fold
by means of OCT microspheres with polyoxyethylene-24-cholesterol
ether (9). Moreover, cationic
polymer-chitosan and its derivatives, such as N-trimethyl chitosan
chloride, could increase OCT bioavailability to five-fold (5) and chenodeoxycholic acid could increase
it to 1.26%, with the absorption rate reaching 20.2% (26). Unexpectedly, the present study found
that the inhibition of P-gp, MRP2 and CYP3A4 is a viable method for
improving the efficacy of orally administered OCT to effectively
decrease PH. Inhibiting P-gp, MRP2 and CYP3A4 may represent a
general method for improving polypeptide absorption by oral
administration.
In the present study, we did not clarify the optimal
proportion of OCT and mixed inhibitors, and no preliminary toxicity
studies were performed for pharmaceutical safety testing to
consider the side effects. In addition, the specific molecular
mechanisms by which P-gp/MRP2/CYP3A4 inhibitors are regulated in
the intestines of PH rats remain unclear. It may be associated with
the pregnane X receptor, retinoid X receptor alpha, protein kinase
C, radixin protein, nuclear factor kB, human cAMP response
element-binding protein or CAATT box enhancer binding protein,
which are reported to be the key factors involved in regulating
CYP3A4, P-gp or MRP2 (19,27–31).
Further studies are required to address these questions to
ultimately achieve the oral absorption of OCT.
In conclusion, the present results suggest that
P-gp, MRP2 and CYP3A4 may be involved in prohibiting the intestinal
absorption of OCT. The effective inhibition of P-gp, MRP2 and
CYP3A4 could be regarded as a targeted therapy to improve the oral
absorption of OCT. The current study is also partially elucidates
the oral absorption of polypeptides under pathological conditions
of PH, thus supporting a basic theory to increase oral
bioavailability by overcoming intestinal first-pass effects.
Further studies are required to investigate the transportation and
enzymolysis mechanisms that occur in the intestines of patients
with cirrhosis complicated by PH. The appropriate clinical
combination of OCT with the presently investigated inhibitors may
yield an improved treatment approach for PH.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant no. 30970886) and the
Science and Technology Project of Dalian City (grant no.
2008E13SF193).
References
|
1
|
Spahr L, Giostra E, Frossard JL, Morard I,
Mentha G and Hadengue A: A 3-month course of long-acting repeatable
octreotide (sandostatin LAR) improves portal hypertension
inpatients with cirrhosis: A randomized controlled study. Am J
Gastroenterol. 102:1397–1405. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Biron E, Chatterjee J, Ovadia O,
Langenegger D, Brueggen J, Hoyer D, Schmid HA, Jelinek R, Gilon C,
Hoffman A and Kessler H: Improving oral bioavailability of peptides
by multiple N-methylation: Somatostatin analogues. Angew Chem Int
Ed Engl. 47:2595–2599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maggio ET and Grasso P: Oral delivery of
octreotide acetate in Intravail® improves uptake,
half-life, and bioavailability over subcutaneous administration in
male Swiss Webster mice. Regul Pept. 167:233–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li YL, Duan ZJ, Tian Y, Liu Z and Wang QM:
A novel perspective and approach to intestinal octreotide
absorption: Sinomenine-mediated reversible tight junction opening
and its molecular mechanism. Int J Mol Sci. 14:12873–12892. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thanou M, Verhoef JC, Marbach P and
Junginger HE: Intestinal absorption of octreotide: N-trimethyl
chitosan chloride (TMC) ameliorates the permeability and absorption
properties of the somatostatin analogue in vitro and in vivo. J
Pharm Sci. 89:951–957. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tuvia S, Atsmon J, Teichman SL, Katz S,
Salama P, Pelled D, Landau I, Karmeli I, Bidlingmaier M,
Strasburger CJ, et al: Oral octreotide absorption in human
subjects: Comparable pharmacokinetics to parenteral octreotide and
effective growth hormone suppression. J Clin Endocrinol Metab.
97:2362–2369. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDevitt CA and Callaghan R: How can we
best use structural information on P-glycoprotein to design
inhibitors? Pharmacol Ther. 113:429–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gomes P, Vale N and Moreira R:
Cyclization-activated prodrugs. Molecules. 12:2484–2506. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Drewe J, Fricker G, Vonderscher J and
Beglinger C: Enteral absorption of octreotide: Absorption
enhancement by polyoxyethylene-24-cholesterol ether. Br J
Pharmacol. 108:298–303. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ghosh RD, Chakraborty P, Banerjee K,
Adhikary A, Sarkar A, Chatterjee M, Das T and Choudhuri SK: The
molecular interaction of a copper chelate with human
P-glycoprotein. Mol Cell Biochem. 364:309–320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu L, Chen J, Synold TW, Forman BM and
Kane SE: Bioimaging real-time PXR-dependent mdr1a gene regulation
in mdr1a.fLUC reporter mice. J Pharmacol Exp Ther. 345:438–445.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo SB, Duan ZJ, Li Q and Sun XY: Effect
of heme oxygenase-1 on renal function in rats with liver cirrhosis.
World J Gastroenterol. 17:322–328. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thanou M, Verhoef JC, Verheijden JH and
Junginger HE: Intestinal absorption of octreotide using trimethyl
chitosan chloride: Studies in pigs. Pharm Res. 18:823–828. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miao Q, Liu Q, Wang C, Meng Q, Guo X, Peng
J, Kaku T and Liu K: Inhibitory effect of zinc on the absorption of
JBP485 via the gastrointestinal oligopeptide transporter (PEPT1) in
rats. Drug Metab Pharmacokinet. 26:494–502. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
16
|
Gutmann H, Miller DS, Droulle A, Drewe J,
Fahr A and Fricker G: P-glycoprotein- and mrp2-mediated octreotide
transport in renal proximal tubule. Br J Pharmacol. 129:251–256.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fricker G, Nobmann S and Miller DS:
Permeability of porcine blood brain barrier to somatostatin
analogues. Br J Pharmacol. 135:1308–1314. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fang HM, Xu JM, Mei Q, Diao L, Chen ML,
Jin J and Xu XH: Involvement of cytochrome P450 3A4 and
P-glycoprotein in first-pass intestinal extraction of omeprazole in
rabbits. Acta pharmacol Sin. 30:1566–1572. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zollner G, Fickert P, Zenz R, Fuchsbichler
A, Stumptner C, Kenner L, Ferenci P, Stauber RE, Krejs GJ, Denk H,
et al: Hepatobiliary transporter expression in percutaneous liver
biopsies of patients with cholestatic liver diseases. Hepatology.
33:633–646. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kneuer C, Honscha W, Gäbel G and Honscha
KU: Adaptive response to increased bile acids: Induction of MDR1
gene expression and P-glycoprotein activity in renal epithelial
cells. Pflugers Arch. 454:587–594. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Liao ZX, Chen M and Hu XL: Effects
of hepatic fibrosis on ofloxacin pharmacokinetics. Pharmacol Res.
53:28–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zollner G, Fickert P, Silbert D,
Fuchsbichler A, Marschall HU, Zatloukal K, Denk H and Trauner M:
Adaptive changes in hepatobiliary transporter expression in primary
biliary cirrhosis. J Hepatol. 38:717–727. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Barnes SN, Aleksunes LM, Augustine L,
Scheffer GL, Goedken MJ, Jakowski AB, Pruimboom-Brees IM,
Cherrington NJ and Manautou JE: Induction of hepatobiliary efflux
transporters in acetaminophen-induced acute liver failure cases.
Drug Metab Dispos. 35:1963–1969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho YA, Lee W and Choi JS: Effects of
curcumin on the pharmacokinetics of tamoxifen and its active
metabolite, 4-hydroxytamoxifen, in rats: Possible role of CYP3A4
and P-glycoprotein inhibition by curcumin. Pharmazie. 67:124–130.
2012.PubMed/NCBI
|
|
25
|
Scaglione F: New oral anticoagulants:
Comparative pharmacology with vitamin K antagonists. Clin
Pharmacokinet. 52:69–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fricker G, Fahr A, Beglinger C, Kissel T,
Reiter G and Drewe J: Permeation enhancement of octreotide by
specific bile salts in rats and human subjects: In vitro, in vivo
correlations. Br J Pharmacol. 117:217–223. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kullak-Ublick GA, Baretton GB, Oswald M,
Renner EL, Paumgartner G and Beuers U: Expression of the hepatocyte
canalicular multidrug resistance protein (MRP2) in primary biliary
cirrhosis. Hepatol Res. 23:78–82. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Eldesoky ES, Kamel SI, Farghaly AM,
Bakheet MY, Hedaya MA and Siest JP: Study of the urinary ratio of 6
beta-hydroxycortisol/cortisol as a biomarker of CYP3A4 activity in
Egyptian patients with chronic liver diseases. Biomarker Insights.
1:157–164. 2007.PubMed/NCBI
|
|
29
|
Orlando R, Piccoli P, De Martin S, Padrini
R and Palatini P: Effect of the CYP3A4 inhibitor erythromycin on
the pharmacokinetics of lignocaine and its pharmacologically active
metabolites in subjects with normal and impaired liver function. Br
J Clin Pharmacol. 55:86–93. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aihua L, Ping L, Fenghua LI, Yongping MU,
Guangli DU and Lei W: Dynamic changes of cholestatic cirrhosis in
rats and its significance. Chin J Integr Tradit Western Nephrol.
16:87–89. 2006.
|
|
31
|
Medina-Díaz IM, Estrada-Muñiz E,
Reyes-Hernández OD, Ramírez P, Vega L and Elizondo G: Arsenite and
its metabolites, MMA (III) and DMA (III), modify CYP3A4, PXR and
RXR alpha expression in the small intestine of CYP3A4 transgenic
mice. Toxicol Appl Pharmacol. 239:162–168. 2009. View Article : Google Scholar : PubMed/NCBI
|