Introduction
Osteosarcoma is a highly malignant primary tumor of
the bone. Often occurring in the bones and skeleton or their
auxiliary tissues, osteosarcoma displays characteristics of osteoid
tissues directly formed by tumor cells (1). The majority of patients with
osteosarcoma undergo amputation to remove the affected bone;
however, post-amputation five-year survival rates remain low at
5%-20% (2). Therefore, patients are
administered high-dose chemotherapy, including cisplatin,
adriamycin and methotrexate, prior to the operation (3), and radical surgery remains the
first-line therapeutic strategy for the treatment of osteosarcoma.
With the promotion of radical surgery and high-dose chemotherapy,
the five-year survival rates for patients with osteosarcoma have
increased to ~70% (4). The majority
of patients with late osteosarcoma present with tumor metastasis,
in particular lung metastasis, in addition to multiple organ
failure. However, the exact mechanism of osteosarcoma has yet to be
elucidated, despite the continual efforts made by researchers
(5). The occurrence and development
of osteosarcoma is regulated by multiple interconnected pathways
involving several types of mRNA and microRNA (miRNA/miR). One study
reported that miR-195 was able to inhibit the invasion and
migration of osteosarcoma by regulating fatty acid synthase factor
(6).
Synuclein γ (SNCG), also known as breast cancer
specific gene 1, belongs to the synuclein gene family, which is
highly conserved in mammals (7).
SNCG was initially isolated from breast cancer (8). Subsequently, a study revealed that SNCG
is involved in the occurrence and development of breast cancer
(7). In recent years, it has been
identified that SNCG is also important in other types of tumors,
including breast, gallbladder, pancreatic and uterine cancer
(9–13). However, whether SNCG has regulatory
effects on the proliferation and migration of osteosarcoma cells
has yet to be determined. In the present study, the effects of SNCG
on the proliferation and invasion of osteosarcoma, in addition to
the regulatory mechanism of miR-497 on SNCG, were investigated.
Materials and methods
Patients
Between December 2010 and August 2013, a total of 36
patients were diagnosed with osteosarcoma at The Second Hospital of
Shandong University (Jinan, China), according to the 2002 World
Health Organization (WHO) classification standards (14). During this time period at the same
hospital, 26 healthy subjects were enrolled as the control group to
provide control blood samples. The staging of the disease was
determined according to the Enneking staging standard, as follows:
Stage I (low-grade malignancy), stage II (high-grade malignancy)
and stage III (metastasis) (15).
Patients with stage III osteosarcoma (lung metastasis; n=15) were
selected to undergo tissue resection. Only patients with lung
metastases at stage III were included in the present study.
Patients received resection of osteosarcoma and lung metastases at
our hospital, and received no hormones, Chinese traditional drugs,
or radiochemotherapy prior to surgery. Additional inclusion and
exclusion criteria were set according to the standards outlined by
the WHO (14). All procedures were
approved by the Ethics Committee of Shandong University (Jinan,
China). Written informed consent was obtained from all patients or
their families.
Samples
Fasting peripheral blood was collected from all 36
patients in the morning. Serum was separated by density gradient
centrifugation of 10–15 ml peripheral blood at 400 × g for 10 min,
and was subsequently stored in liquid nitrogen. Stage III patients
with osteosarcoma (n=15) underwent surgery for osteosarcoma
(amputation or limb salvage surgery) and lung metastasis tissue
resection, with negative adjacent tissues (>5 cm away from tumor
foci) used as controls. Tissue samples were stored in liquid
nitrogen.
Bioinformatics
Bioinformatics is the predominant method used for
the functional analysis of miRNA. A previous study revealed
evidence of the regulation of SNCG by one of its upstream mRNAs,
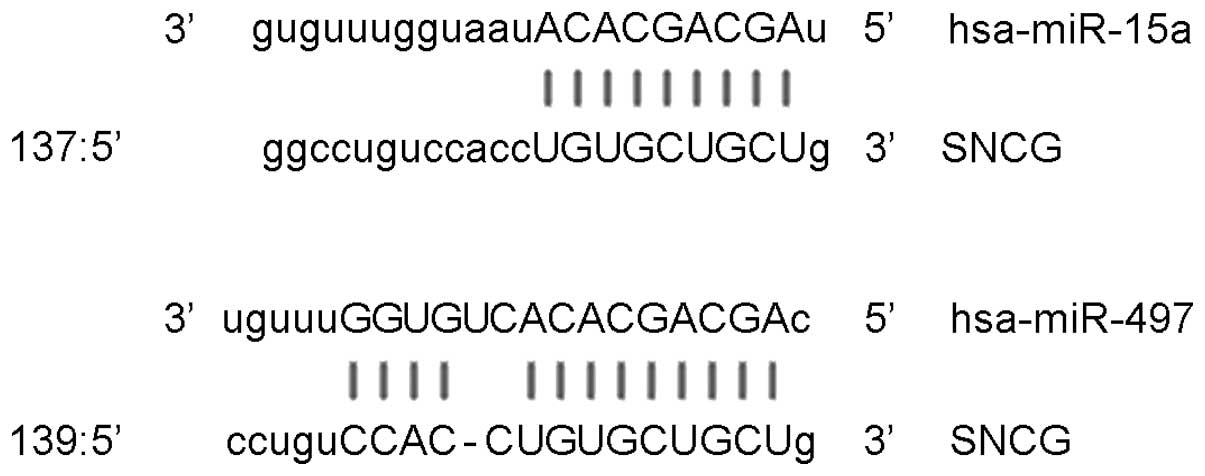
miR-15a (16). Using miRanda
(www.microrna.org/microrna/getExprForm.do; version
2010), TargetScan (www.targetscan.org/vert_61; version 6.2), PieTar
(www.pictar.mdc-berlin.de; version 2007)
and BibiServ (www.bibiserv.techfak.uni-bielefield.be/bibi/Tools.html;
version 2013) databases, the miR-15 family genes were screened
(miR-15a, miR-15b, miR-16, miR-195, miR-424 and miR-497), and
miR-497 was selected for evaluation in the present study, due to it
having similar SNCG-binding sites to miR-15a (Fig. 1).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (cat
no. 10606ES60; Yeasen, Shanghai, China) and purified using a
miRNeasy Serum/Plasma kit (Guangzhou Jianlun Biological Technology
Co., Ltd., Guangzhou, China), according to the manufacturer's
protocol. The purity of RNA was determined by the absorbance ratio
at 260/280 nm using ultraviolet spectrophotometry (NanoDrop
ND-1000; Thermo Fisher Scientific, Waltham, MA, USA). Next, cDNA
was obtained by reverse transcription (TIANScript RT kit; Tiangen
Biotech Co., Ltd., Beijing, China) of 1 µg RNA and stored at −20°C.
RT-PCR was performed using a SuperReal PreMix kit (FP204; Tiangen
Biotech Co., Ltd.). The primers for SNCG were as follows: Upstream,
5′-ACACCCACCATGGATGTCTT-3′ and downstream,
5′-ACAGTGTTGACGCTGCTCAC-3′. The primers for β-actin were as
follows: Upstream, 5′-TGTTTGAGACCTTCAACACCC-3′ and downstream,
5′-AGCACTGTGTGTTGGCGTACAG-3′. PCR amplification was performed on an
iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.
Hercules, CA, USA), as follows: Initial denaturation at 95°C for 60
sec; 45 cycles of denaturation at 95°C for 5 sec, annealing at 60°C
for 15 sec and elongation at 72°C for 5 sec. The quantification
cycle (Cq) method (2−ΔΔCq) was used to calculate
SNCG/β-actin expression ratio (17).
miR-497 was isolated from the total RNA using a
miRcute miRNA Isolation kit (Tiangen Biotech Co., Ltd.), according
to the manufactuer's instructions, and cDNA was obtained using a
miRcute miRNA First-Strand cDNA Synthesis kit (Tiangen Biotech Co.,
Ltd.). RT-qPCR was performed using a miRcute miRNA Detection kit
(Tiangen Biotech Co., Ltd.). The primers for miR-497 were as
follows: Forward, 5′-TCGGGCAGCAGCACACTGTG, and universal reverse,
5′-GTGCAGGGTCCGAGGT-3′. The primers for U6 were as follows:
Forward, 5′-CGCTTCGGCAGCACATATAC-3′ and reverse,
5′-TTCACGAATTTGCGTGTCAT-3′. PCR amplification was performed on an
iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Inc.), as
follows: Initial denaturation at 95°C for 3 min; and 40 cycles of
denaturation at 95°C for 5 sec, and annealing at 60°C for 20 sec.
The 2−ΔΔCq method was used to calculate the miR-497/U6
expression ratio.
Western blotting
Total proteins were extracted from serum and tissues
using radio-immunoprecipitation assay lysis buffer (P0013B;
Beyotime Institute of Biotechnology, Shanghai, China). Protein
concentration was determined using a bicinchoninic acid protein
concentration determination kit [cat no. RTP7102; Real-Times
(Beijing) Biotechnology Co., Ltd., Beijing, China]. After boiling
with loading buffer for 5 min, protein samples (20 µg) were
subjected to 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis at 65 V until the target proteins had travelled to
1 cm above the edge of the gel. Next, the resolved proteins were
transferred to polyvinylidene difluoride membranes on ice (100 V, 2
h) and blocked with 5% skimmed milk at room temperature for 1 h.
Subsequently, the membranes were incubated with SNCG rabbit
anti-human (1:1,000; ab55424) and internal reference rabbit
anti-human β-actin polyclonal primary antibodies (1:5,000;
ab129348; both Abcam, Cambridge, MA, USA) at 4°C overnight. After
extensive washing with phosphate-buffered saline with Tween-20
(CW0041; CWBIO, Beijing, China), the membranes were incubated with
goat anti-rabbit horseradish peroxidase conjugated-IgG secondary
antibody (1:3,000; ab6721; Abcam) for 1 h at room temperature.
Then, the membrane was developed using an enhanced
chemiluminescence detection kit (Sigma-Aldrich, St. Louis, MO, USA)
for imaging. Image Lab software (version 3.0; Bio-Rad Laboratories,
Inc.) was used to acquire and analyze imaging signals. The relative
expression of SNCG protein was expressed as the ratio of SNCG to
β-actin.
Statistical analysis
All statistical analyses were performed using SPSS
software for Windows (version 18.0; SPSS, Inc., Chicago, IL, USA).
Results are expressed as means ± standard deviation for tests of
normality. Multi-group measurements were subjected to one-way
analysis of variance. In cases of homogeneity of variance, Fisher's
Least Significant Difference and Student-Newman-Keuls methods were
used; in cases of heterogeneity of variance, Tamhane's T2 or
Dunnett's T3 methods were used. P<0.05 was considered to
indicate a statistically significant difference.
Results
SNCG mRNA and protein expression
levels are upregulated in osteosarcoma tissues
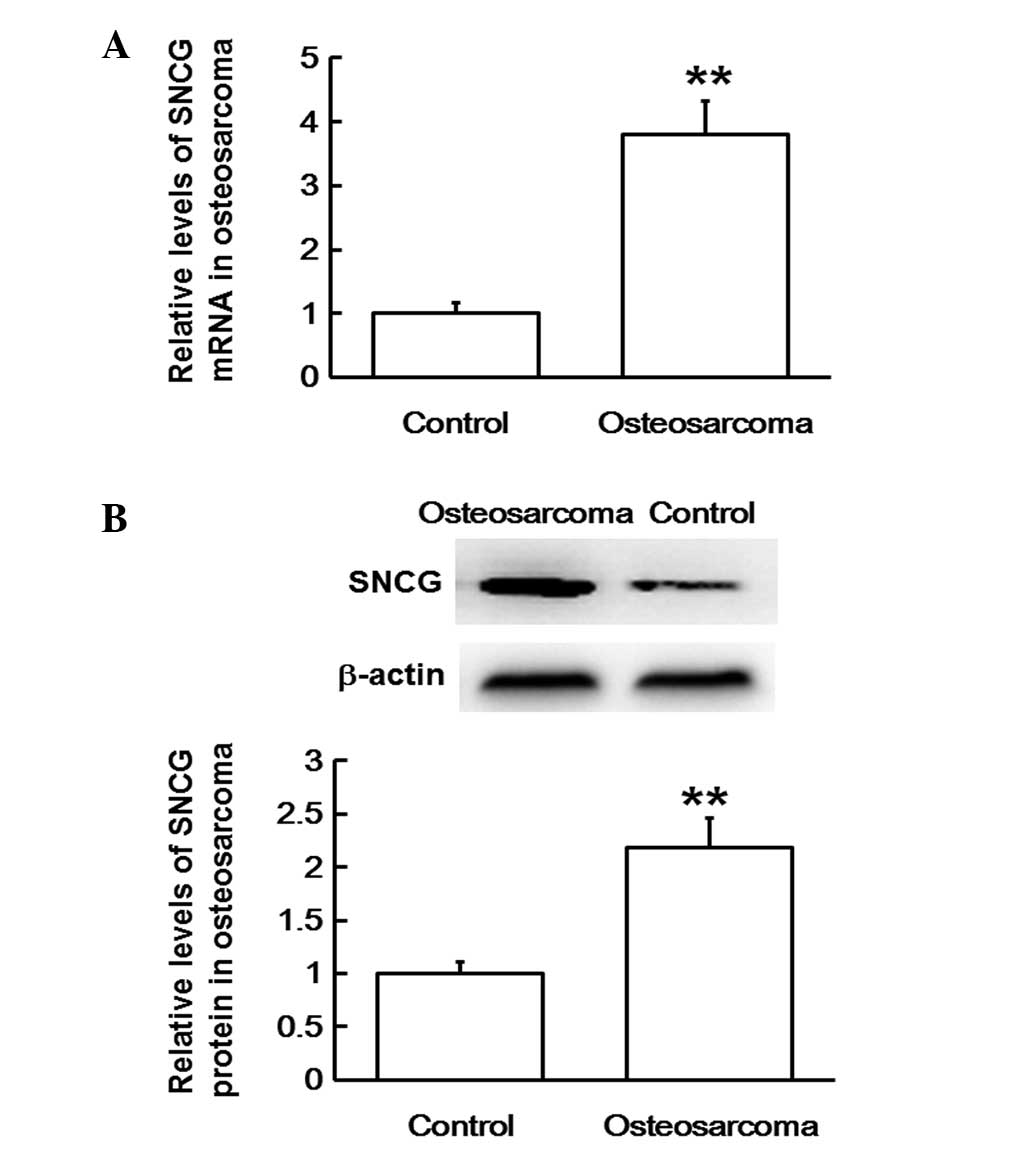
In order to evaluate SNCG mRNA and protein
expression levels in osteosarcoma tissues, RT-qPCR and western
blotting analyses were used, respectively. The RT-qPCR data
revealed that mRNA expression levels in osteosarcoma tissues were
significantly upregulated compared with those in adjacent tissues
(P<0.01; Fig. 2A). In agreement,
western blots indicated that SNCG protein expression levels in
osteosarcoma tissues were also significantly higher compared with
those of the adjacent tissues (P<0.01; Fig. 2B). The results suggest that both mRNA
and protein expression levels of SNCG are upregulated in
osteosarcoma tissues.
SNCG mRNA and protein expression
levels are upregulated in the blood of patients with
osteosarcoma
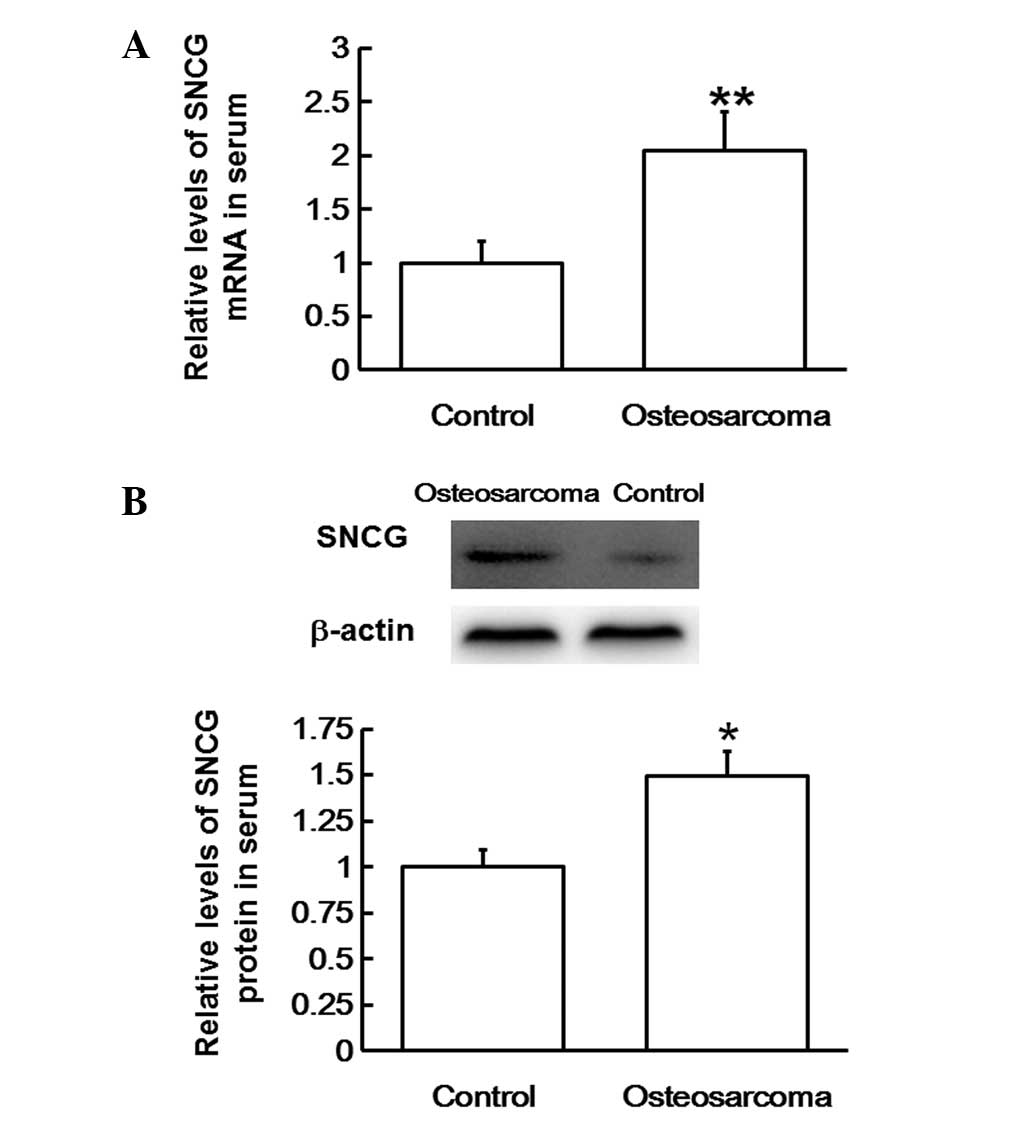
To examine SNCG mRNA and protein levels in the
serum, RT-qPCR and western blotting analyses were performed,
respectively. The RT-qPCR data revealed that SNCG mRNA levels in
the serum of patients with osteosarcoma were significantly higher
compared with those in healthy subjects (P<0.01; Fig. 3A). In addition, western blots
revealed that SNCG protein content in the serum of patients with
osteosarcoma was also significantly higher compared with that in
healthy subjects (P<0.05; Fig.
3B). The results indicate that SNCG mRNA and protein levels are
upregulated in the blood of patients with osteosarcoma.
SNCG mRNA and protein expression
levels are upregulated in lung metastatic tissues
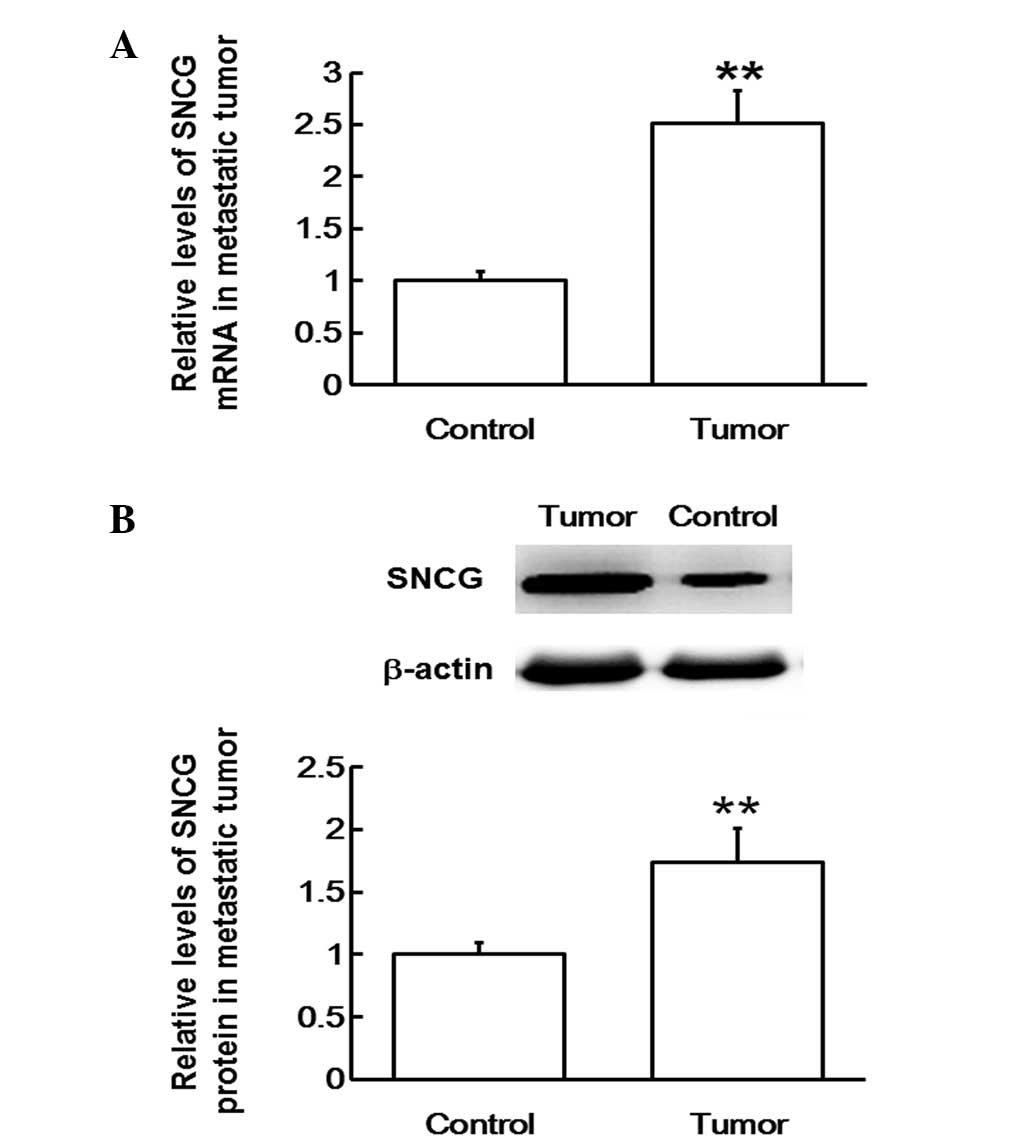
To measure SNCG mRNA and protein expression levels
in lung metastatic tissues, RT-qPCR and western blotting analyses
were performed, respectively. The RT-qPCR data revealed that SNCG
mRNA expression levels in lung metastatic tissues were
significantly upregulated compared with those in the adjacent
tissues (P<0.01; Fig. 4A). In
addition, western blots indicated that SNCG protein expression in
lung metastatic tissues was also significantly elevated compared
with that in the adjacent tissues (P<0.01; Fig. 4B). The results suggest that SNCG mRNA
and protein expression levels are upregulated in lung metastatic
tissues.
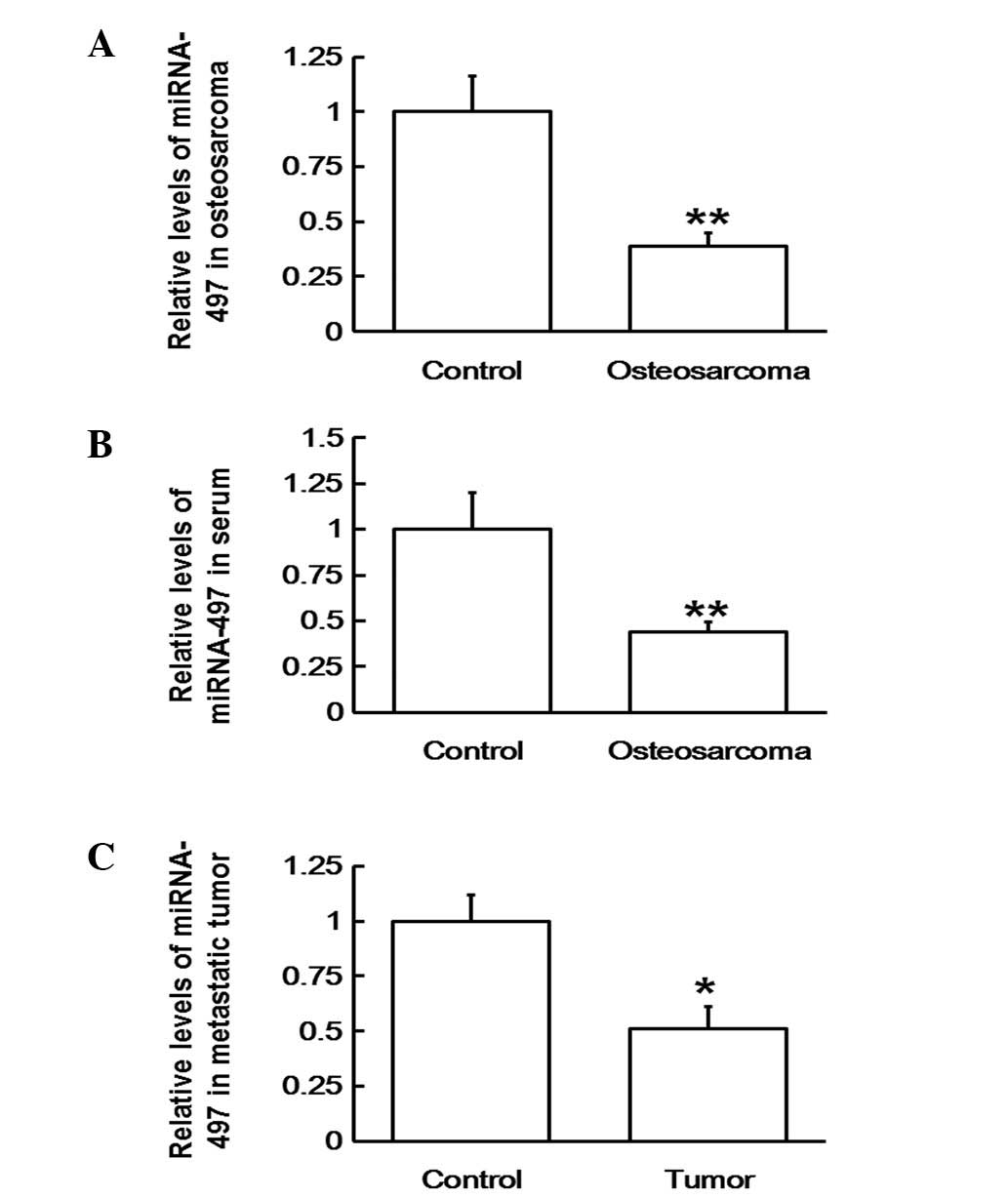
Downregulation of miR-497 results in
the occurrence and development of osteosarcoma and its metastasis
by upregulating SNCG mRNA
To determine the levels of miR-497 in blood,
osteosarcoma and metastatic tissues, RT-qPCR was employed. The
results revealed that the expression levels of miR-497 were
significantly lower in osteosarcoma tissue (P<0.01; Fig. 5A), serum (P<0.01; Fig. 5B) and metastatic tissues (P<0.05;
Fig. 5C). Thus, the results indicate
that the downregulation of miR-497 may cause the upregulation of
SNCG mRNA, resulting in the occurrence, development and metastasis
of osteosarcoma.
Discussion
Osteosarcoma typically occurs in the area
surrounding the knees in patients between 15 and 25 years of age,
and hormone disorders during adolescence are considered to be a
cause of osteosarcoma in young people(<20 years old) (18). Osteosarcoma can metastasize
throughout the body and can be classified as one of three types:
Adjacent organ invasion, implantation metastasis and distant
metastasis. As a result of the abundant blood flow to the lungs and
brain, the two organs are common sites for distant metastasis of
osteosarcoma via the blood circulation. This can result in severe
consequences, including tumors of the brain and lung (19,20).
Factors detectable in the blood, such as miRNA-26a, miRNA-27a and
miRNA-191, may also indirectly affect the malignancy and metastasis
of osteosarcoma (21,22). Therefore, the elucidation of the
molecular mechanisms and gene regulatory pathways involved in this
process, in addition to the means to inhibit them, are important
for the prevention of osteosarcoma occurrence, proliferation and
invasion.
SNCG is widely distributed throughout the
presynaptic region of the central nervous system, and has tissue
specificity. However, SNCG is also a proto-oncogene, and its
overexpression results in the interruption of mitotic checkpoints,
leading to multiple nuclei in the cells. Consequently, the abnormal
proliferation of these cells results in tumor formation (23). SNCG is involved in the invasion and
metastasis of tumors, including those of the breast, gallbladder,
pancreas and uterus (9–13), where high expression levels of SNCG
have been observed. The aforementioned characteristics make SNCG a
suitable tumor biomarker. The present study, which investigated
SNCG levels in tumor tissues and blood samples from patients with
lung metastasis of osteosarcoma, revealed abnormally upregulated
SNCG expression levels. This may be important in the regulation of
osteosarcoma proliferation and metastasis.
The upstream regulatory genes of SNCG were also
investigated in the present study. In vertebrates, the 5′-end
sequences of certain miRNAs, particularly the nucleotides at
positions 2–7 (or 8), are similar. These sequences, also termed
seed regions, are pairing sequences for miRNAs and their target
mRNAs, and determine their functions. miRNAs that have highly
similar sequences, characteristic seed regions and common target
genes, are classified into a superfamily. The miR-15 superfamily is
located in the 13q14 region of human chromosomes, and includes
miR-15a, miR-15b, miR-16, miR-195, miR-424 and miR-497. A previous
study revealed that miRNA-15a regulates the cell cycle and
apoptosis of breast cancer cells through its target gene, SNCG
(16). Using bioinformatic tools,
the present study revealed that miR-497 is located within close
proximity of miR-15a and possibly participates in the regulation of
SNCG. Other studies have indicated that miR-497 is able to inhibit
features of colon cancer by targeting insulin-like growth factor-1
receptor (24); suppress
angiogenesis in ovarian cancer by targeting the vascular
endothelial growth factor gene, and regulating the
phosphatidylinositol 3-kinase/protein kinase B and
mitogen-activated protein kinase/extracellular signal-regulated
kinase signaling pathways (25); and
reduce the proliferation of cervical cancer cells by targeting
cyclin-E1 (26). Further studies
have revealed its ability to hinder the proliferation of gastric
cancer cells by targeting eukaryotic initiation factor 4E (27) and suppresses liver cancer cells by
negatively regulating checkpoint kinase 1 (28). Based on these studies, we hypothesize
that miR-497 may have similar regulatory effects in osteosarcoma.
The current results revealed that the expression of miR-497 was
reduced in blood, osteosarcoma and lung metastatic tissues, leading
to a reduced regulatory effect on the tumor. This may explain the
abnormal cell proliferation observed in osteosarcoma. Combining the
present data on SNCG mRNA and protein expression, downregulated
miR-497 may be associated with the upregulation of SNCG expression
levels. The imbalance between SNCG and miR-497 may lead to the
occurrence and development of osteosarcoma. Notably, the trends in
expression levels of miR-497 in the blood are consistent with
trends in SNCG expression levels in tumor tissues. Therefore,
miR-497 in the blood may also be used for the prediction and
prevention of osteosarcoma metastasis. However, a number of factors
may have influenced the present study, including the limited number
of patients, different surgical approaches (amputation or limb
salvage surgery) and regions of origin, varying health conditions
and complications, as well as numerous other factors associated
with osteosarcoma (29–32). Therefore, further studies on cells,
animals and clinical trails are necessary in order to understand
the exact mechanism of action of miR-497 in osteosarcoma.
In conclusion, miR-497 may exert its inhibitory
effect on the proliferation and invasion of osterosarcoma by
regulating the expression levels of SNCG. As such, miR-497 may be a
potential marker and therapeutic target for the treatment of
osteosarcoma; however, future studies are required in order to
fully elucidate the underlying mechanisms.
Acknowledgements
The present study was supported by Dr Shengtian
Zhao, the director of The Second Hospital of Shandong University
(Jinan, China). During the write-up, we received help and
constructive suggestions from Dr Wenguang Liu and Dr Haipeng Si of
Shandong University.
References
|
1
|
Lei P, Xie J, Wang L, Yang X, Dai Z and Hu
Y: microRNA-145 inhibits osteosarcoma cell proliferation and
invasion by targeting ROCK1. Mol Med Rep. 10:155–160.
2014.PubMed/NCBI
|
|
2
|
Jaffe N: Osteosarcoma: Review of the past,
impact on the future. The American experience. Cancer Treat Res.
152:239–262. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferguson WS and Goorin AM: Current
treatment of osteosarcoma. Cancer Invest. 19:292–315. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hameed M and Dorfman H: Primary malignant
bone tumours - recent developments. Semin Diagn Pathol. 28:86–101.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li R, Liu J, Wu H, Liu L, Wang L and Zhang
S: TIKI2 suppresses growth of osteosarcoma by targeting
Wnt/β-catenin pathway. Mol Cell Biochem. 392:109–116. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao JH, Zhou RP, Peng AF, Liu ZL, Huang
SH, Long XH and Shu Y: microRNA-195 suppresses osteosarcoma cell
invasion and migration in vitro by targeting FASN. Oncol Lett.
4:1125–1129. 2012.PubMed/NCBI
|
|
7
|
Jiang Y, Liu YE, Goldberg ID and Shi YE:
Gamma synuclein, a novel heat-shock protein-associated chaperone,
stimulates ligand-dependent estrogen receptor alpha signaling and
mammary tumorigenesis. Cancer Res. 64:4539–4546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao
G, Joseph BK, Rosen C and Shi YE: Identification of a breast
cancer-specific gene, BCSG1, by direct differential cDNA
sequencing. Cancer Res. 57:759–764. 1997.PubMed/NCBI
|
|
9
|
Morgan J, Hoekstra AV, Chapman-Davis E,
Hardt JL, Kim JJ and Buttin BM: Synuclein-γ (SNCG) may be a novel
prognostic biomarker in uterine papillary serous carcinoma. Gynecol
Oncol. 114:293–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hibi T, Mori T, Fukuma M, Yamazaki K,
Hashiguchi A, Yamada T, Tanabe M, Aiura K, Kawakami T, Ogiwara A,
et al: Synuclein-γ is closely involved in perineural invasion and
distant metastasis in mouse models and is a novel prognostic factor
in pancreatic cancer. Clin Cancer Res. 15:2864–2871. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han S, She F, Wang D, Yao X, Jiang L and
Chen Y: SNCG gene silencing in gallbladder cancer cells inhibits
key tumorigenic activities. Front Biosci (Landmark Ed).
17:1589–1598. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan ZZ, Bruening W, Giasson BI, Lee VM and
Godwin AK: Gamma-synuclein promotes cancer cell survival and
inhibits stress- and chemotherapy drug-induced apoptosis by
modulating MAPK pathways. J Biol Chem. 277:35050–35060. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C,
Wang L, Zhao W, Jiang JD and Liu J: Loss of epigenetic control of
synuclein-γ gene as a molecular indicator of metastasis in a wide
range of human cancers. Cancer Res. 65:7635–7643. 2005.PubMed/NCBI
|
|
14
|
Dorfman HD, Czemiak B and Kotz R: World
Health Organization Classification of TumoursWorld Health
Organization Classification of Tumours, Pathology and Genetics of
Tumours of Soft Tissue and Bone. Fletcher CDM, Unni KK and Mertens
F: IARC Press; Lyon, France: pp. 227–232. 2002
|
|
15
|
Enneking WF: Staging musculoskeletal
tumorsMusculoskeletal Tumor Surgery. Churchill Livingstone; New
York: pp. 69–122. 1983
|
|
16
|
Li P, Xie XB, Chen Q, Pang GL, Luo W, Tu
JC, Zheng F, Liu SM, Han L, Zhang JK, et al: MiRNA-15a mediates
cell cycle arrest and potentiates apoptosis in breast cancer cells
by targeting synuclein-gamma. Asian Pac J Cancer Prev.
15:6949–6954. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Jing J, Peng J, Mao W, Zheng Y,
Wang D, Wang X, Liu Z and Zhang X: Expression and clinical
significance of galectin-3 in osteosarcoma. Gene. 546:403–407.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shweikeh F, Bukavina L, Saeed K, Sarkis R,
Suneja A, Sweiss F and Drazin D: Brain metastasis in bone and soft
tissue cancers: A review of incidence, interventions, and outcomes.
Sarcoma. 2014:4751752014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Link MP, Gebhardt MC and Meyers PA:
Chapter 35: OsteosarcomaPrinciples and Practice of Pediatric
Oncology. Pizzo PA and Poplack DG: 5th. Lippincott Williams &
Wilkins; Philadelphia, PA: pp. 1051–1089. 2006
|
|
21
|
Taheriazam A, Bahador R, Karbasy SH,
Jamshidl SM, Torkaman A, Yahaghi E and Shakeri M: Down-regulation
of microRNA-26a and up-regulation of microRNA-27a contributes to
aggressive progression of osteosarcoma. Diagn Pathol. 10:1662015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang T, Ji F, Dai Z, Xie Y and Yuan D:
Increased expression of microRNA-191 as a potential serum biomarker
for diagnosis and prognosis in human osteosarcoma. Cancer Biomark.
15:543–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ninkina NN and Bukhman VL: Synucleins - to
have or not to have. Genetika. 36:1487–1491. 2000.(In Russian).
PubMed/NCBI
|
|
24
|
Guo ST, Jiang CC, Wang GP, Li YP, Wang CY,
Guo XY, Yang RH, Feng Y, Wang FH, Tseng HY, et al: MicroRNA-497
targets insulin-like growth factor 1 receptor and has a tumour
suppressive role in human colorectal cancer. Oncogene.
32:1910–1920. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang W, Ren F, Wu Q, Jiang D, Li H and Shi
H: MicroRNA-497 suppresses angiogenesis by targeting vascular
endothelial growth factor A through the PI3K/AKT and MAPK/ERK
pathways in ovarian cancer. Oncol Rep. 32:2127–2133.
2014.PubMed/NCBI
|
|
26
|
Han J, Huo M, Mu M, Liu J and Zhang J:
miR-497 suppresses proliferation of human cervical carcinoma HeLa
cells by targeting cyclin E1. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
30:597–600. 2014.(In Chinese). PubMed/NCBI
|
|
27
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie Y, Wei RR, Huang GL, Zhang MY, Yuan YF
and Wang HY: Checkpoint kinase 1 is negatively regulated by miR-497
in hepatocellular carcinoma. Medical Oncology. 31:8442014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ohba T, Cates JM, Cole HA, Slosky DA, Haro
H, Ando T, Schwartz HS and Schoenecker JG: Autocrine VEGF/VEGFR1
Signaling in a Subpopulation of Cells Associates with Aggressive
Osteosarcoma. Molecular Cancer Research molcanres. 0037.2014. 2014.
View Article : Google Scholar
|
|
30
|
Liu H, Huang L, Zhang Z, Zhang Z, Yu Z,
Chen X, Chen Z, Zen Y, Yang D, Han Z, et al: LIM mineralization
protein-1 inhibits the malignant phenotypes of human osteosarcoma
cells. International journal of molecular sciences. 15:7037–7048.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He H, Ni J and Huang J: Molecular
mechanisms of chemoresistance in osteosarcoma. Oncol Lett.
7:1352–1362. 2014.(Review). PubMed/NCBI
|
|
32
|
Zhang H, Yin Z, Ning K, Wang L, Guo R and
Ji Z: Prognostic value of microRNA-223/epithelial cell transforming
sequence 2 signaling in patients with osteosarcoma. Hum Pathol.
45:1430–1436. 2014. View Article : Google Scholar : PubMed/NCBI
|