Introduction
Statistically, male sterility accounts for 40–50% of
total infertility. Low sperm motility and asthenozoospermia,
account for up to 30–50% of male sterility (1). Analysis of differential proteins by
sperm dimensional electrophoresis found that GP130 and Proteasomeβ3
(PSMβ3) were expressed abnormally (2). PSMβ3, also known as plasma membrane
calcium (Ca2+) ATPase (PMCA3), is an important enzyme
for Ca2+ transport in and out of cells (3). Ca2+ plays an important role
in a series of processes, including sperm maturation, capacitation
and sperm-egg binding (4). The
PMCA3 gene, located on the X chromosome, is thought to be
associated with sperm motility (5).
Research on GP130 found that the Janus kinase (JAK)-signal
transduction and activator of transcription (STAT) and RAS-MAPK
signaling pathways play vital roles in the pathogenic mechanism of
asthenozoospermia (6). The present
study examined the possible mechanism of JAK-STAT/PSMβ3 signal
transduction pathways in asthenozoospermia, providing new
theoretical bases and therapeutic targets for clinical study.
Materials and methods
Patients
Thirty cases of male sterility, diagnosed with
asthenozoospermia at the General Hospital of Beijing Military
Region (Beijing, China) from June 2015 to January 2016, were
selected. After 3–5 days of abstinence, 3 ml semen samples obtained
by masturbation were analyzed by a sperm quality analyzer, BD-8000G
(Xuzhou City Beidou Technology & Trading Co., Ltd., Xuzhou,
China). With reference to World Health Organization (WHO) standards
on the diagnostic criteria of asthenozoospermia, the percentage of
forward moving sperm (grade A + B) was <50% or grade A sperm
movement was <25%. The age range of patients was 21–33 years,
with an average age of 26.4±5.5 years. At the same time, 30 healthy
controls with normal semen samples, according to physical exam were
chosen, aged 20–34 years, with an average age of 26.2±5.3 years.
The age difference between the two groups was not statistically
significant (P>0.05).
The present study was approved by the Ethics
Committee of the General Hospital of Beijing Military Region and we
received informed consent from all participating patients.
Percoll density gradient
centrifugation for sperm collection
One and a half milliliters of 90% and 1.5 ml of 45%
Percoll solutions were added to 15 ml centrifuge tubes (90% below,
45% above). Subsequently, 3 ml of semen was added. After
centrifugation (200 × g for 20 min), the supernatant was removed
and sperm sediment at the bottom was washed with IVF-20 culture
medium twice. The sperm precipitate was collected for later
use.
Detection of JAK, STAT and PSMβ3 mRNA
by reverse transcriptase quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted using RNA Pure, a high
purity, total RNA, rapid extraction TRIzol reagent (Sigma-Aldrich,
St. Louis, MO, USA), and cDNA was synthesized after assessing the
purity and concentration of RNA. The primer concentrations were 10
pmol/µl. Primers were designed using Primer Premier 5.0 and were
produced by Sangon Biotech Co. Ltd. (Shanghai, China). Primer
sequences used were: JAK forward, 5′-TGCTGTCCAGACAAGAATGC-3′ and
reverse, 5′-TTCTGCAACCGTCTCTTCCT-3′; STAT forward,
5′-TAACGAGGAGCTGGTGGAGT-3′ and reverse, 5′-GCTTGCGTGTCAGAAAAGTT-3′;
PSMβ3 forward, 5′-GAAATCGCAGCCATAGTATC-3′ and reverse,
5′-CTGATGACGGTGAACTTCTG-3′; and β-actin forward,
5′-ATGTTTGAGACCTTCAACAC-3′ and reverse,
5′-GGCCATCTCTTGCTCGAAGTC-3′. According to the Applied Biosystems
StepOne system, the reaction mixture contained 10 µl of 2X Smart
Green PCR mix, 0.2 µl of each 10 pmol/µl primer, 1 µl of cDNA
template, and 8.6 µl of ddH2O for a total volume of 20
µl. The thermal profile was 40 cycles of pre-denaturation at 95°C
for 10 min, denaturation at 94°C for 15 sec, 60°C for 1 min and
extension at 72°C for 30 sec. The 2−∆∆Cq relative
quantitative method was used to show the relative expression levels
of each target gene.
Measurement of p-JAK, p-STAT and PSMβ3
by western blot analysis
Each well of the plate was treated by 200 µl lysis
buffer and maintained in an ice bath for 1 h. Sample solutions were
then centrifuged for 15 min at 4°C. Protein concentration of the
centrifuged supernatant was determined by Coomassie Brilliant Blue
staining, and preserved at −80°C. Total protein (50 µg) from each
sample was separated by electrophoresis. The samples were then
transferred to PVDF membranes (90 V, 1.5 h at low temperature).
Rabbit anti-human p-JAK (dilution: 1:500; cat. no.: ab138005),
rabbit monoclonal p-STAT (dilution: 1:500; cat no.: ab32143) and
mouse monoclonal PSMβ3 (dilution: 1:500; cat no.: ab128094),
purchased from Abcam (Cambridge, MA, USA), were added after
blocking and incubated at 4°C overnight, following four washes with
phosphate-buffered saline (PBS). Horseradish peroxidase-conjugated
goat anti-rabbit IgG (dilution: 1/2000; Abcam; cat no.: ab6721) was
added to membranes and incubated at 4°C overnight. After three
washes with PBS, the ECL enhanced chemiluminescence reagent kit was
used for signal development. After thorough scanning of negative
film, a semi-quantitative analysis was carried out on the bands by
Shanghai Tianneng gel imaging processing system software. Data were
normalized to levels of β-actin.
JAK inhibitor intervention
Cases without asthenozoospermia were randomly
divided into the non-treated control group (n=15) and JAK
inhibitor-treated group (n=15). The levels of PSMβ3 mRNA and
protein were measured after application of the JAK inhibitor,
AG-490. Immunofluorescent staining of PSMβ3 was observed by a laser
confocal microscope. The cells were stained as follows: 25-min
incubation with 0.25% H2O2, 15 min with 0.3%
Triton X-100, three washes with PBS for 5 min, and 30-min
incubation with 10% normal goat serum. Rabbit anti-human PSMβ3
monoclonal antibody (1:200) was then added and allowed to incubate
at 4°C overnight. The samples were washed three times for 5 min,
and biotin-labeled goat anti-rabbit IgG (1:100) was added and left
to incubate at room temperature for 30 min. Three 5 min washes with
PBS were performed again, and streptavidin-biotin
complex-fluorescein isothiocyanate (SABC-FITC)-labeled secondary
antibody (1:100) was added, and incubated at room temperature for
30 min. Next, we performed three washes with PBS for 5 min. The
samples were stained with DAPI for 5 min, washed in PBS, and
mounted using antifade mounting solution. Staining was observed
under a confocal microscope (Nikon, Tokyo, Japan). For the blank
control group, PBS was used instead of PSMβ3 antibody as described
above.
Statistical analysis
Data were analyzed using SPSS 19.0 statistical
software (Chicago, IL, USA), and quantitative data are presented as
mean ± standard deviation. Comparisons between groups were analyzed
by one-way ANOVA, and pairwise comparisons were analyzed using LSD
or Bonferroni test. Differences were considered to indicate a
statistically significant difference when P<0.05.
Results
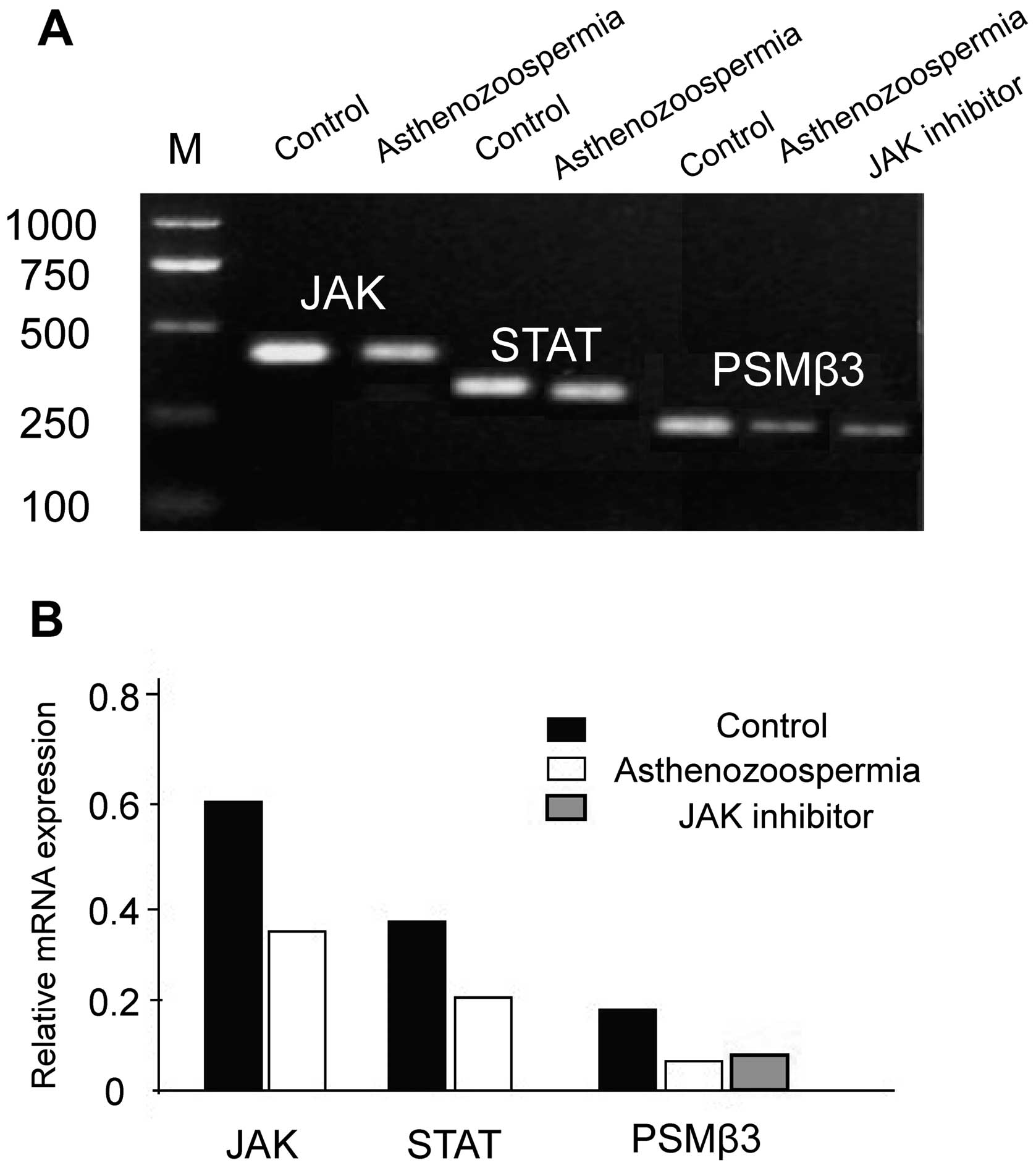
Comparison of mRNA expression
levels
The mRNA levels of JAK, STAT and PSMβ3 in
asthenozoospermia were significantly lower than in the control
group (P<0.05), and the levels of PSMβ3 mRNA in the JAK
inhibitor group were significantly downregulated when compared with
the control non-treated group (P<0.05), which were approximately
equal to the asthenozoospermia group (P>0.05) (Fig. 1).
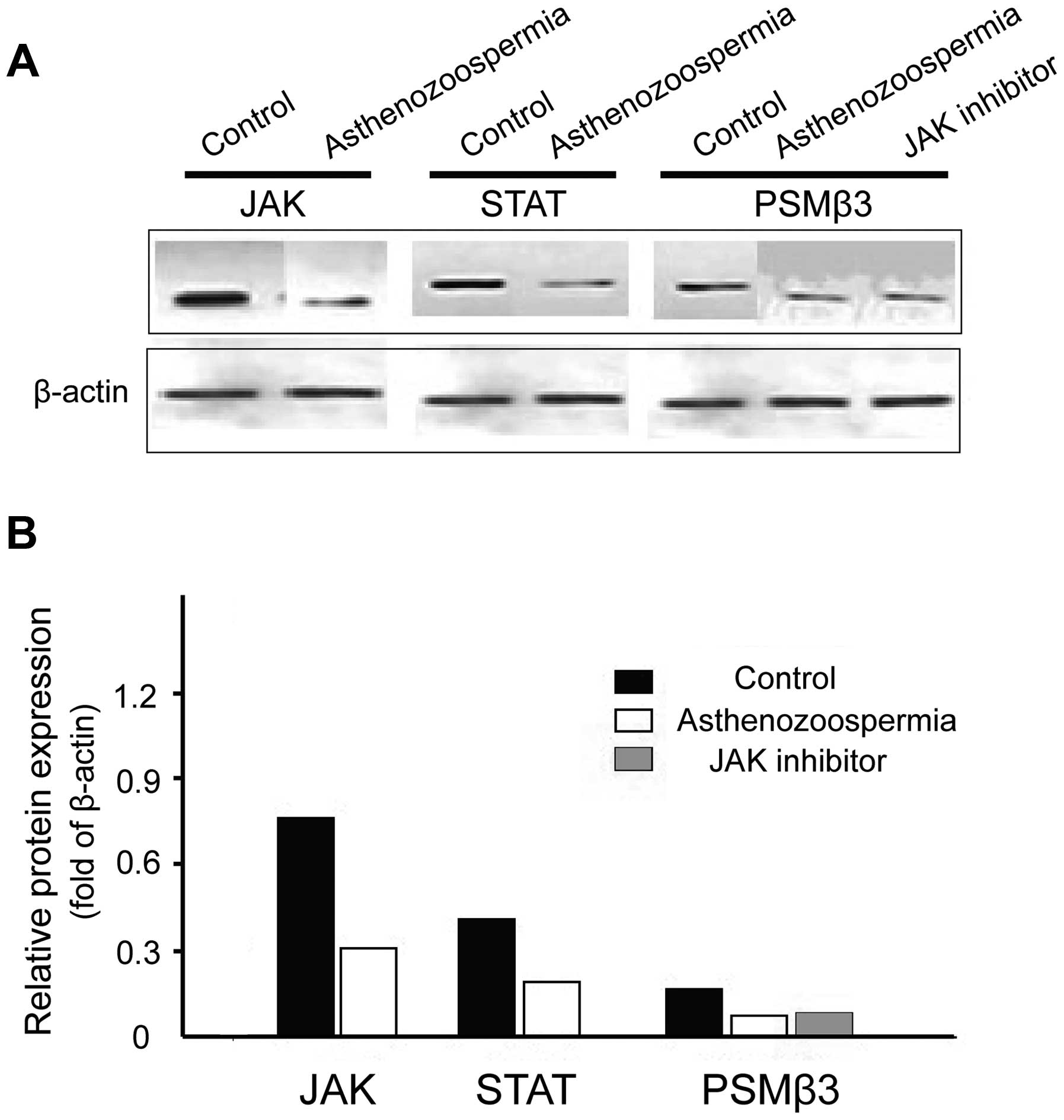
Comparison of protein expression
levels
The protein levels of JAK, STAT and PSMβ3 in
asthenozoospermia were significantly lower than the control group
(P<0.05), and the levels of PSMβ3 protein in the JAK inhibitor
intervention group were significantly lower when compared with the
control non-treated-group (P<0.05), which were approximately
equal to the asthenozoospermia group (P> 0.05) (Fig. 2).
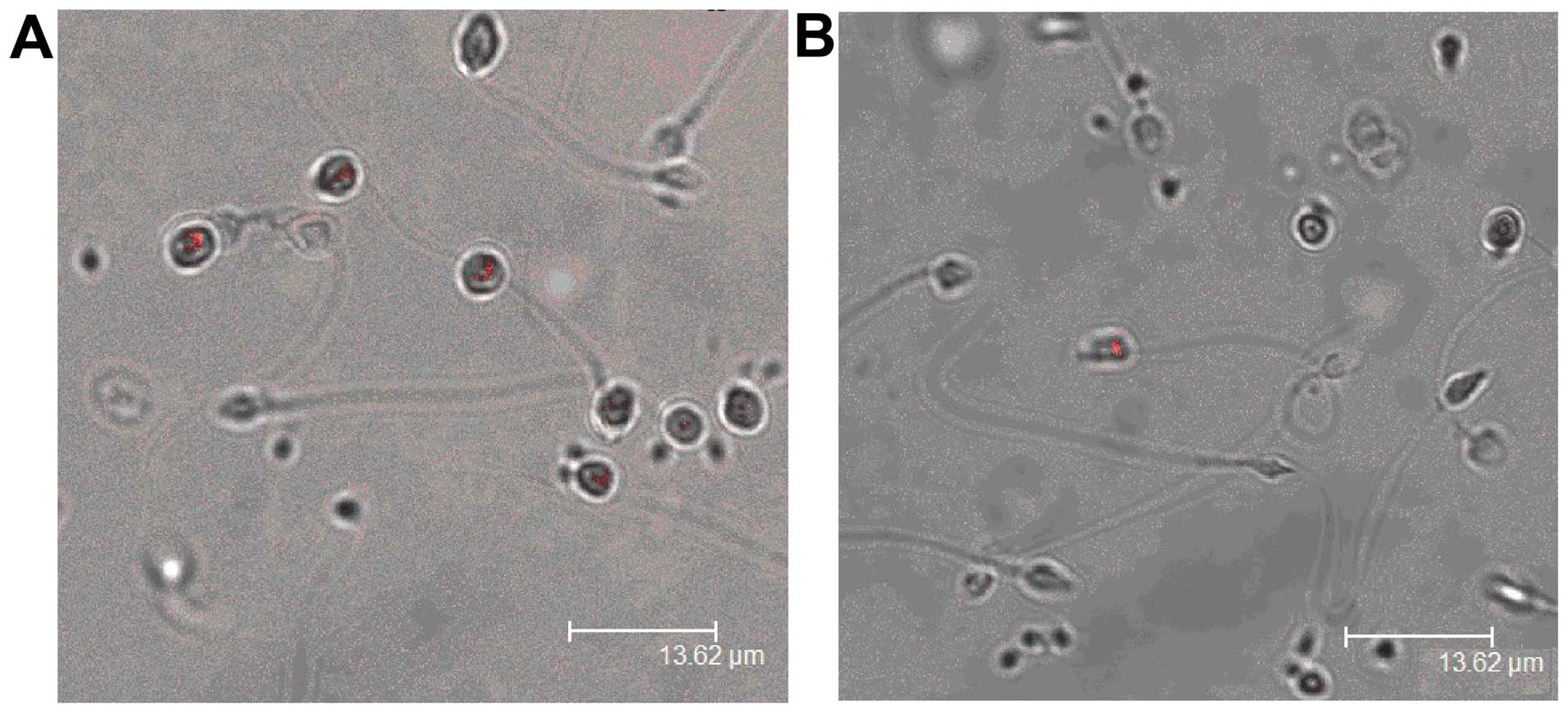
Immunofluorescent staining of
PSMβ3
PSMβ3 was mainly expressed in round-headed sperm,
and less expressed in asthenozoospermia (Fig. 3).
Discussion
JAKs are a family of protein tyrosine kinases that
are involved in cell signaling downstream of cytokine receptors,
which can activate signal transduction and activator of
transcription (STAT) proteins. A previous study reported that the
JAK-STAT pathway was one of the most common signaling pathways
in vivo (7). JAK-STAT
signaling is involved in cell proliferation, differentiation,
survival, apoptosis, mediating immune disorders and tumor
formation. Wawersik et al found that the JAK-STAT pathway
participates in the process of male germ cell proliferation and
differentiation (8). Issigonis et
al reported that the JAK-STAT pathway functions in maintaining
the microenvironment necessary for germ cells to survive (9).
Previous studies have confirmed that the membrane
voltage-dependent Ca2+ channels are involved in the
capacitation of sperm and the acrosome reaction (10). In addition, the
Ca2+-dependent regulation of sperm motility is mainly
mediated by related Ca2+ channels on the sperm cell
membrane. Ca2+ channels are transmembrane protein
complexes, generally composed of four subunits: α1, α2/δ, β and γ.
The most important subunit is α1, which is the infiltration pathway
component of all voltage-gated Ca2+ channels and is also
the binding site of voltage-sensitive, channel-specific drugs and
toxins (11). Mutations in the α1
subunit can lead to reduced sperm motility, causing
asthenozoospermia and resulting in male infertility (12). Other studies found that sperm cation
channel (CatSper) family proteins were specifically expressed on
sperm cell membranes, and played an important role in the
regulation of sperm hyperactivation (13). CatSper channels are known
Ca2+ channels that are expressed in spermatogenic cells
and mature sperm. CatSper1 and CatSper2 are considered to be
necessary for mouse sperm motility and fertility, and their absence
can result in infertility (14).
There are two main modes for Ca2+ transport out of
cells: Sodium-Ca2+ exchangers (NCX) and PMCAs. PMCAs
have a high affinity for Ca2+, but small capacity. PMCAs
are mainly responsible for the fine control of Ca2+
transport and play an important role in cell signal transduction
(15). PMCAs belongs to the p-type
ATPase family and can form a high-energy phosphorylated
intermediate in the reaction cycle. Phosphorylated PMCAs exist in
two conformational states, E1 and E2, each being alternative for
the other (16). PMCAs in mammals
are encoded by four genes that encode PMCA1-4, and are located on
human chromosomes 12, 3, 1 and X. The most important method of
regulating PMCA activity is interaction with calmodulin (17). The combination of calmodulin and PMCA
depends on Ca2+. When Ca2+ is lower than the
Km value needed for the combination of PMCA and calmodulin, PMCA
remains inactivated by calmodulin. Only when Ca2+ is
higher than the Km value, can the two bind with each other in a
Ca2+-dependent manner. PMCAs play an important role in
regulating the spatial and temporal dynamic distribution of
intracellular Ca2+. Furthermore, they participate in
multiple signaling pathways related to intracellular
Ca2+. The interaction between PMCAs and other PDZ
domain-containing proteins is one of the ways by which PMCAs
participate in signal transduction (18).
Research subjects in most related studies are
animals such as mice; thus, it remains to be further analyzed
whether sperm vitality, Ca2+ distribution,
Ca2+ channel status and Ca2+-dependent
regulation of PMCAs apply to humans (19). The present study demonstrates that
the levels of JAK, STAT and PSMβ3 mRNA in asthenozoospermia were
decreased significantly, and the levels of p-JAK, p-STAT and PSMβ3
protein in asthenozoospermia were also reduced. The differences
were statistically significant. The mRNA and protein levels of
PSMβ3 were decreased after the application of a JAK inhibitor in
the control group, and were approximately equal to the
asthenozoospermia group. PSMβ3 was mainly expressed in round-headed
sperm, and less expressed in asthenozoospermia. This finding
suggests that the JAK-STAT/PSMβ3 signal transduction pathway may be
involved in the pathogenic mechanism of asthenozoospermia.
Specifically how the JAK-STAT pathway regulates the expression of
PSMβ3 requires further exploration.
References
|
1
|
Pan T and Huang YH: Ouabain and
asthenospermia. Zhonghua Nan Ke Xue. 21:1129–1133. 2015.(In
Chinese). PubMed/NCBI
|
|
2
|
Shen S, Wang J, Liang J and He D:
Comparative proteomic study between human normal motility sperm and
idiopathic asthenozoospermia. World J Urol. 31:1395–1401. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bader M and Steller H: Regulation of cell
death by the ubiquitin-proteasome system. Curr Opin Cell Biol.
21:878–884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gunaratne HJ, Neill AT and Vacquier VD:
Plasma membrane calcium ATPase is concentrated in the head of sea
urchin spermatozoa. J Cell Physiol. 207:413–419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Segawa K, Kurata S and Nagata S: Human
type IV P-type ATPases that work as plasma membrane phospholipid
flippases and their regulation by caspase and calcium. J Biol Chem.
291:762–772. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding YQ, Jiang H and Wang CL: ERK and
P38MAPK expressions and human sperm motility. Zhonghua Nan Ke Xue.
17:809–812. 2011.(In Chinese). PubMed/NCBI
|
|
7
|
Proia DA, Foley KP, Korbut T, Sang J,
Smith D, Bates RC, Liu Y, Rosenberg AF, Zhou D, Koya K, et al:
Multifaceted intervention by the Hsp90 inhibitor ganetespib
(STA-9090) in cancer cells with activated JAK/STAT signaling. PLoS
One. 6:e185522011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wawersik M, Milutinovich A, Casper AL,
Matunis E, Williams B and Van Doren M: Somatic control of germline
sexual development is mediated by the JAK/STAT pathway. Nature.
436:563–567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Issigonis M, Tulina N, de Cuevas M,
Brawley C, Sandler L and Matunis E: JAK-STAT signal inhibition
regulates competition in the Drosophila testis stem cell niche.
Science. 326:153–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou CX, Zhang YL, Xiao L, Zheng M, Leung
KM, Chan MY, Lo PS, Tsang LL, Wong HY, Ho LS, et al: An
epididymis-specific beta-defensin is important for the initiation
of sperm maturation. Nat Cell Biol. 6:458–464. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Catterall WA, Striessnig J, Snutch TP and
Perez-Reyes E: International Union of Pharmacology: International
Union of Pharmacology. XL. Compendium of voltage-gated ion
channels: calcium channels. Pharmacol Rev. 55:579–581. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang MG and Campbell KP: Gamma subunit of
voltage-activated calcium channels. J Biol Chem. 278:21315–21318.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xia J, Reigada D, Mitchell CH and Ren D:
CATSPER channel-mediated Ca2+ entry into mouse sperm
triggers a tail-to-head propagation. Biol Reprod. 77:551–559. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tamburrino L, Marchiani S, Minetti F,
Forti G, Muratori M and Baldi E: The CatSper calcium channel in
human sperm: relation with motility and involvement in
progesterone-induced acrosome reaction. Hum Reprod. 29:418–428.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andrews RE, Galileo DS and Martin-DeLeon
PA: Plasma membrane Ca2+-ATPase 4: Interaction with
constitutive nitric oxide synthases in human sperm and prostasomes
which carry Ca2+/CaM-dependent serine kinase. Mol Hum
Reprod. 21:832–843. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alexander RT, Beggs MR, Zamani R,
Marcussen N, Frische S and Dimke H: Ultrastructural and
immunohistochemical localization of plasma membrane
Ca2+-ATPase 4 in Ca2+-transporting epithelia.
Am J Physiol Renal Physiol. 309:F604–F616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Strehler EE: Plasma membrane calcium
ATPases: from generic Ca(2+) sump pumps to versatile systems for
fine-tuning cellular Ca(2+). Biochem Biophys Res Commun. 460:26–33.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
DeMarco SJ and Strehler EE: Plasma
membrane Ca2+-ATPase isoforms 2b and 4b interact
promiscuously and selectively with members of the
membrane-associated guanylate kinase family of PDZ (PSD95/Dlg/ZO-1)
domain-containing proteins. J Biol Chem. 276:21594–21600. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pérez-Cerezales S, López-Cardona AP and
Gutiérrez-Adán A: Progesterone effects on mouse sperm kinetics in
conditions of viscosity. Reproduction. 151:501–507. 2016.
View Article : Google Scholar : PubMed/NCBI
|