Introduction
Hypertension is a multifactorial disease associated
with various types of cells and affected by multiple factors
(1,2). It is characterized by elevated arterial
pressure, and may be complicated by damage to the heart, blood
vessels, brain, kidneys, retinas and other target organs, and
metabolic changes in systemic disease (3). Patients with hypertension exhibit an
increased incidence of heart and cerebrovascular disease and
associated mortality; therefore, the ultimate goal of
antihypertensive therapy is to reduce these risks by controlling
blood pressure (4).
In 1970, Ebringer and Doyle reported that 30% of
patients with hypertension exhibited increased serum immunoglobulin
levels (5) and, with the rapid
development of clinical immunology, it has been demonstrated that
hypertension is associated with immune dysfunction, which suggests
that immune factors are associated with the complications of
hypertension (6). Previous studies
have revealed that T cells are able to induce high blood pressure,
vascular disorders and kidney disease and that possible mechanisms
for this may include the release of cytokines, which directly
affect vascular and renal function or indirectly stimulate cells to
release additional cytokines, and induce the infiltration of
inflammatory cells (7,8). The thymus is a primary immune organ, in
which T cells are generated and matured (9); therefore, high blood pressure is likely
to be associated with thymus function. A recent study has revealed
that the thymus atrophies and becomes dysfunctional with age
(10). A thymus transcription
factor, Forkhead box protein N1 (Foxn1) is an important factor for
complete physiological function of the thymus (11). As the thymus atrophies, the
expression of the thymus aging-associated gene Foxn1 decreases,
leading to a downregulation of Foxn1 with age (12). Increased expression of Foxn1 has been
shown to improve thymus function, and potentially promote
regeneration of the thymus (13),
suggesting that Foxn1 may serve a role in high blood pressure. A
previous study reported that atrophy of the thymus was observed in
mice with hypertension (14);
however, the specific changes in thymus function remain to be
elucidated.
The autoimmune regulator (AIRE) is expressed in the
thymus, particularly in thymic medullary epithelial cells. The
expression profile of peripheral tissue antigens is affected by
AIRE (15), and AIRE expression
levels have been shown to have functional effects in the thymus
(16).
The thymus is a vital immune organ, serving an
important role in human health. With increasing age, the thymus
gradually shrinks and its functionality declines or is lost
completely (17). Thymus function
may be used as a proxy to indicate the condition of a patient's
immune system to a certain degree. At the
CD4−CD8−-phase, thymocytes begin the
rearrangement of their T cell receptor (TCR) α chain, and 95% of T
cells are TCRαβ (18). The
signal-joint (sj) TCR excision circle (TREC) is an
extra-chromosomal DNA fragment ring, which is generated when the
TCRδ gene is deleted prior to TCRα gene rearrangement. It is stable
but not replicated, so with increased cell generation and dilution,
the level of sjTREC is highest in T cell populations most recently
generated, and is lower in cell groups after the cell division
cycle (19–21). The prevalence of sjTREC therefore
provides an indication of the number of naive T cells during
functional TCR formation. The presence of naive T cells, which
enable the immune system to recognize all antigens, serves as an
indicator of the generation and output of T cells; therefore, the
level of sjTREC may reflect the functional status of the thymus
(22,23).
To date, research into hypertension has
predominantly focused on the changes in the renin-angiotensin
system (24,25), with less investigation of changes in
immune mechanisms and organs being performed. Investigating the
changes to immune organs in patients with hypertension may identify
a novel target for the treatment of hypertension through immune
function.
Materials and methods
Animals
A total of 60 8-week-old C57BL/6J male mice with a
mean weight of 20 g were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). Animals were housed in a
climate-controlled, light-regulated space with 12-hour day and
night cycles. They were fed a normal mouse chow and water ad
libitum. Animal protocols were approved by the ethics committee of
Second Military Medical University (Shanghai, China), and performed
according to the Guide for the Care and Use of Laboratory Animals
published by the United States National Institutes of Health (NIH
Publication no. 85–23, revised 1996).
Induction of two-kidney, one-clip
hypertension
Following 1 week of acclimation, mice were randomly
divided into the control, sham surgery and two-kidney, one-clip
(HTN) groups (n=20 in each). As previously described (26), the mice in the sham surgery and HTN
groups were anesthetized with sodium pentobarbital (50 mg/kg;
intraperitoneally; cat. no. P3761; Sigma-Aldrich; Merck Millipore,
Darmstadt, Germany) the left kidney was exposed via flank incision,
renal arteries were separated and a silver clip was placed around
the left renal artery. Mice in the sham surgery group underwent an
identical surgical procedure with the exception of the arterial
clip. At 4 weeks post-surgery, 10 mice from each group were fasted
overnight, anesthetized with sodium pentobarbital (50 mg/kg;
intraperitoneally) and sacrificed via carotid puncture. The
remaining mice were fasted overnight and sacrificed as above at 8
weeks post-surgery. Blood samples were harvested from the carotid
artery, and organs of interest (spleen, kidneys and thymus) were
immediately harvested, blotted dry, weighed and stored at −80°C
until required. Blood samples and spleens were prepared as
previously described (27,28), and organ indices were calculated
using the following formula: Organ index=organ weight/body weight
×1,000.
Measurement of blood pressure
The systolic blood pressure (SBP) and diastolic
blood pressure (DBP) of mice in each group were measured weekly
using a non-invasive computerized tail-cuff system (ALC-NIBP;
Shanghai Alcott Biotech Co., Ltd., Shanghai, China). The SBP and
DBP of each mouse were measured three times and the mean was
calculated.
DNA and RNA extraction, and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the thymus of each
mouse using TRIzol reagent. The purity of obtained RNA was
estimated using a NanoDrop 2000c spectrophotometer (Thermo Fisher
Scientific, Inc., Wilmington, DE, USA), and RNA with an A260/A280
ratio >1.8 was used for cDNA synthesis. First strand cDNA
synthesis was performed using the First Strand cDNA Synthesis kit
(cat. no. 6210A; Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. qPCR was performed using SYBR Green PCR
Master Mix iQ (cat. no. 170-8882AP; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and the CFX Connect Real-Time PCR System
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's protocols. qPCR conditions were as follows: 3 min at
95°C, followed by two-step PCR at 95°C for 10 sec and 58°C for 30
sec for 50 cycles, with fluorescence monitoring at the end of each
elongation step. All primers were obtained from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA), and are listed in Table I. Relative mRNA expression of target
genes was calculated using the 2−∆∆Cq method (29). Target sequences were normalized to
β-actin in multiplexed reactions performed in duplicate.
 | Table I.Oligonucleotide primers used for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Oligonucleotide primers used for
reverse transcription-quantitative polymerase chain reaction.
| Gene | Forward | Reverse |
|---|
| m β-actin |
5′-GGTCATCACTATTGGCAACG-3′ |
5′-ACGGATGTCAACGTCACACT-3′ |
| m Foxn1 |
5′-TGACGGAGCACTTCCCTTAC-3′ |
5′-GACAGGTTATGGCGAACAGAA-3′ |
| m AIRE |
5′-GGTTCCTCCCCTTCCATC-3′ |
5′-GGCACACTCATCCTCGTTCT-3′ |
| m sjTREC |
5′-CATTGCCTTTGAACCAAGCTG-3′ |
5′-TTATGCACAGGGTGCAGGTG-3′ |
| m RAG2 |
5′-TGACGTGGTGTATAGTCGA-3′ |
5′-TCCTGAAGTTCTGGGAGA-3′ |
Quantification of TRECs
Genomic DNA was extracted from thymocytes using the
Wizard Genomic DNA Purification kit (cat. no. A1125; Promega
Corporation, Madison, WI, USA). qPCR was performed using a CFX
Connect real-time PCR System (Bio-Rad Laboratories, Inc.) to detect
the number of TRECs. Each amplification was performed using
SYBR-Green PCR Master Mix iQ (cat. no. 170-8882AP; Bio-Rad
Laboratories, Inc.) according to the manufacturer's protocol. The
PCR conditions were as follows: 5 min at 95°C, followed by
three-step PCR: 95°C for 15 sec, 60°C for 40 sec and 72°C for 30
sec for 45 cycles, with fluorescence monitoring at the end of each
elongation step. PCR data was analyzed using SDS software (version
2.2.3; Applied Biosystems; Thermo Fisher Scientific, Inc.). The PCR
product of sjTREC and recombination activating protein 2
(RAG2) sequence was cloned into the PCR3.1 plasmid
(Invitrogen; Thermo Fisher Scientific, Inc.) at known
concentrations: 106, 105, 104,
103 and 102 copies/µl. Target sequences were
normalized to β-actin in multiplexed reactions performed in
duplicate. Each sample was performed in triplicate and the data
expressed as the number of copies of sjTREC/105 cells
based on the mouse genome of 2.6×109 bp (30). All primers were obtained from Thermo
Fisher Scientific, Inc., and primer sequences are displayed in
Table I.
Flow cytometric analysis
Blood cells and splenocytes were incubated with the
appropriate antibodies as previously described (31,32) and
flow cytometry was performed using a FACSCalibur cell analyzer (BD
Biosciences, Franklin Lakes, NJ, USA). Antibodies (all BioLegend,
Inc., San Diego, CA, USA) used are as follows: Alexa Fluor 488
anti-mouse CD3 (cat. no. 100210), PreCP/Cy5.5 anti-mouse CD4 (cat.
no. 100540), Alexa Fluor 647 anti-mouse/human CD44 (cat. no.
103018) and PE anti-mouse CD62 L (cat. no. 104408). All antibodies
were used at a dilution of 1:12. Data were analyzed using FlowJo
software, (version 10.0.7; Tree Star, Inc., Ashland, OR, USA).
Hematoxylin and eosin (H&E)
staining
Standard H&E staining was performed according to
the manufacturer's protocol (Beijing Xin Hua Luyuan Science and
Technology, Ltd., Beijing , China), and viewed under an Olympus
IX51 fluorescence microscope (OLYMPUS CORPORATION, Japan).
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Data analysis was performed using Student's t-test for
comparison between two groups and one-way analysis of variance was
performed to test for differences among multiple groups, followed
by Fisher's least significant difference test using SPSS 19.0 (IBM
SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Blood pressure
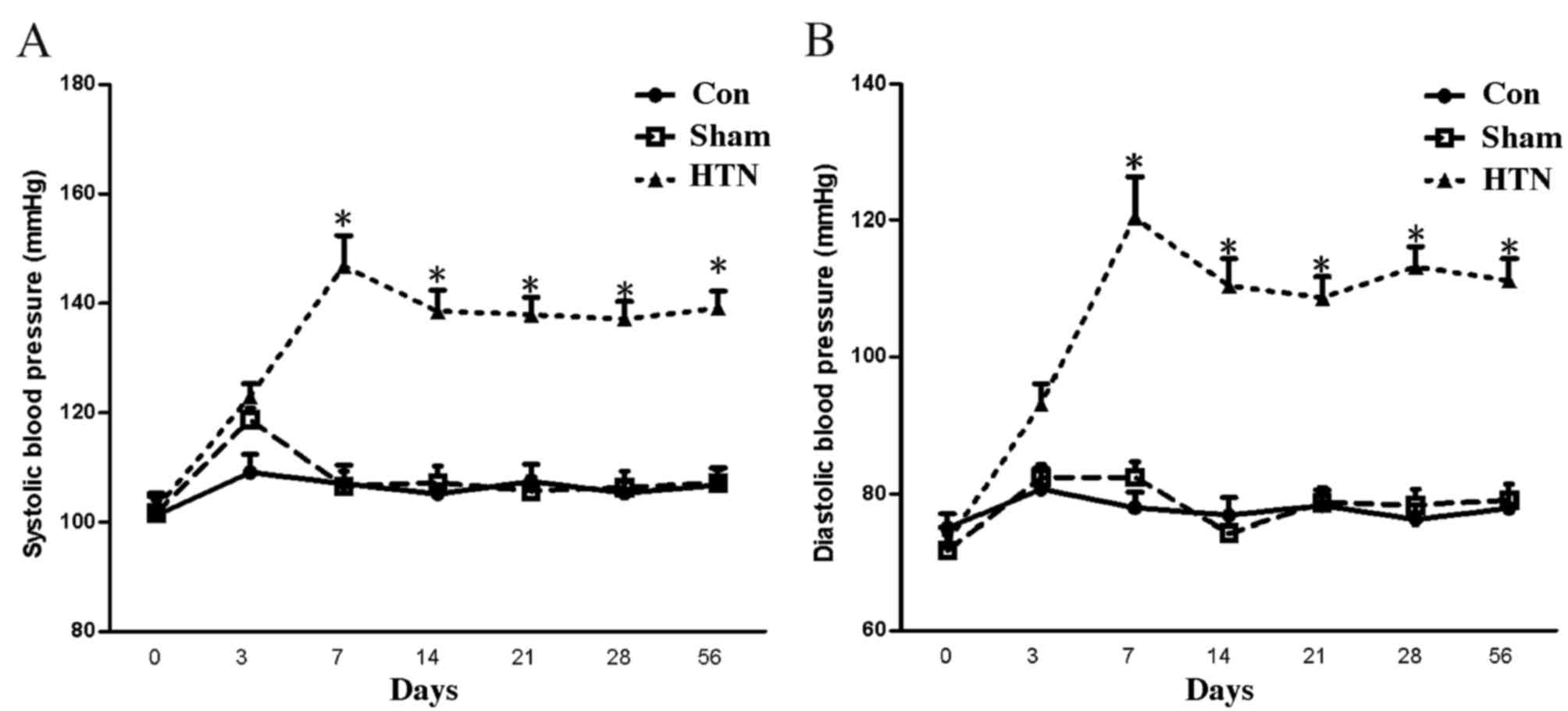
At 4 weeks post-surgery, the SBP and DBP of the HTN
group were significantly increased (P<0.001) compared with those
of the sham and control groups, and reached the hypertension
criteria (SBP >140 mmHg; Fig. 1)
(27), indicating that the
hypertensive models were successfully established.
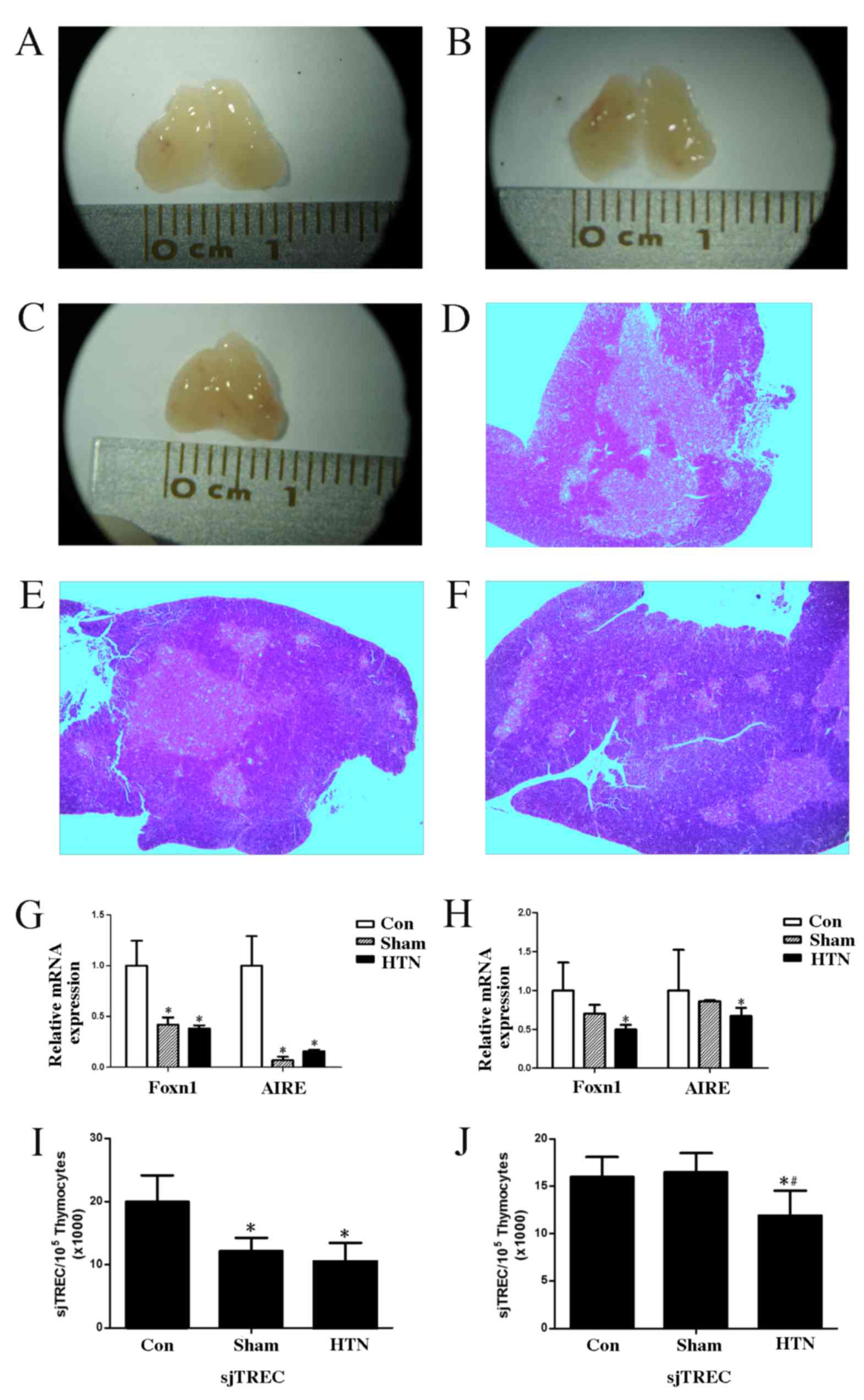
Changes in thymus function
Organs of interest (spleen, kidneys and thymus) were
immediately removed following sacrifice and the organ indices were
calculated. Thymus atrophy was observed in the HTN group as
illustrated in Fig. 2A-C. The thymic
index of the HTN group exhibited no significant differences at 4 or
8 weeks post-surgery compared with the control and sham groups
(Table II).
 | Table II.Body, thymus and spleen weight, and
thymus index of mice. |
Table II.
Body, thymus and spleen weight, and
thymus index of mice.
|
| Control group | Sham group | HTN group |
|---|
|
|
|
|
|
|---|
| Parameters | Wk 4 (n=10) | Wk 8 (n=10) | Wk 4 (n=10) | Wk 8 (n=10) | Wk 4 (n=10) | Wk 8 (n=10) |
|---|
| Body weight, g | 21.11±0.82 | 26.32±3.62 | 20.32±0.76 | 25.67±1.99 | 20.40±1.22 | 26.33±2.17 |
| Thymus weight,
g | 0.043±0.051 | 0.039±0.011 | 0.038±0.0029 | 0.037±0.0061 | 0.041±0.0071 | 0.038±0.008 |
| Spleen weight,
g | 0.079±0.013 | 0.087±0.02 | 0.076±0.0058 | 0.079±0.004 | 0.084±0.0097 | 0.086±0.0082 |
| Thymus index | 1.83±0.475 | 1.588±0.374 | 1.818±0.266 | 1.506±0.22 | 1.834±0.354 | 1.575±0.225 |
H&E staining of the thymus revealed
stereotypical deterioration of the thymic epithelial compartment,
reduced distinction between the cortical and medullary regions and
a reduction in the number of medullary islets per thymic lobe in
the HTN group (Fig. 2D-F).
At 4 and 8 weeks post-surgery, the expression of
Foxn1 and AIRE mRNA in the thymus was significantly downregulated
(4 weeks, Foxn1 and AIRE, P<0.001; 8 weeks, Foxn1 P<0.001 and
AIRE P<0.05) in the HTN group compared with the control group
(Fig. 2G and H). There was no
significant difference in the expression of Foxn1 and AIRE mRNA at
8 weeks post-surgery between the sham and HTN groups; however,
there was a significant difference (P<0.001) between the sham
and control groups at 4 weeks post-surgery. Patterns of sjTREC
expression were similar to those of Foxn1 and AIRE, as shown in
Fig. 2I and J. Expression of sjTREC
in the thymus was significantly downregulated in the HTN group
compared with the control group at 4 and 8 weeks post-surgery (both
P<0.001), and a significant difference (P<0.001) was observed
between the sham and HTN groups at 8 weeks post-surgery.
Furthermore, a significant difference (P<0.001) was observed
between the control and sham groups at 4 weeks post-surgery.
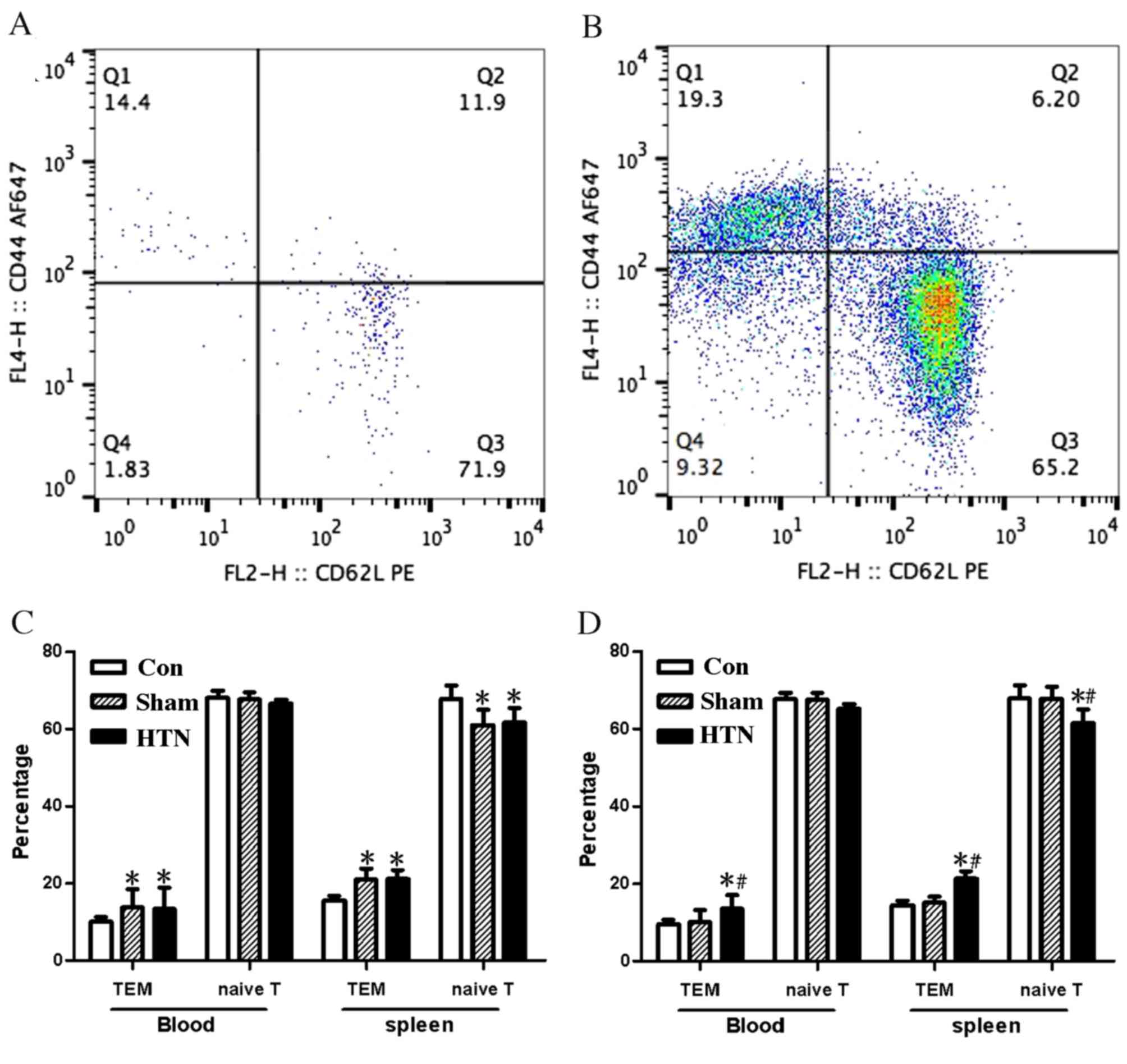
Changes in T cell subsets evaluated by
flow cytometry
Flow cytometric analysis was performed on blood and
splenocytes, as shown in Fig. 3A and
B. Significant differences were observed between the T cell
subset percentages in the blood and splenocytes of the control
group compared with the other groups at 4 weeks post-surgery [blood
and splenocytes, effector memory T cells (TEM) P<0.001;
splenocytes, naïve T cells P<0.05], whereas there was no
significant difference between the HTN and sham groups (Fig. 3C). T cell subsets in the HTN group
were significantly different (blood, TEM P<0.05; splenocytes,
TEM and naïve T cells P<0.001) compared with those in the sham
and control groups, whereas the other two groups exhibited no
significant differences from each other 8 weeks post-surgery
(Fig. 3D).
Discussion
Various cells and influencing factors are associated
with the progression of hypertension. Previous studies have
demonstrated that hypertension is associated with immunity. The
present study assessed the changes in thymus function in mice with
hypertension, and the results demonstrated that thymus
functionality is significantly decreased in mice with hypertension,
suggesting that the immune system is associated with the process of
hypertension. Therefore, treating the thymus may improve
functionality, promote immune system function and ameliorate
hypertension, potentially providing a novel therapeutic target for
the treatment of hypertension.
The present study demonstrated that mice with
hypertension exhibited had decreased immune function due to atrophy
of the thymus, based on observations of the gross thymus, thymic
pathology slices, the thymus index and expression of the
anti-thymus aging related gene Foxn1.
As the development and maturation of T cell occurs
in the thymus, changes to the T cell subsets are also indicative of
abnormal thymus function (33–35).
sjTREC may be used to evaluate the output of naive T cells and
thereby serve as a proxy for thymus output function, indirectly
demonstrating thymus dysfunction (36–38).
Hypertension is acknowledged to be a chronic disease (39,40);
however, in the present study hypertension was induced in mice via
the two-kidney one-clip method only 4 or 8 weeks prior to
sacrifice, which may explain why the thymic index showed no
significant differences between the three groups. In future
research, it may be better to allow a longer period of time between
surgery and sacrifice to investigate whether the thymic index is
significantly altered.
Previous studies have demonstrated that Foxn1 is
exclusively expressed in the thymus (41–43).
Increasing Foxn1 expression may induce partial or total recovery of
thymus function (13); therefore,
increasing the expression of Foxn1 may raise the cure rate and
provide new diagnostic methods for hypertension and other
immune-related diseases, such as asthenic bulbar paralysis.
A number of studies have suggested that stress may
induce a decline in thymus function (44–46), by
demonstrating that stress induces a transient reduction in thymus
functionality; however, when individuals recover to a non-stressed
state, thymus function is restored. However, these studies did not
explore the mechanisms responsible for the transient effects of
stress on thymus function. This may be associated with the increase
of angiotensin, renin angiotensin and thyroid hormone, and may also
be associated with stimulation of sympathetic nerves. Further
research is required to confirm this hypothesis.
In the present study, the two-kidney, one-clip model
of hypertension was used, which is secondary renal hypertension;
therefore the results demonstrate that the immune system may be
associated with the pathological process of renal hypertension.
However, it remains to be determined whether the immune system has
an association with primary hypertension and other types of
secondary hypertension.
In conclusion, in vivo experiments on mice
with hypertension suggest that there may be a correlation between
hypertension and immune dysfunction. Improving immune system or
thymus function may enhance the therapeutic effects of treatments
for hypertension, which may provide a novel therapeutic target and
treatment concept for treating hypertension, and potentially lead
to the development of an anti-hypertension vaccine.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81130065, 81072981,
30971101, 31171130 and 30900528), the Shanghai Pujiang Talent
Program (D-15), the Shanghai Key Basic Research Program (grant no.
10411956,500), and the Shanghai Project of International
Cooperation and Exchange (grant no. 10410701700).
References
|
1
|
Vaziri ND, Wang XQ, Oveisi F and Rad B:
Induction of oxidative stress by glutathione depletion causes
severe hypertension in normal rats. Hypertension. 36:142–146. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vinh A, Chen W, Blinder Y, Weiss D, Taylor
WR, Goronzy JJ, Weyand CM, Harrison DG and Guzik TJ: Inhibition and
genetic ablation of the B7/CD28 T cell costimulation axis prevents
experimental hypertension. Ccirculation. 122:2529–2537. 2010.
View Article : Google Scholar
|
|
3
|
Cavasin MA, Liao TD, Yang XP, Yang JJ and
Carretero OA: Decreased endogenous levels of Ac-SDKP promote organ
fibrosis. Hypertension. 50:130–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Neutel JM, Giles T, Punzi H, Weiss RJ, Li
H and Finck A: Long-term safety of nebivolol and valsartan
combination therapy in patients with hypertension: An open-label,
single-arm, multicenter study. J Am Soc Hypertens. 8:915–920. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ebringer A and Doyle AE: Raised serum IgG
levels in hypertension. Br Med J. 2:146–148. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Ciuceis C, Rossini C, La Boria E,
Porteri E, Petroboni B, Gavazzi A, Sarkar A, Rosei EA and Rizzoni
D: Immune Mechanisms in Hypertension. High Blood Press Cardiovasc
Prev. 21:227–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leibowitz A and Schiffrin EL: Immune
mechanisms in hypertension. Curr Hypertens Rep. 13:465–472. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sonmez A, Kisa U, Uckaya G, Eyileten T,
Comert B, Koc B, Kocabalkan F and Ozata M: Effects of losartan
treatment on T-cell activities and plasma leptin concentrations in
primary hypertension. J Renin Angiotensin Aldosterone Syst.
2:112–116. 2001.PubMed/NCBI
|
|
9
|
Walters SN, Webster KE, Daley S and Grey
ST: A role for intrathymic B cells in the generation of natural
regulatory T cells. J Immunol. 193:170–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ruan L, Zhang Z, Mu L, Burnley P, Wang L,
Coder B, Zhuge Q and Su DM: Biological significance of FoxN1
gain-of-function mutations during T and B lymphopoiesis in juvenile
mice. Cell Death Dis. 5:e14572014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bredenkamp N, Ulyanchenko S, O'Neill KE,
Manley NR, Vaidya HJ and Blackburn CC: An organized and functional
thymus generated from FOXN1-reprogrammed fibroblasts. Nat Cell
Biol. 16:902–908. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zook EC, Krishack PA, Zhang S, Zeleznik-Le
NJ, Firulli AB, Witte PL and Le PT: Overexpression of Foxn1
attenuates age-associated thymic involution and prevents the
expansion of peripheral CD4 memory T cells. Blood. 118:5723–5731.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bredenkamp N, Nowell CS and Blackburn CC:
Regeneration of the aged thymus by a single transcription factor.
Development. 141:1627–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukuda S, Tsuchikura S and Iida H:
Age-related changes in blood pressure, hematological values,
concentrations of serum biochemical constituents and weights of
organs in the SHR/Izm, SHRSP/Izm and WKY/Izm. Exp Anim. 53:67–72.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lovewell TR, McDonagh AJ, Messenger AG,
Azzouz M and Tazi-Ahnini R: The AIRE-230Y Polymorphism affects aire
transcriptional activity: Potential influence on aire function in
the thymus. PLoS One. 10:e01274762015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liston A, Gray DH, Lesage S, Fletcher AL,
Wilson J, Webster KE, Scott HS, Boyd RL, Peltonen L and Goodnow CC:
Gene dosage-limiting role of Aire in thymic expression, clonal
deletion, and organ-specific autoimmunity. J Exp Med.
200:1015–1026. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Berthiaume F, Aparicio CL, Eungdamrong J
and Yarmush ML: Age- and disease-related decline in immune
function: An opportunity for ‘thymus-boosting’ therapies. Tissue
Eng. 5:499–514. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lang PO, Govind S, Dramé M and Aspinall R:
Measuring the TREC ratio in dried blood spot samples: Intra- and
inter-filter paper cards reproducibility. J Immunol Methods.
389:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lynch HE and Sempowski GD: Molecular
measurement of T cell receptor excision circles. Methods Mol Biol.
979:147–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hug A, Korporal M, Schröder I, Haas J,
Glatz K, Storch-Hagenlocher B and Wildemann B: Thymic export
function and T cell homeostasis in patients with relapsing
remitting multiple sclerosis. J Immunol. 171:432–437. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sodora DL, Douek DC, Silvestri G,
Montgomery L, Rosenzweig M, Igarashi T, Bernacky B, Johnson RP,
Feinberg MB, Martin MA and Koup RA: Quantification of thymic
function by measuring T cell receptor excision circles within
peripheral blood and lymphoid tissues in monkeys. Eur J Immunol.
30:1145–1153. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiarini M, Sottini A, Bertoli D, Serana
F, Caimi L, Rasia S, Capra R and Imberti L: Newly produced T and B
lymphocytes and T-cell receptor repertoire diversity are reduced in
peripheral blood of fingolimod-treated multiple sclerosis patients.
Mult Scler. 21:726–734. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Al-Harthi L, Marchetti G, Steffens CM,
Poulin J, Sékaly R and Landay A: Detection of T cell receptor
circles (TRECs) as biomarkers for de novo T cell synthesis using a
quantitative polymerase chain reaction-enzyme linked immunosorbent
assay (PCR-ELISA). J Immunol Methods. 237:187–197. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiménez PM, Conde C, Casanegra A, Romero
C, Tabares AH and Orías M: Association of ACE genotype and
predominantly diastolic hypertension: A preliminary study. J Renin
Angiotensin Aldosterone Syst. 8:42–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campagnaro BP, Gava AL, Meyrelles SS and
Vasquez EC: Cardiac-autonomic imbalance and baroreflex dysfunction
in the renovascular Angiotensin-dependent hypertensive mouse. Int J
Hypertens. 2012:9681232012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou YB, Sun HJ, Chen D, Liu TY, Han Y,
Wang JJ, Tang CS, Kang YM and Zhu GQ: Intermedin in paraventricular
nucleus attenuates sympathetic activity and blood pressure via
nitric oxide in hypertensive rats. Hypertension. 63:330–337. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wei Z, Spizzo I, Diep H, Drummond GR,
Widdop RE and Vinh A: Differential phenotypes of
tissue-infiltrating T cells during angiotensin II-induced
hypertension in Mice. PLoS One. 9:e1148952014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guzik TJ, Hoch NE, Brown KA, McCann LA,
Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG: Role of
the T cell in the genesis of angiotensin II-induced hypertension
and vascular dysfunction. J Exp Med. 204:2449–2460. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-tie quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ortman CL, Dittmar KA, Witte PL and Le PT:
Molecular characterization of the mouse involuted thymus:
Aberrations in expression of transcription regulators in thymocyte
and epithelial compartments. Int Immunol. 14:813–822. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McDonald CA, Payne NL, Sun G, Moussa L,
Siatskas C, Lim R, Wallace EM, Jenkin G and Bernard CC:
Immuno-suppressive potential of human amnion epithelial cells in
the treatment of experimental autoimmune encephalomyelitis. J
Neuroinflammation. 12:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ammirati E, Cianflone D, Vecchio V, Banfi
M, Vermi AC, De Metrio M, Grigore L, Pellegatta F, Pirillo A,
Garlaschelli K, et al: Effector memory t cells are associated with
atherosclerosis in humans and animal models. J Am Heart Assoc.
1:27–41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bernardi AI, Andersson A, Stubelius A,
Grahnemo L, Carlsten H and Islander U: Selective estrogen receptor
modulators in T cell development and T cell dependent inflammation.
Immunobiology. 220:1122–1128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Petrie HT: Cell migration and the control
of post-natal T-cell lymphopoiesis in the thymus. Nat Rev Immunol.
3:859–866. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klausmann S, Sydler T, Summerfield A,
Lewis FI, Weilenmann R, Sidler X and Brugnera E: T-cell
reprogramming through targeted CD4-coreceptor and T-cell receptor
expression on maturing thymocytes by latent Circoviridae family
member porcine circovirus type 2 cell infections in the thymus.
Emerg Microbes Infect. 4:e152015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ringhoffer S, Rojewski M, Döhner H, Bunjes
D and Ringhoffer M: T-cell reconstitution after allogeneic stem
cell transplantation: Assessment by measurement of the sjTREC/βTREC
ratio and thymic naïve T cells. Haematologica. 98:1600–1608. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ou XL, Gao J, Wang H, Wang HS, Lu HL and
Sun HY: Predicting human age with bloodstains by sjTREC
quantification. PLoS One. 7:e424122012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Douek DC, McFarland RD, Keiser PH, Gage
EA, Massey JM, Haynes BF, Polis MA, Haase AT, Feinberg MB, Sullivan
JL, et al: Changes in thymic function with age and during the
treatment of HIV infection. Nature. 396:690–695. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Feng YJ, Wang HC, Li YC and Zhao WH:
Hypertension screening and follow-up management by primary health
care system among chinese population aged 35 years and above.
Biomed Environ Sci. 28:330–340. 2015.PubMed/NCBI
|
|
40
|
Yang PR, Shih WT, Chu YH, Chen PC and Wu
CY: Frequency and co-prescription pattern of Chinese herbal
products for hypertension in Taiwan: A Cohort study. BMC Complement
Altern Med. 15:1632015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Romano R, Palamaro L, Fusco A, Giardino G,
Gallo V, Del Vecchio L and Pignata C: FOXN1: A master regulator
gene of thymic epithelial development program. Front Immunol.
4:1872013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Romano R, Palamaro L, Fusco A, Iannace L,
Maio S, Vigliano I, Giardino G and Pignata C: From murine to human
nude/SCID: the thymus, T-cell development and the missing link.
Clin Dev Immunol. 2012:4671012012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jin X, Nowell CS, Ulyanchenko S, Stenhouse
FH and Blackburn CC: Long-Term Persistence of functional thymic
epithelial progenitor cells in vivo under conditions of low FOXN1
expression. PLoS One. 9:e1148422014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Misa-Agustiño MJ, Leiro-Vidal JM,
Gomez-Amoza JL, Jorge-Mora MT, Jorge-Barreiro FJ, Salas-Sánchez AA,
Ares-Pena FJ and López-Martín E: EMF radiation at 2450 MHz triggers
changes in the morphology and expression of heat shock proteins and
glucocorticoid receptors in rat thymus. Life Sci. 127:1–11. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gupta S, Haldar C and Ahmad R:
Photoperiodic regulation of nuclear melatonin receptor RORα in
lymphoid organs of a tropical rodent Funambulus pennanti: Role in
seasonal oxidative stress. J Photochem Photobiol B. 142:141–153.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Novoselova EG, Lunin SM, Khrenov MO,
Parfenyuk SB, Novoselova TV, Shenkman BS and Fesenko EE: Changes in
immune cell signalling, apoptosis and stress response functions in
mice returned from the BION-M1 mission in space. Immunobiology.
220:500–509. 2015. View Article : Google Scholar : PubMed/NCBI
|