Introduction
Acute respiratory distress syndrome (ARDS) is a
severe and life-threatening medical condition that is common in
critically ill patients and has a high mortality rate (1). It is a main reason for acute
respiratory failure, and is characterized by widespread
inflammation in the lungs (1,2). ARDS
can induce pathophysiological mechanisms of alveolar collapse,
hyoxemia, vascular dysfunction and elevated dead space fraction
[the ratio of dead space volume to tidal volume (VD/VT)] (3,4).
Currently, the lung-protection strategy for
ventilation involves the use of high positive end-expiratory
pressure (PEEP) levels combined with low tidal volumes to prevent
end expiratory alveolar collapse, increase functional residual
capacity, reduce VD/VT and attenuate hypoxemia (5,6).
However, the application of higher levels of PEEP may not be
necessarily beneficial, since it increases the inflation of lung
regions. Additionally, it will also increase the risk of
hemodynamic abnormalities as well as the lung injury induced by
ventilation (7,8). Numerous studies have attempted to
define the optimal PEEP level on the basis of a variety of methods
during a recruitment maneuver (RM) with decreasing PEEP (9–11).
A number of studies have applied the VD/VT method to
assess the effects of lung recruitment and PEEP titration in
patients with severe ARDS (9–11). VD/VT
is a specific value based on the relatively high diffusibility of
CO2 across tissue membranes (12), and the exchange of CO2
depends strictly on alveolar ventilation volume (13). However, some studies did not find a
similar effect on VD/VT during PEEP titration (14,15).
Therefore, the application of the lowest VD/VT method to titrate
the optimal PEEP in patients with ARDS remains to be
investigated.
In the present study, an oleic acid lung-injury
model in swine was used to evaluate the effect of varying the PEEP
level on dead space fraction. The aim was to realize the changes in
VD/VT induced by different PEEP levels in the ARDS swine model and
to explore the feasibility of using the VD/VT ratio to guide the
optimal PEEP titration.
Materials and methods
Animals and anesthesia
The study was a prospective, sham-controlled and
in vivo animal study, and was approved by the animal ethics
committee of Beijing Shijitan Hospital, affiliated to Capital
Medical University (Beijing, China).
Twelve healthy male swine (age, 11–13 months) with
an average weight of 39.13±3.27 kg were provided by the animal
center of Pinggu Hospital of Capital Medical University [licence:
SYXK (B) 2010–0016]. The animals were housed at 21–27°C with a
humidity of 45–55%, with free access to food and water. Swine were
fasted for 24 h and then were orotracheally intubated in the supine
position during deep intramuscular anesthesia with ketamine (35
mg/kg; Jiangsu Hengrui Medicine Co. Ltd., Lianyungang, China), 3%
pentobarbital sodium (30 mg/kg; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and diazepam (1.5 mg/kg; Sigma-Aldrich). A
double cavity central venous catheter was inserted into the right
internal jugular vein using the Seldinger technique (16) and connected to the monitoring system.
Following line placement, the anesthetic was switched to total
intravenous anesthesia with continuous infusion of pentobarbital
sodium (2 mg/kg/h), ketamine (3 mg/kg/h) and pipecurium bromide
(0.03 mg/kg/h; Gedeon Richter Plc., Budapest, Hungary). In all
swine, a 4-French gauge arterial thermodilution catheter was
inserted via the left femoral artery. The arterial catheter was
connected to a computer for pulse contour analysis (Pulsion Medical
Systems, Munich, Germany) for the clinical monitoring of
hemodynamic measurements.
Monitoring
The respiration parameters of alveolar partial
pressure of O2 (PAO2)/fraction of inspiration
O2 (FiO2) ratio (P/F), arterial
CO2 partial pressure (PaCO2) and arterial
O2 saturation (SaO2) were directly measured
through arterial blood gas analysis using the (GEM Premier 3000;
Instrumentation Laboratory, Inc., Lexington MA, USA) (17). Lung maximum dynamic tidal respiratory
compliance (Cdyn) was monitored using a SERVI-i ventilator (Siemens
Maquet Critical Care AB, Slona, Sweden).
The VD/VT ratio was measured via the single-breath
analysis of CO2 (18)
(NICO Cardiopulmonary Management System; Novametrix; Philips
Respironics, Murrysville, PA, USA). With this method, the partial
pressure of mixed-expired CO2 was calculated followed by
the Enghoff modification of the Bohr equation as follows:
VD/VT=(PaCO2-PeCO2)/PaCO2, where
PeCO2 represents mixed expired CO2 (19). An arterial blood gas sample was
obtained when the PeCO2 variability on the NICO monitor
was ≤1 mmHg within 5 min. The NICO sensor fitted between the
Y-piece and the endotracheal tube.
The intrapulmonary shunt fraction (Qs/Qt) was
calculated according to the standard formulae:
Qs/Qt = (CcO2 -
CaO2)/(CcO2 - CvO2)
CaO2 = Hb × SaO2 × 1.34 +
PAO2 × 0.0031
CvO2 = Hb × SvO2 × 1.34 +
PvO2 × 0.0031
CcO2 = Hb × ScO2 × 1.34 +
PcO2 × 0.0031
ScO2 ≈ 100%
PcO2 ≈ PAO2
PAO2 = PiO2 -
PaCO2/R
PiO2 = (PB - PH2O) ×
FiO2
where Qs represents shunted pulmonary blood flow; Qt
represents total pulmonary blood flow; CcO2 represents
pulmonary capillary O2 content; PvO2 represents mixed
venous O2 partial pressure; CaO2 represents arterial
O2 content; CvO2 represents mixed venous
O2 content; Hb represents hemoglobin; PiO2
represents partial pressure of inspired O2; R represents
respiratory quotient (0.8); PB represents barometric pressure (~100
kPa on sea level); PH2O represents saturation vapor
pressure (6.3 kPa at 37°C).
The hemodynamic parameters of cardiac output index
(CI), global end-diastolic volume index (GEDI), extravascular lung
water index (ELWI), intra-thoracic blood volume index (ITBI) and
systemic vascular resistance index (SVRI) were directly measured by
the thermodilution method (20)
using the PICCO system (Pulsion Medical Systems). The central
venous pressure (CVP) was monitored with the central venous
catheter in the right internal jugular vein.
Protocol
Following intubation, lungs were ventilated in a
volume-controlled ventilation mode, with the following initial
parameters: VT of 8 ml/kg, FiO2 of 1.0, PEEP of 5 cm
H2O, respiratory rate of 40 breaths/min, and inspiratory
to expiratory time ratio (I:E) of 1:2. These settings were
maintained for 30 min to achieve stabilization.
ARDS induction
After recording pre-injury hemodynamic, gas
exchange, respiratory mechanics measurements and oxygen metabolism,
0.2 ml/kg oleic acid (Sigma-Aldrich) in 40 ml saline was slowly
(within 15 min) injected in the right atrium via the central venous
catheter. After a 90-min injury stabilization period, the
experimental protocol was initiated. A successful model of ARDS was
defined by P/F <200 mmHg for 90 min following oleic acid
infusion (21). Each swine was
infused continuously with intravenous saline at a rate of 100
ml/h.
Lung recruitment maneuver
The swine were stabilized for 15 min on the
following ventilator settings and followed by data gathering:
Pressure control ventilation (PCV) peak pressure, 35 cm
H2O; PEEP, 20 cm H2O; inspiratory time, 0.6
sec; rate, 40/min and FiO2 1.0. The PEEP was then set to
20 cm H2O and pressure control set to a peak airway
pressure of 40 cm H2O. These settings were maintained
for 2 min, followed by a 15-min stabilization period with a peak
pressure 35 cm H2O. Data were gathered if
PAO2 + PaCO2 was >400 mmHg. If
PAO2 + PaCO2 was <400 mmHg, the PEEP
setting remained unchanged and pressure control was increased to
obtain a peak airway pressure of 45 cm H2O. This pattern
was sustained for 2 min, followed by a 15-min stabilization period
with peak pressure 35 cm H2O. If PAO2 +
PaCO2 was >400 mmHg, the lung recruitment was
considered complete (22,23).
PEEP titration
When the sum of PAO2 and PaCO2
was >400 mmHg, all swine underwent a decremental PEEP titration
in volume control mode. PEEP was decreased in 2 cm H2O
steps (from 20 to 0 cm H2O) and was maintained at each
level for 10 min. Cdyn was measured at each step using a VT of 8
ml/kg and a frequency of 40/min. Additionally, the physiological
data including Cdyn, VD/VT and P/F were gathered following each
step. The optimal open-lung PEEP was identified by the lowest VD/VT
method, which was achieved as determined by a reduction in VD/VT
and then a rise with each PEEP step.
The study consisted of the following seven
experimental periods: i) Pre-injury period, which involved
introduction of catheters and mechanical ventilation using the
initial parameters; ii) injury period, when ARDS was induced by the
intravenous administration of oleic acid; iii) PEEP period 1
(Po-4), 4 cm H2O below the optimal PEEP; iv) PEEP period
2 (Po-2), 2 cm H2O below the optimal PEEP; v) PEEP
period 3 (Po), optimal PEEP; vi) PEEP period 4 (Po+2), 2 cm
H2O above the optimal PEEP; vii) PEEP period 5 (Po+4). 4
cm H2O above the optimal PEEP.
Statistical analysis
All data were analyzed using IBM-SPSS version 19.0
statistics software (IBM SPSS, Armonk, NY, USA) and were expressed
as mean ± standard deviation. Analysis of non-parametric
repeated-measures ANOVA test was used for comparison of all
variables collected during the seven assessment periods. P<0.05
was considered to indicate a statistically significant
difference.
Results
Optimal PEEP
The optimal PEEP identified by the lowest VD/VT
method was 13.25±1.36 cm H2O.
VD/VT changes induced by different
PEEP levels
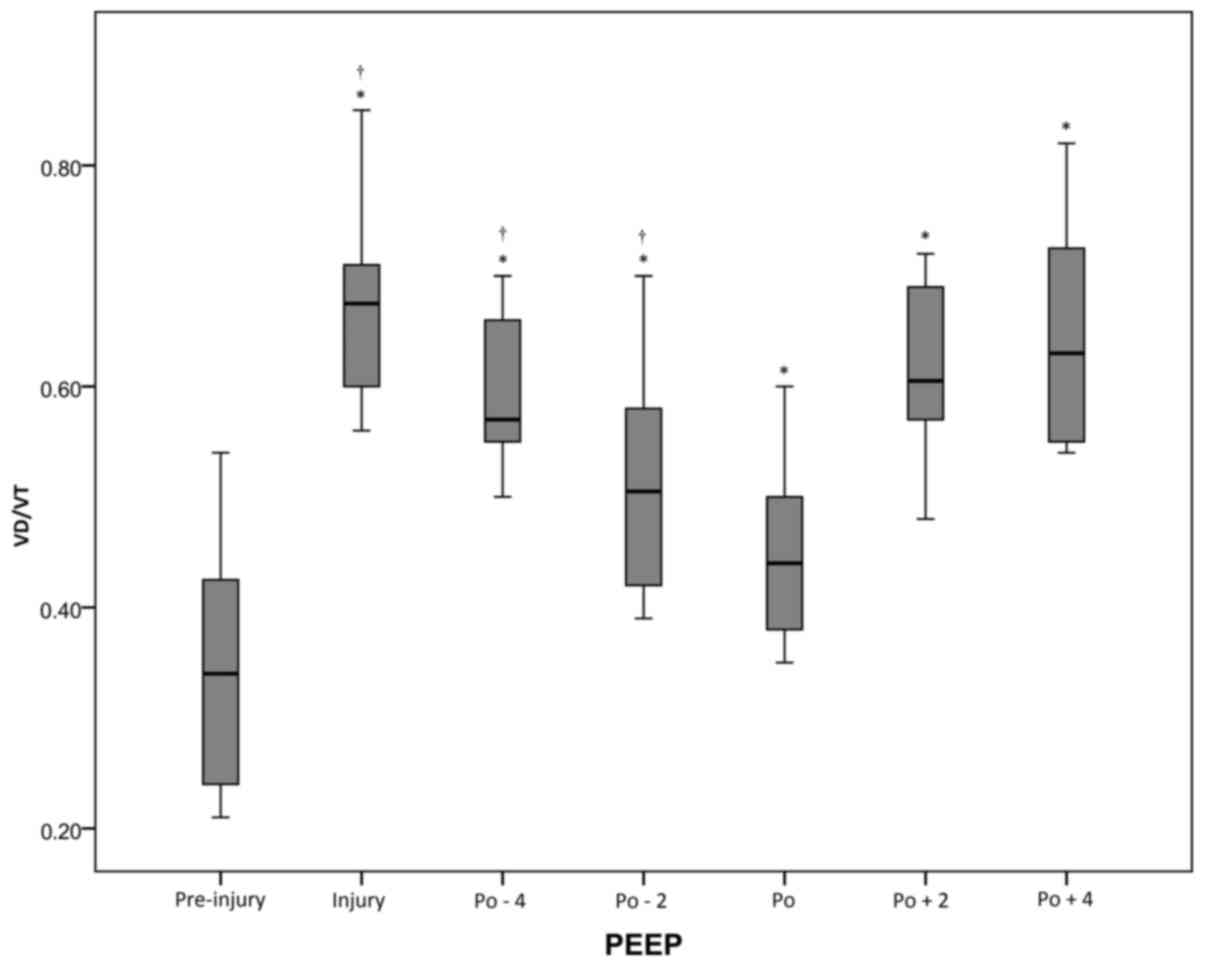
There was a significant (P<0.05) increase in
VD/VT from the pre-injury period (0.35±0.11) to the injury period
(0.68±0.10). Following the RM, VD/VT decreased to the lowest value
of 0.44±0.08 (vs. injury, P<0.05) at the optimal PEEP. When PEEP
decreased to Po-4 cm H2O, VD/VT significantly increased
to 0.60±0.07 (P<0.05). However, at the Po+4 cm H2O,
VD/VT was higher (0.64±0.10; Fig. 1
and Table I).
 | Table I.Respiration parameters of the acute
respiratory distress syndrome swine model under different
conditions (mean ± standard deviation). |
Table I.
Respiration parameters of the acute
respiratory distress syndrome swine model under different
conditions (mean ± standard deviation).
| Parameter | Pre-injury | Injury | Po-4 | Po-2 | Po | Po+2 | Po+4 |
|---|
| VD/VT | 0.35±0.11 |
0.68±0.10a |
0.60±0.07a,b |
0.52±0.11a,b |
0.44±0.08a,b |
0.61±0.08a |
0.64±0.10a |
| PaCO2
(mmHg) | 34.80±6.73 |
43.81±8.02a |
47.73±10.33a |
49.05±12.47a |
49.19±11.82a |
47.71±12.28a |
47.62±12.89a |
| SaO2
(%) | 99.86±0.05 |
88.40±11.25a |
96.79±1.52b |
98.58±0.48b |
98.75±2.55b |
97.78±0.96b |
96.34±2.50b |
| Qs/Qt (%) | 1.88±1.14 |
21.06±15.62a |
7.50±4.14a,b |
5.01±1.53b |
2.77±2.53b |
6.68±2.86b |
9.55±5.85a,b |
| P/F (mmHg) | 562±162 | 75±21a |
166±109a | 291±62a,b |
342±144a,b |
365±133a,b |
294±170a,b |
| Cdyn (ml/cm
H2O) | 38.17±6.97 |
15.17±5.37a |
17.83±3.81a |
18.00±4.97a |
20.67±5.58a,b |
19.33±4.44a,b |
17.50±3.34a |
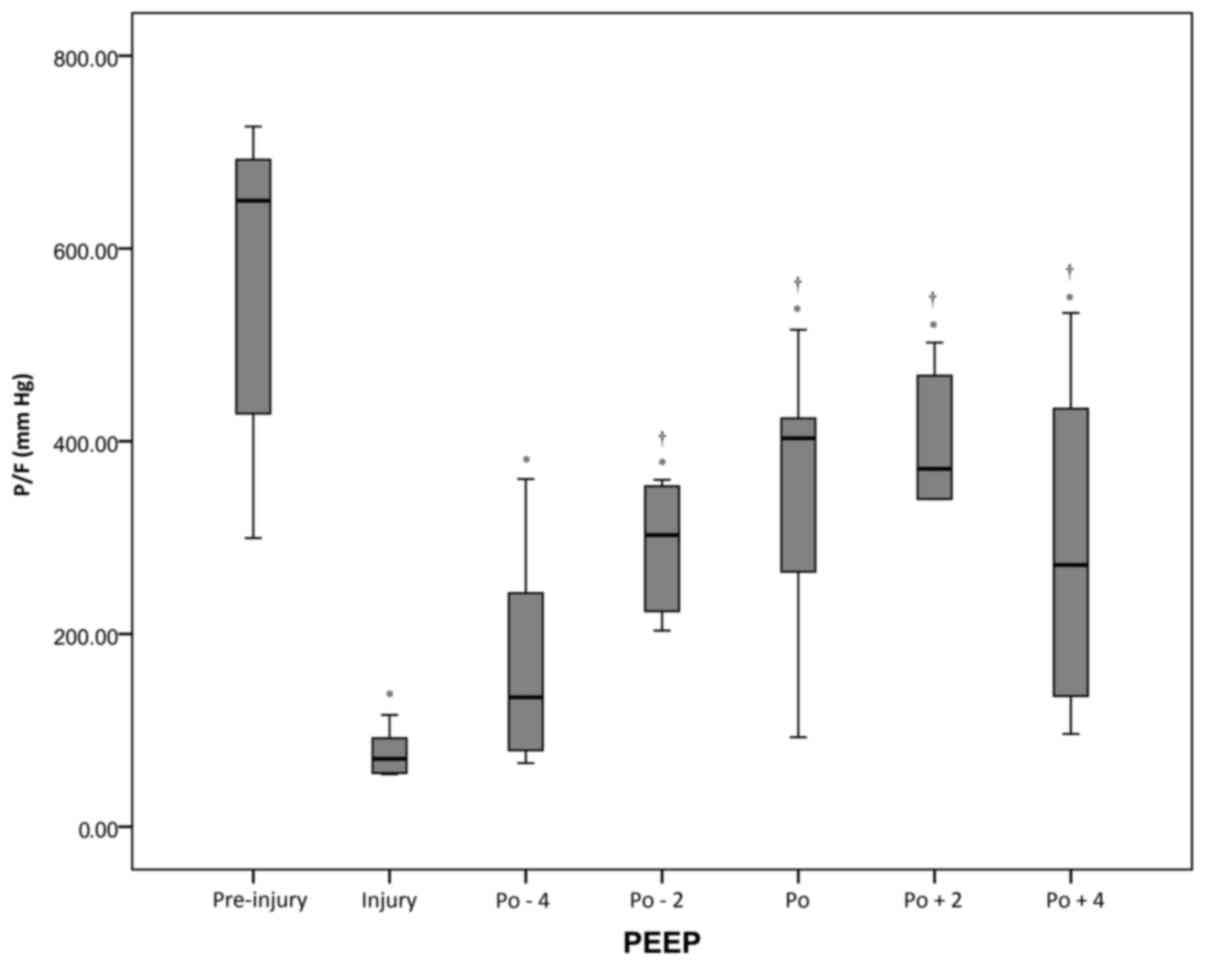
Changes of P/F during PEEP
decrement
There was a statistically significant (P<0.05)
reduction in P/F from the pre-injury period (562±162 mmHg) to the
injury period (75±21 mmHg). Following the RM, P/F values
significantly increased from 166±109 to 365±133 mmHg when the PEEP
increased from the Po-4 cm H2O to Po+2 cm
H2O. However, P/F decreased again at Po+4 cm
H2O (Fig. 2 and Table I).
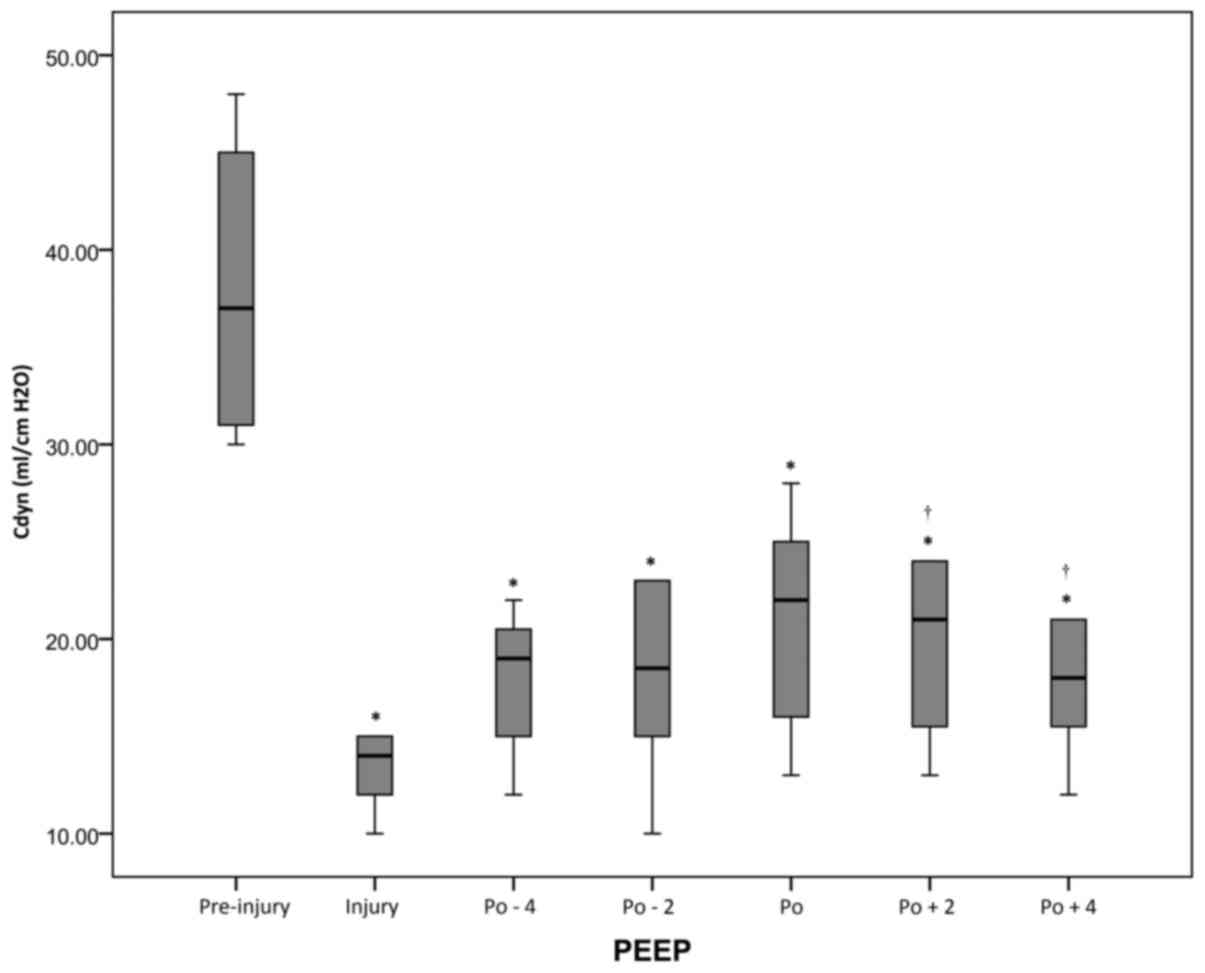
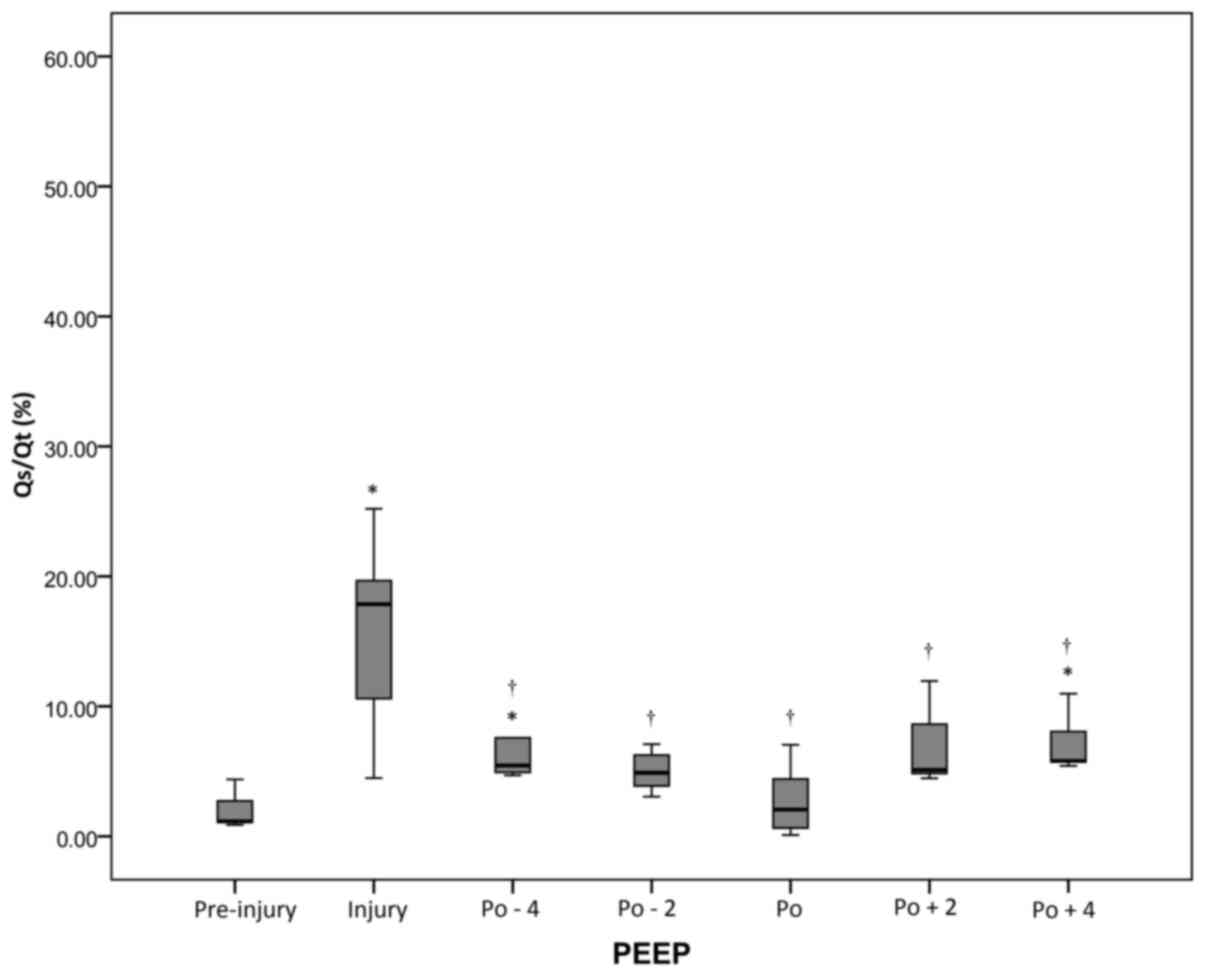
Changes of Cdyn and Qs/Qt during PEEP
decrement
At all periods after injury, the Cdyn values were
decreased compared with the pre-injury value (P<0.05), but the
highest post-RM values were observed at the pressure level of
optimal PEEP. However, Qs/Qt values were significantly (P<0.05)
lower at the pressure level of optimal PEEP compared with the
levels at the injury period (Figs. 3
and 4 and Table I).
Hemodynamic changes induced by
different PEEP levels
The CI, ITBI, GEDI and SVRI did not change
significantly during the pre-injury, injury and variable PEEP
periods, although a downtrend was observed in CI with the increase
of PEEP. For CVP, a significant (P<0.05) increment was observed
during the variable PEEP period relative to the pre-injury and
injury period. In addition, CVP increased markedly as PEEP
increased. In comparison with the pre-injury period, EVLWI values
were significantly higher during the injury and variable PEEP
periods (P<0.05; Table II).
 | Table II.Hemodynamics parameters of the acute
respiratory distress syndrome swine model under different
conditions (mean ± standard deviation). |
Table II.
Hemodynamics parameters of the acute
respiratory distress syndrome swine model under different
conditions (mean ± standard deviation).
| Parameter | Pre-injury | Injury | Po-4 | Po-2 | Po | Po+2 | Po+4 |
|---|
| CVP (mmHg) | 6.58±2.08 |
8.67±2.84a |
10.67±1.72a,b |
10.83±2.67a,b |
10.75±2.83a,b |
11.33±2.06a,b |
11.83±1.90a,b |
| CI
(l/min/m2) | 4.84±2.08 | 3.36±1.62 | 3.80±1.86 | 3.75±2.00 | 3.75±2.09 | 2.96±1.46 | 2.67±1.22 |
| ITBI
(ml/m2) | 694±219 | 664±201 | 561±240 | 690±229 | 611±202 | 597±201 | 590±130 |
| GEDI
(ml/m2) | 555±175 | 532±161 | 449±192 | 552±183 | 488±162 | 478±160 | 472±104 |
| EVLWI (ml/kg) | 10.69±4.01 |
17.61±5.71a |
15.33±3.00a |
17.14±7.13a |
16.85±6.05a |
16.69±4.97a |
16.94±4.81a |
| SVRI
(dyn.sec.cm−5.m2) | 1,430±590 | 2,113±1,012 | 2,469±948 | 2,541±1,366 | 2,179±1,439 | 2,152±1,532 | 1,970±1,237 |
Discussion
Currently, many methods exist in the literature for
identifying the PEEP to set in patients with ARDS following a lung
RM. The detection parameters include Cdyn, PAO2, maximum
PAO2 + PaCO2, as well as the inflation lower
inflection point (Pflex) and deflation upper Pflex on the
pressure-volume curve (22).
However, controversy over the approach for setting PEEP has existed
since 1967 when Ashbaugh et al (24) first used PEEP to manage ARDS. A
previous study has reported that an increased VD/VT ratio is one of
the markers of early ARDS, and furthermore, an elevated VD/VT ratio
is associated with an increased risk of mortality (10). In the present study, a decremental
PEEP procedure was performed following an RM in swine with ARDS. It
was observed that PEEP caused significant changes of VD/VT, Qs/Qt,
Cdyn and P/F. The results indicated that in cases of recruitment
maneuver and a PEEP titration procedure, VD/VT might become a
clinically useful tool for assessing collapsed alveolar opening and
titrating the optimal PEEP in ARDS.
A markedly elevated VD/VT may be detected in early
ARDS, which is suggested to be due to the obstruction of pulmonary
blood flow in the extra-alveolar pulmonary circulation (25), and increasing areas with a low
ventilation (26). Importantly,
injury of pulmonary capillaries by inflammation and thrombus can
also result in increased VD/VT (27,28). As
shown in Fig. 1, VD/VT was
significantly higher during the injury period than during the
pre-injury period, which was in accordance with results from
previous studies (29,30). In addition, the results showed that
different PEEP levels following RM caused significant changes in
VD/VT, which was in agreement with the findings of Maisch et
al (31), who showed that
different PEEP levels after RM caused significant changes in VD/VT
and P/F, as well as compliance in patients with ARDS. With the
increase of PEEP, VD/VT showed a trend of decline. However, higher
PEEP may lead to an increase of the VD/VT ratio, which might be
caused by the regional over-distention of well-ventilated alveoli
(26) or by a reduction in cardiac
output (32). As can be seen from
the formula used to calculate VD/VT, VD/VT is inversely related to
CO2 elimination. The elimination of CO2 by
the lung is influenced by effective alveolar surface area, alveolar
ventilation and cardiac output (33,34).
Following the RM and optimal PEEP, the CO2 elimination
capacity of the lung is increased, because alveolar ventilation is
markedly increased. The present study also showed that in the PEEP
levels ranging from Po-4 to Po, VD/VT was gradually reduced.
However, when the PEEP levels ranged from Po+2 to Po+4 cm
H2O, VD/VT increased again. A previous study
demonstrated that VD was significantly increased in piglets with
higher PEEP (20 cm H2O), which was induced by
hyperinflation of the lung region (35).
In ARDS, the alveolar collapse causes a deficiency
of alveolar ventilation while the blood flow does not significantly
decrease and VD/VT increases, which leads to a decline of the
ventilation and blood flow and increase of Qs/Qt. In the present
study, the Qs/Qt ratio achieved its maximum value with the increase
of VD/VT in ARDS conditions. Under the application of PEEP, the
Qs/Qt ratio showed a trend of decline to approach the base value
with the reduction of VD/VT. Furthermore, Qs/Qt reached its minimum
under the optimal PEEP state.
Higher PEEP increases VD/VT via a reduction in
cardiac output (32,36). In the present study, following the
application of PEEP, CVP increased as the PEEP increased,
indicating that PEEP significantly affected the loading conditions
of the right atrium to reduce the volume of returned blood. The
increase of CVP might be due to the augmentation of intrapleural
pressure and vena cava reflux resistance that were induced by the
high PEEP levels. Moreover, the reduction of returned blood volume
gives rise to a reduction of left atrium cardiac output, which is
in accordance with the present study's findings that the CI
gradually declined with the increase of PEEP. Notably, CI had no
evident significant difference among PEEP states, because the PEEP
range in this study was <20 cm H2O.
In conclusion, measurement of VD/VT is valuable in
assessing the effects of lung recruitment. The minimal VD/VT can be
used as one of many options for the assessment of PEEP titration in
ARDS. In the context of RM and a PEEP titration procedure, a
reduction in VD/VT and Qs/Qt, and an increase in Cdyn and P/F
indicate a maximum amount of effectively expanded alveoli. The
VD/VT may be prospectively used in future clinical trials,
particularly when the goal is to evaluate the benefit of an
open-lung protective ventilation strategy in patients with
ARDS.
However, there are certain limitations to the
present study. In the context of RM and PEEP, alveolar ventilation
volume could not be assessed by direct computed tomography methods.
In addition, PEEP was not evaluated at >20 cm H2O
after RM in the ARDS model, so it is impossible to comment on the
effect of higher PEEP on VD/VT and Qs/Qt under those circumstances.
Finally, the sample size of 12 swine is relatively small, and
arguably underpowered to detect an important effect.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (grant no.
81372043).
References
|
1
|
Phua J, Badia JR, Adhikari NK, Friedrich
JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, et
al: Has mortality from acute respiratory distress syndrome
decreased over time? A systematic review. Am J Respir Crit Care
Med. 179:220–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petty TL: Acute respiratory distress
syndrome (ARDS). Dis Mon. 36:1–58. 1990.PubMed/NCBI
|
|
3
|
Villar J, Blanco J, Añón JM, Santos-Bouza
A, Blanch L, Ambrós A, Gandía F, Carriedo D, Mosteiro F, Basaldúa
S, et al: The ALIEN study: Incidence and outcome of acute
respiratory distress syndrome in the era of lung protective
ventilation. Intensive Care Med. 37:1932–1941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bull TM, Clark B, McFann K and Moss M:
National Institutes of Health/National Heart, Lung, and Blood
Institute ARDS Network: Pulmonary vascular dysfunction is
associated with poor outcomes in patients with acute lung injury.
Am J Respir Crit Care Med. 182:1123–1128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lachmann B: Open up the lung and keep the
lung open. Intensive Care Med. 18:319–321. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amato MB, Barbas CS, Medeiros DM, Magaldi
RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D,
Munoz C, Oliveira R, et al: Effect of a protective-ventilation
strategy on mortality in the acute respiratory distress syndrome. N
Engl J Med. 338:347–354. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levy MM: PEEP in ARDS-how much is enough?
N Engl J Med. 351:389–391. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sakuramoto H, Shimojo N, Jesmin S, Unoki
T, Kamiyama J, Oki M, Miya K, Kawano S and Mizutani T: Repeated
open endotracheal suctioning causes gradual desaturation but does
not exacerbate lung injury compared to closed endotracheal
suctioning in a rabbit model of ARDS. BMC Anesthesiol. 13:472013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Charron C, Repesse X, Bouferrache K,
Bodson L, Castro S, Page B, Jardin F and Vieillard-Baron A: PaCO2
and alveolar dead space are more relevant than PaO2/FiO2 ratio in
monitoring the respiratory response to prone position in ARDS
patients: A physiological study. Crit Care. 15:R1752011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fengmei G, Jin C, Songqiao L, Congshan Y
and Yi Y: Dead space fraction changes during PEEP titration
following lung recruitment in patients with ARDS. Respir Care.
57:1578–1585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gattinoni L, Caironi P, Cressoni M,
Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R
and Bugedo G: Lung recruitment in patients with the acute
respiratory distress syndrome. N Engl J Med. 354:1775–1786. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tusman G, Suarez-Sipmann F, Böhm SH, Pech
T, Reissmann H, Meschino G, Scandurra A and Hedenstierna G:
Monitoring dead space during recruitment and PEEP titration in an
experimental model. Intensive Care Med. 32:1863–1871. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Uttman L, Bitzén U, De Robertis E,
Enoksson J, Johansson L and Jonson B: Protective ventilation in
experimental acute respiratory distress syndrome after
ventilator-induced lung injury: A randomized controlled trial. Br J
Anaesth. 109:584–594. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beydon L, Uttman L, Rawal R and Jonson B:
Effects of positive end-expiratory pressure on dead space and its
partitions in acute lung injury. Intensive Care Med. 28:1239–1245.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blanch L, Lucangelo U, Lopez-Aguilar J,
Fernandez R and Romero P: Volumetric capnography in patients with
acute lung injury: Effects of positive end-expiratory pressure. Eur
Respir J. 13:1048–1054. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Higgs Z, Macafee D, Braithwaite B and
Maxwell-Armstrong C: The Seldinger technique: 50 years on. Lancet.
366:1407–1409. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marshall W: Arterial blood gas analysis.
Ann Clin Biochem. 47:283. 2010. View Article : Google Scholar
|
|
18
|
Fletcher R, Jonson B, Cumming G and Brew
J: The concept of deadspace with special reference to the single
breath test for carbon dioxide. Br J Anaesth. 53:77–88. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fowler WS: Lung function studies; The
respiratory dead space. Am J Physiol. 154:405–416. 1948.PubMed/NCBI
|
|
20
|
Hofer CK, Furrer L, Matter-Ensner S,
Maloigne M, Klaghofer R, Genoni M and Zollinger A: Volumetric
preload measurement by thermodilution: A comparison with
transoesophageal echocardiography. Br J Anaesth. 94:748–755. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Quintel M, Pelosi P, Caironi P, Meinhardt
JP, Luecke T, Herrmann P, Taccone P, Rylander C, Valenza F,
Carlesso E and Gattinoni L: An increase of abdominal pressure
increases pulmonary edema in oleic acid-induced lung injury. Am J
Respir Crit Care Med. 169:534–541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caramez MP, Kacmarek RM, Helmy M, Miyoshi
E, Malhotra A, Amato MB and Harris RS: A comparison of methods to
identify open-lung PEEP. Intensive Care Med. 35:740–747. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Borges JB, Okamoto VN, Matos GF, Caramez
MP, Arantes PR, Barros F, Souza CE, Victorino JA, Kacmarek RM,
Barbas CS, et al: Reversibility of lung collapse and hypoxemia in
early acute respiratory distress syndrome. Am J Respir Crit Care
Med. 174:268–278. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ashbaugh DG, Bigelow DB, Petty TL and
Levine BE: Acute respiratory distress in adults. Lancet. 2:319–323.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greene R, Zapol WM, Snider MT, Reid L,
Snow R, O'Connell RS and Novelline RA: Early bedside detection of
pulmonary vascular occlusion during acute respiratory failure. Am
Rev Respir Dis. 124:593–601. 1981.PubMed/NCBI
|
|
26
|
Dantzker DR, Brook CJ, Dehart P, Lynch JP
and Weg JG: Ventilation-perfusion distributions in the adult
respiratory distress syndrome. Am Rev Respir Dis. 120:1039–1052.
1979.PubMed/NCBI
|
|
27
|
Idell S, Mazar AP, Bitterman P, Mohla S
and Harabin AL: Fibrin turnover in lung inflammation and neoplasia.
Am J Respir Crit Care Med. 163:578–584. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tomashefski JF Jr, Davies P, Boggis C,
Greene R, Zapol WM and Reid LM: The pulmonary vascular lesions of
the adult respiratory distress syndrome. Am J Pathol. 112:112–126.
1983.PubMed/NCBI
|
|
29
|
Nuckton TJ, Alonso JA, Kallet RH, Daniel
BM, Pittet JF, Eisner MD and Matthay MA: Pulmonary dead-space
fraction as a risk factor for death in the acute respiratory
distress syndrome. N Engl J Med. 346:1281–1286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lucangelo U, Bernabè F, Vatua S, Degrassi
G, Villagrà A, Fernandez R, Romero PV, Saura P, Borelli M and
Blanch L: Prognostic value of different dead space indices in
mechanically ventilated patients with acute lung injury and ARDS.
Chest. 133:62–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maisch S, Reissmann H, Fuellekrug B,
Weismann D, Rutkowski T, Tusman G and Bohm SH: Compliance and dead
space fraction indicate an optimal level of positive end-expiratory
pressure after recruitment in anesthetized patients. Anaesth Analg.
106:175–181. 2008. View Article : Google Scholar
|
|
32
|
Coffey RL, Albert RK and Robertson HT:
Mechanisms of physiological dead space response to PEEP after acute
oleic acid lung injury. J Appl Physiol Respir Environ Exerc
Physiol. 55:1550–1557. 1983.PubMed/NCBI
|
|
33
|
Breen PH and Mazumdar B: How does positive
end-expiratory pressure decrease CO2 elimination from the lung?
Respir Physiol. 103:233–242. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anderson CT and Breen PH: Carbon dioxide
kinetics and capnography during critical care. Crit Care.
4:207–215. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tang R, Huang Y, Chen Q, Hui X, Li Y, Yu
Q, Zhao H, Yang Y and Qiu H: The effect of alveolar dead space on
the measurement of end-expiratory lung volume by modified nitrogen
wash-out/wash-in in lavage-induced lung injury. Respir Care.
57:2074–2081. 2012.PubMed/NCBI
|
|
36
|
Suwa K, Hedley-Whyte J and Bendixen HH:
Circulation and physiologic dead space changes on controlling the
ventilation of dogs. J Appl Physiol. 21:1855–1859. 1966.PubMed/NCBI
|