Introduction
Mesenchymal stem cells are clonogenic, self-renewing
progenitor cells that are able to generate one or more specialized
cell types (1). Stem cells may be
postnatally isolated from different tissues including adipose
tissue, muscle, bone marrow, and periosteum (2). Mesenchymal stem cells are a valuable
source of stem cells for regenerative medicine, and the
transplantation of mesenchymal stem cells is a promising treatment
for many diseases (3,4). However, conventional techniques, which
consist of cells being cultured as a monolayer, typically result in
slow cell proliferation and insufficient yield to fulfill clinical
demands (3). Furthermore, the loss
of stemness, which is the ability to self-renew and differentiate,
of mesenchymal stem cells during in vitro expansion reduces
the therapeutic efficacy (4).
Three-dimensional culture systems have been used to
demonstrate the importance of intercellular interactions in
regulating stem cell self-renewal and differentiation (5). Due to their rich biological content and
superior ability to mimic the in vivo environment compared
with two-dimensional cell cultures, such multi-cellular spheroids
are currently receiving increased attention with regard to their
applications and production (6).
Typically, cells in a spheroid culture exhibit various properties
that are distinct from monolayer cells (7). Cells grow with similar characteristics
to in vivo tissue and these cultures are able to simulate
native tissue behaviors much more accurately than two-dimensional
cultures (7). A recent study has
demonstrated that the stemness properties of mesenchymal stem cells
are retained in the in vivo microenvironment, which
comprises cell-cell interactions, soluble growth factors, and
cell-matrix interactions (4).
Human mesenchymal stem cells have been isolated and
characterized from the periodontium including the gingiva (8), and gingiva-derived stem cells have been
utilized for tissue-engineering purposes (9). Gingiva-derived stem cells from the
maxillofacial region may be considered a favorable source of
mesenchymal stem cells as harvesting stem cells from the mandible
or maxilla may be easily performed under local anesthesia (8,10). The
present study was performed to produce stem-cell spheroids using
concave microwells and to evaluate the maintenance of stemness,
viability, and differentiation potential. To the best of our
knowledge, this study is the first to evaluate the maintenance of
stemness, viability, and differentiation potential of
gingiva-derived stem-cell spheroids.
Materials and methods
Isolation and culturing of
gingiva-derived stem cells
Gingiva-derived stem cells and cultures were
obtained using a previously reported method (8). Gingival tissues were harvested from
healthy patients during crown lengthening procedures from July 2013
to August 2015 visiting the Department of Periodontics, Seoul St.
Mary's Hospital. Exclusion criteria were as follows: i) Severe
medical or psychological disease or ii) hemorrhagic disease. The
design of the present study was reviewed and approved by the
Institutional Review Board of Seoul St. Mary's Hospital, College of
Medicine, Catholic University of Korea (Seoul, Korea; KC11SISI0348)
and informed consent was obtained from the patients.
Attached keratinized gingival tissues were
immediately placed in sterile phosphate-buffered saline (PBS;
Welgene, Daegu, South Korea) with 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany)
at 4°C until use. The tissues were de-epithelialized with a
surgical blade, processed into 1–2 mm2 fragments, and
digested in an α-modified, minimal essential medium (α-MEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
dispase (1 mg/ml) and collagenase IV (2 mg/ml; both from
Sigma-Aldrich; Merck Millipore). The cells were incubated at 37°C
in a humidified incubator with 5% CO2 and 95%
O2 for 24 h. Non-adherent cells were subsequently washed
with PBS (Welgene), replaced with a fresh medium (α-MEM) containing
fetal bovine serum (both from Gibco), and penicillin, streptomycin
and ascorbic acid 2-phosphate (all from Sigma-Aldrich; Merck
Millipore) every 2 to 3 days.
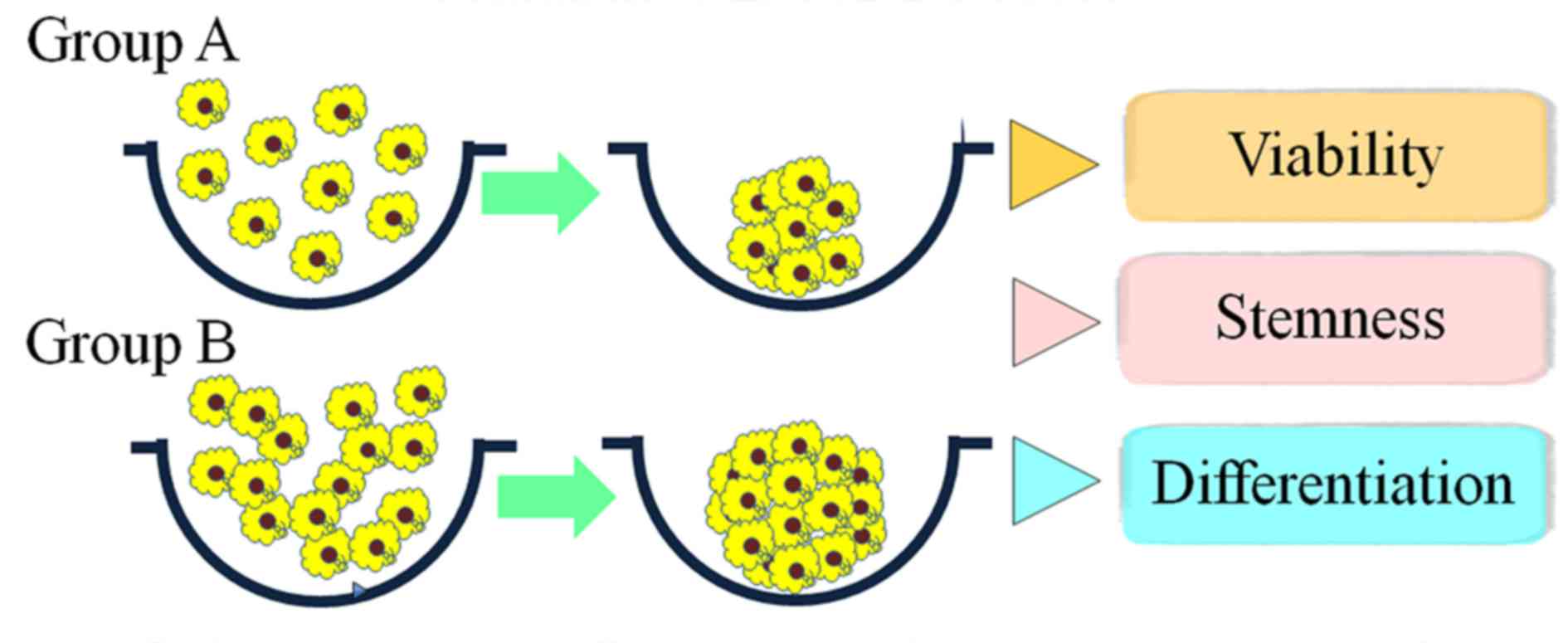
Formation of spheres
Stem-cell spheroids were formed in silicon
elastomer-based concave microwells (StemFIT 3D; MicroFIT, Seonnam,
Republic of Korea) with 600 µm diameters. Subsequently,
4×105 (group A) or 8×105 (group B) stem cells
were seeded in each concave micromold and cultured to investigate
cellular behavior (Fig. 1). The
difference between group A and group B was only the number of cells
per spheroid. Cell aggregation and spheroid formation were observed
and images were captured using an inverted microscope (Leica DM
IRM; Leica Microsystems GmbH, Wetzlar, Germany). The diameters of
spheroids were measured from the captured images.
Determination of cell viability
Viability of spheroids was qualitatively analyzed
using a Live/Dead assay kit (Molecular Probes; Thermo Fisher
Scientific, Inc.). The assay is based on the principle that the
activity of intracellular esterase induces non-fluorescent,
cell-permeant calcein acetoxymethyl to become intensely
fluorescent, giving the viable spheroids a green fluorescence.
Ethidium homodimer enters into damaged cell membrane and binds to
nucleic acids, thereby producing a red fluorescence in dead
cells.
Stem-cell spheroids were cultured at 37°C in α-MEM
containing 15% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin, 100 µg/ml streptomycin, 200 mM
L-glutamine and 10 mM ascorbic acid 2-phosphate (all from
Sigma-Aldrich; Merck Millipore). These spheroids were washed twice
with PBS, followed by suspension in 1 ml α-MEM containing 2 µl 50
mM calcein acetoxymethyl ester working solution and 4 µl 2 mM
ethidium homodimer-1 for 15 min at room temperature. The spheroids
stained with calcein acetoxymethyl ester and ethidium homodimer-1
were observed under a fluorescence microscope (Axiovert 200; Zeiss
AG, Oberkochen, Germany) at days 6 and 12.
A cell-viability analysis was performed on days 1,
3, 6, and 12, by adding
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H
tetrazolium monosodium salt (WST-8; Cell Counting Kit-8; Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) to cultures, and
spheres were incubated for 1 h at 37°C. Viable cells were
identified by the assay, which relies on the ability of
mitochondrial dehydrogenases to oxidize WST-8 into a formazan
product. The spectrophotometric absorbance of the samples was
measured using a microplate reader (BioTek Instruments, Inc.,
Winooski, VT, USA) at 450 nm.
Evaluation of maintenance of
stemness
Following cultivation for six days, spheroids were
retrieved. Antibodies were purchased from R&D Systems, Inc.,
(Minneapolis, MN, USA), and diluted to 50X. Human SSEA-4 conjugated
to NHL493 (green) and human TRA-1-60(R) conjugated to NL557 (red)
antibodies (cat. no. SC023; Live Cell Imaging kit) were used as
positive markers of human stem cells. Human SSEA-1 conjugated to
NL557 (red) antibody (cat. no. SC023; Live Cell Imaging kit) was
used as a negative marker. The cells were incubated for 30 min. The
antibody-containing media were removed, and cells were washed with
fresh media and re-fed with fresh media. The spheroids were
visualized via fluorescence microscopy.
Osteogenic differentiation
Spheroids were grown at 37°C in osteogenic induction
medium (STEMPRO Osteogenesis Differentiation kit; Gibco; Thermo
Fisher Scientific, Inc.). The medium was replaced with a fresh
induction medium every 3 to 4 days. At days 14 and 21, Alizarin Red
S staining (Sigma-Aldrich; Merck Millipore) was performed to detect
calcium formation. Bound dye was solubilized in 10 mM sodium
phosphate containing 10% cetylpyridinium chloride and quantified
spectrophotometrically at 562 nm. The morphological evaluation was
performed using an inverted microscope (Leica DM IRM) and
experiments were performed in triplicate.
Adipogenic differentiation
Spheroids were grown at 37°C with adipogenic
induction medium (STEMPRO Adipogenesis Differentiation kit; Gibco;
Thermo Fisher Scientific, Inc.). The medium was replaced with a
fresh induction medium every 3 to 4 days. Oil Red O staining
(Sigma-Aldrich; Merck Millipore) was performed at days 6 and 18 to
detect the oil globules.
Chondrogenic differentiation
Spheroids were grown at 37°C in a chondrogenic
induction medium (STEMPRO Chondrogenesis Differentiation kit;
Gibco). The medium was replaced with a fresh induction medium every
3 to 4 days. Alcian blue staining (Sigma-Aldrich; Merck Millipore)
was performed following 14 days to detect the presence of
cartilage-specific proteoglycan core protein.
Statistical analysis
Data are presented as means ± standard deviations of
the experiments. A test of normality using a Shapiro-Wilk test was
performed and a Student's t-test or a two-way analysis of variance
with post hoc Tukey testing was performed to determine the
differences between the groups using SPSS 12 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
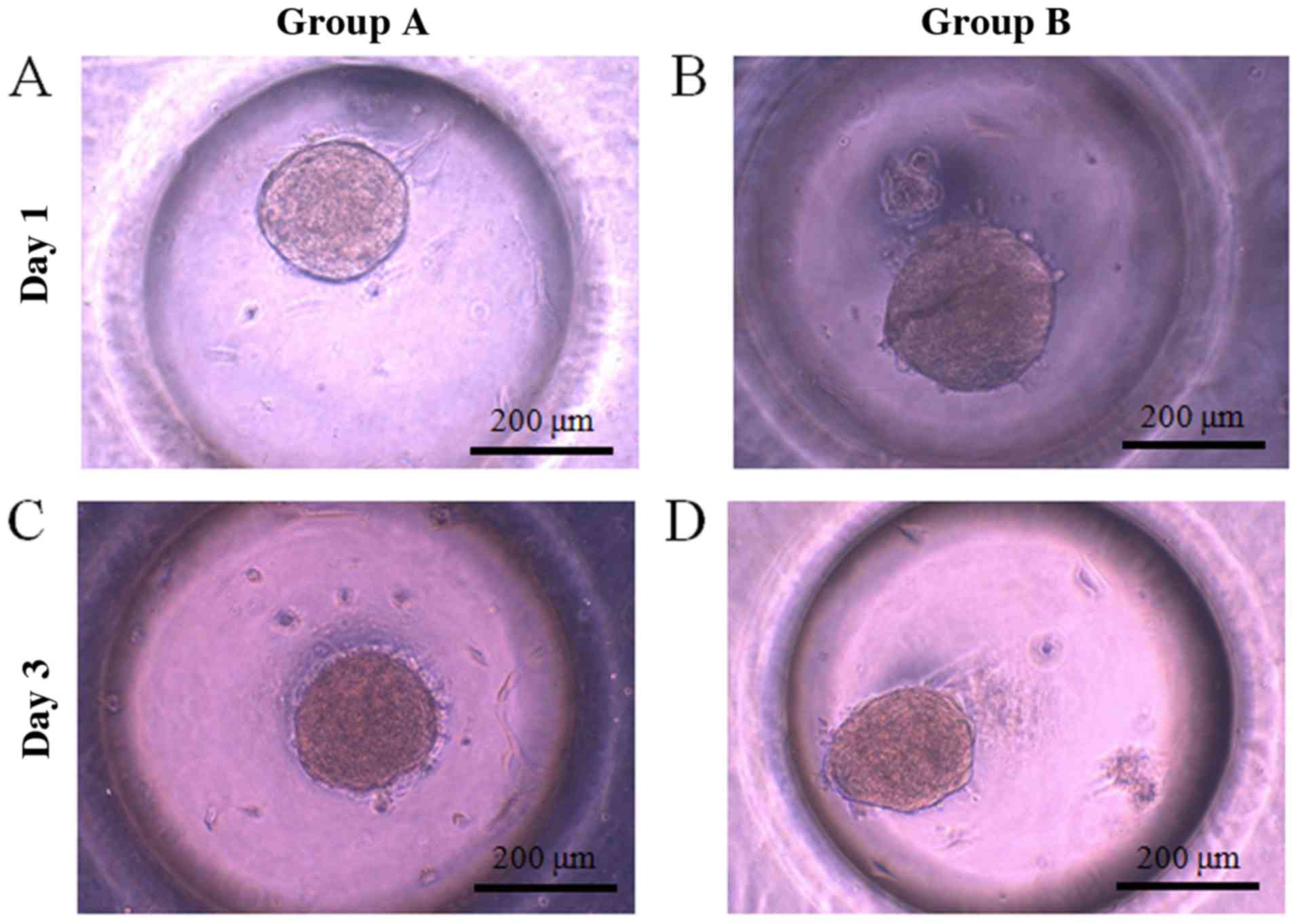
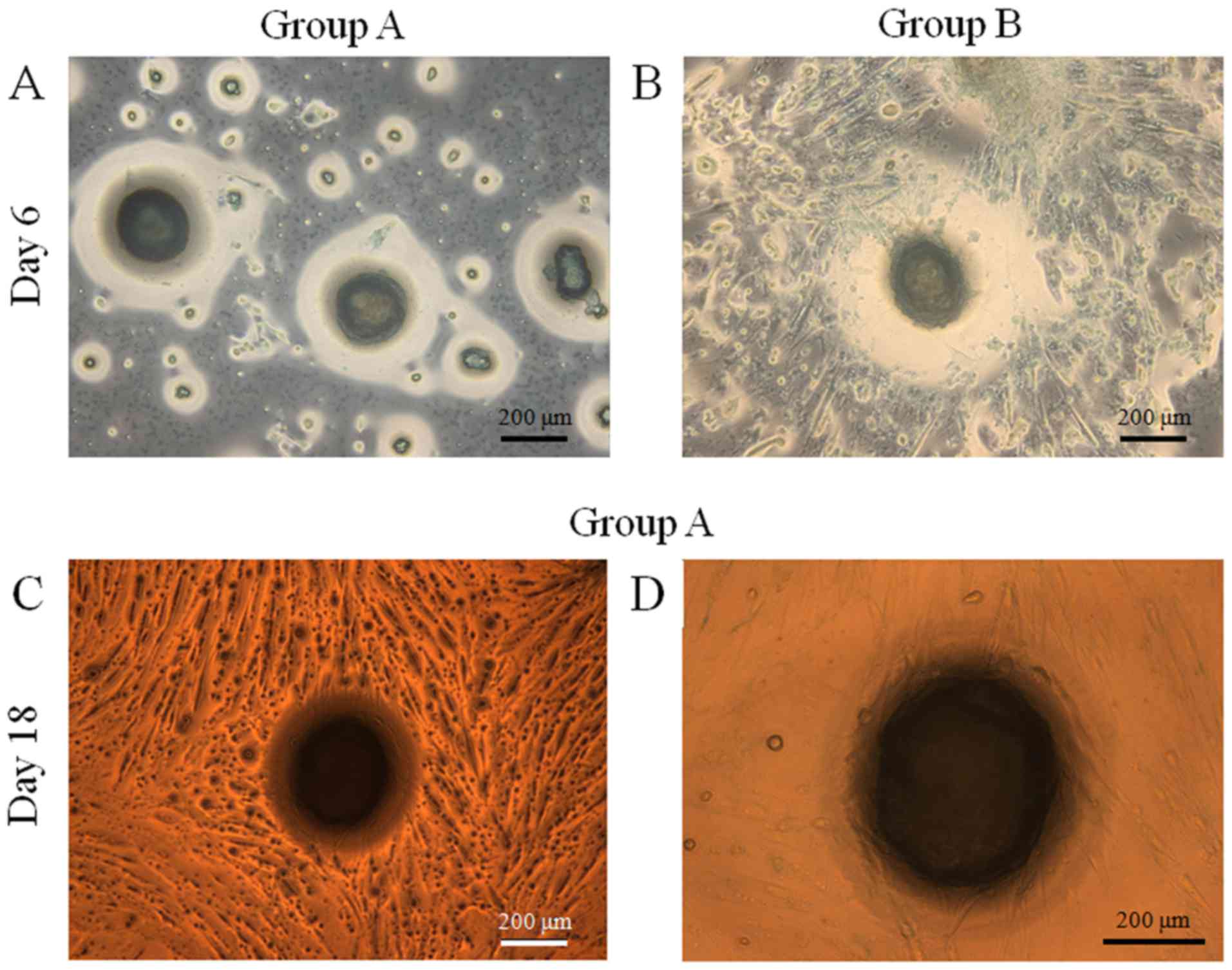
Evaluation of cell morphology
Gingiva-derived stem cells were able to form
spheroids in concave microwells. The morphology of the spheroids at
day 1 is shown in Fig. 2A and B. The
morphology of the spheroids at day 3 was similar to that of day 1
(Fig. 2C-D). The diameters of
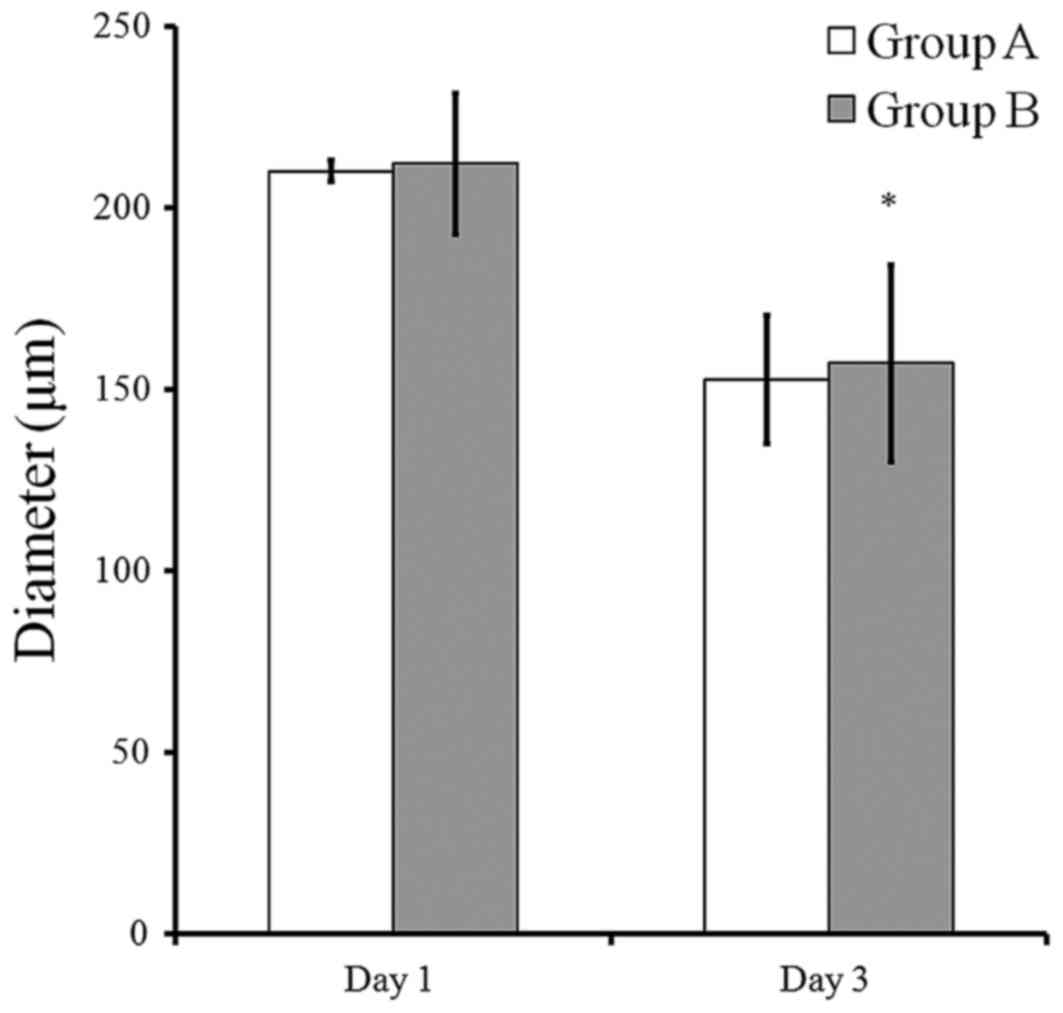
spheroids in group B were larger compared to those of group A. The
mean spheroid diameters in group A were 210.0±3.0 and 152.5±17.8 µm
at days 1 and 3, respectively (Fig.
3). The mean spheroid diameters in group B were 212.0±19.3 and
157.1±27.2 µm at days 1 and 3, respectively.
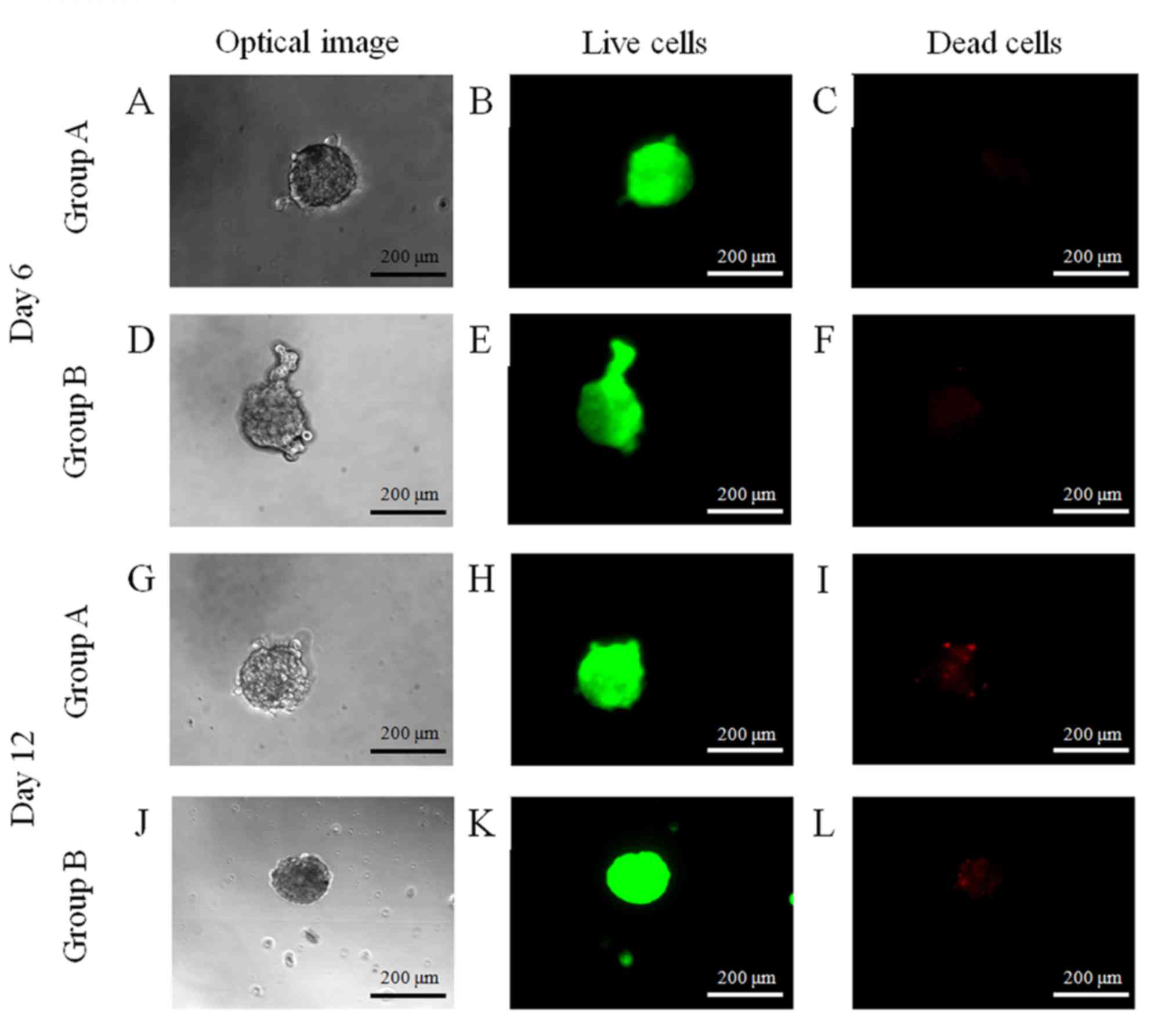
Determination of cell viability
The majority of cells in the spheroids emitted green
fluorescence, and the morphology was round without marked changes
at day 6 (Fig. 4). At day 12, the
majority of cells in the spheroids emitted green fluorescence;
however a small portion of red fluorescence was noted.
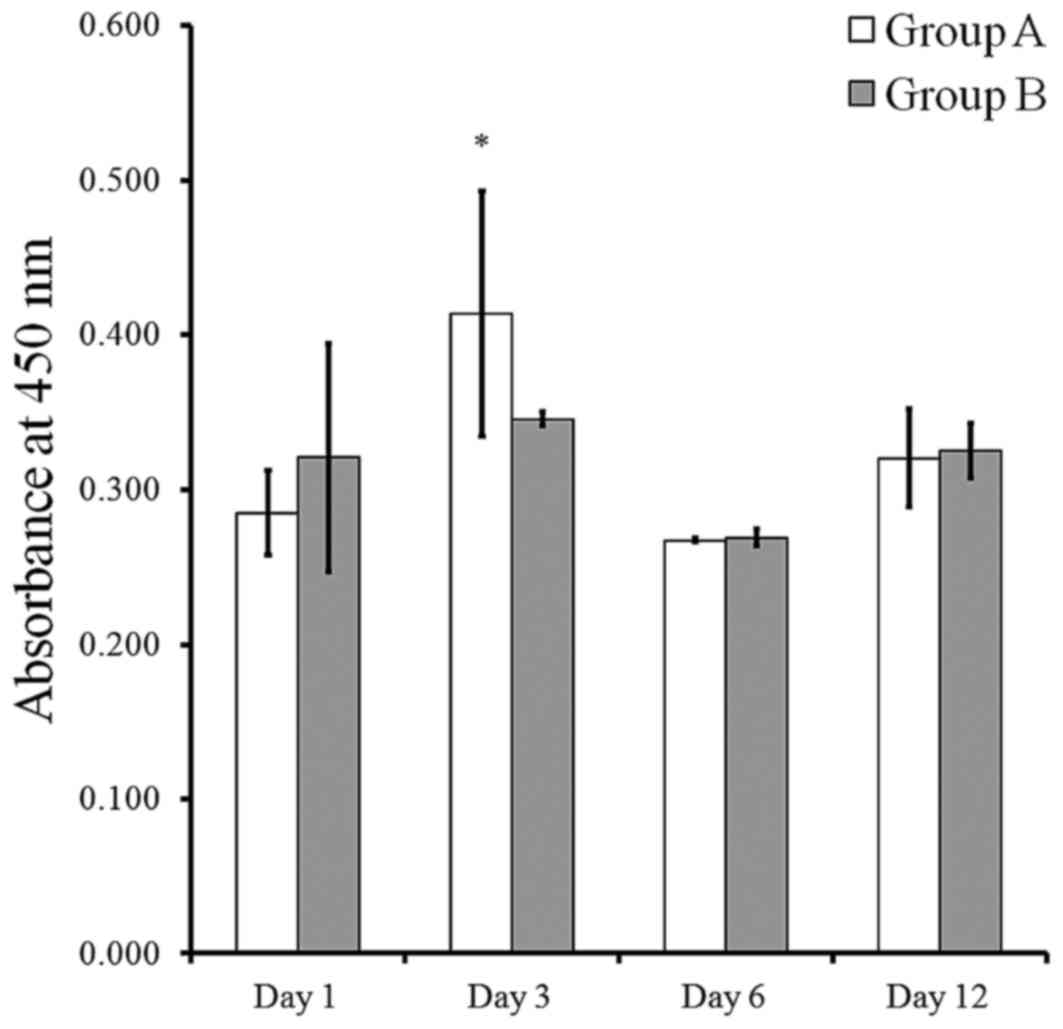
The results of cell viability using Cell Counting
Kit-8 following culturing at days 1, 3, 6, and 12 are presented in
Fig. 5. The cell viability in group
B was typically higher than that in group A at each time point,
although this did not reach statistical significance (P>0.05).
The Cell Counting Kit-8 assay values of groups A and B at day 1
were 0.285±0.027 and 0.320±0.074, respectively. The viability
values of groups A and B at day 3 were 0.413±0.080 and 0.345±0.005,
respectively. The Cell Counting Kit-8 assay values of groups A and
B at day 6 were 0.267±0.001 and 0.269±0.006, respectively. The Cell
Counting Kit-8 assay value of groups A and B at day 12 were
0.320±0.032 and 0.325±0.018, respectively.
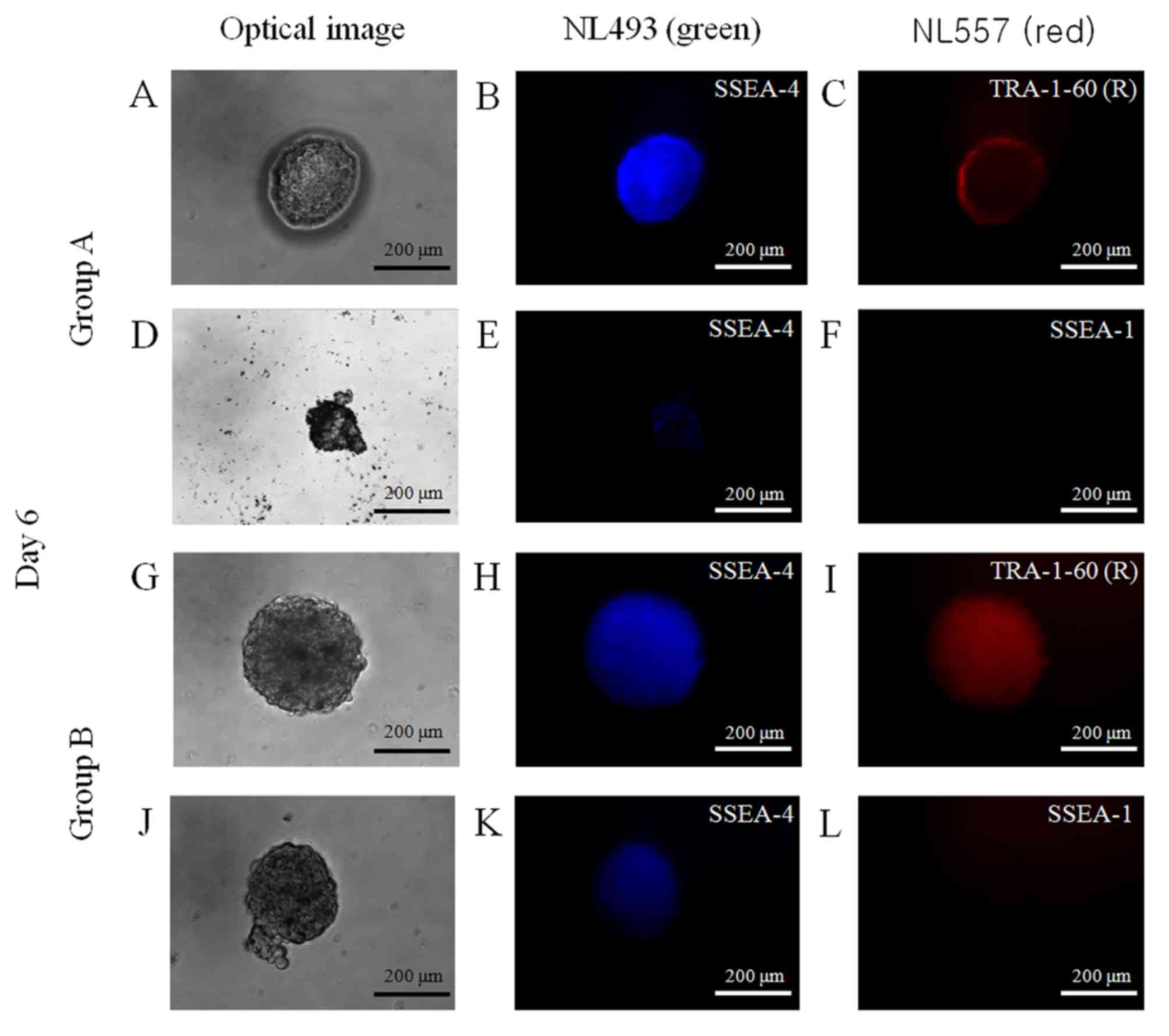
Maintenance of stemness
Spheroids were stained with NL493-conjugated SSEA-4
(green) and NL557-conjugated TRA-1-60(R) (red) antibodies or with
NL493-conjugated SSEA-4 (green) and NL557-conjugated SSEA-1 (red)
antibodies (Fig. 6). The spheroids
were positive for the stem-cell markers SSEA-4 and TRA-1-60(R), and
were negative for SSEA-1, which suggests that these spheroids
primarily contained undifferentiated human stem cells.
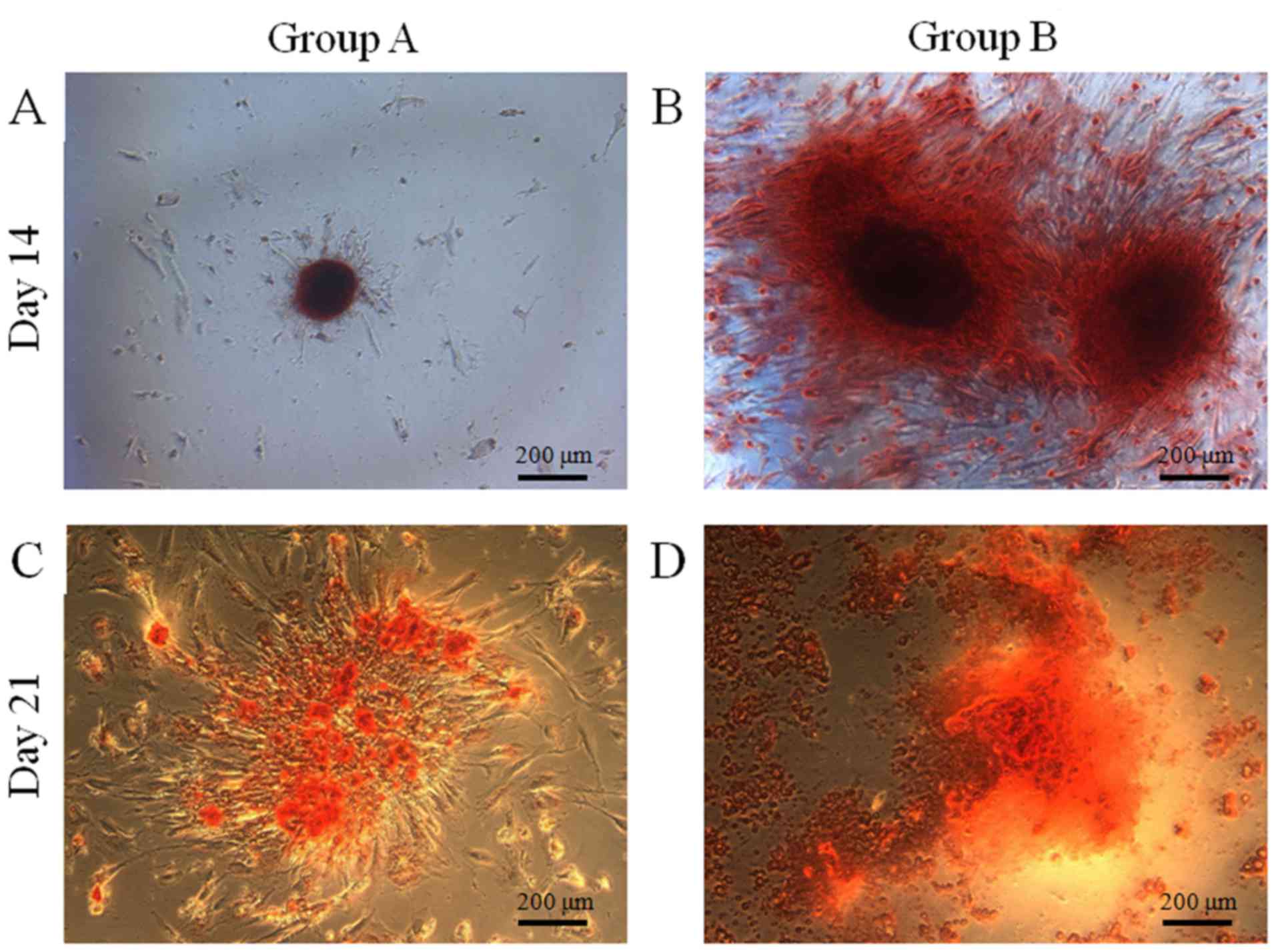
Osteogenic differentiation
Mineralized extracellular deposits were observed
following Alizarin Red S staining at days 14 and 21 (Fig. 7). A marked increase in mineralized
deposits was observed in group B compared with group A. The
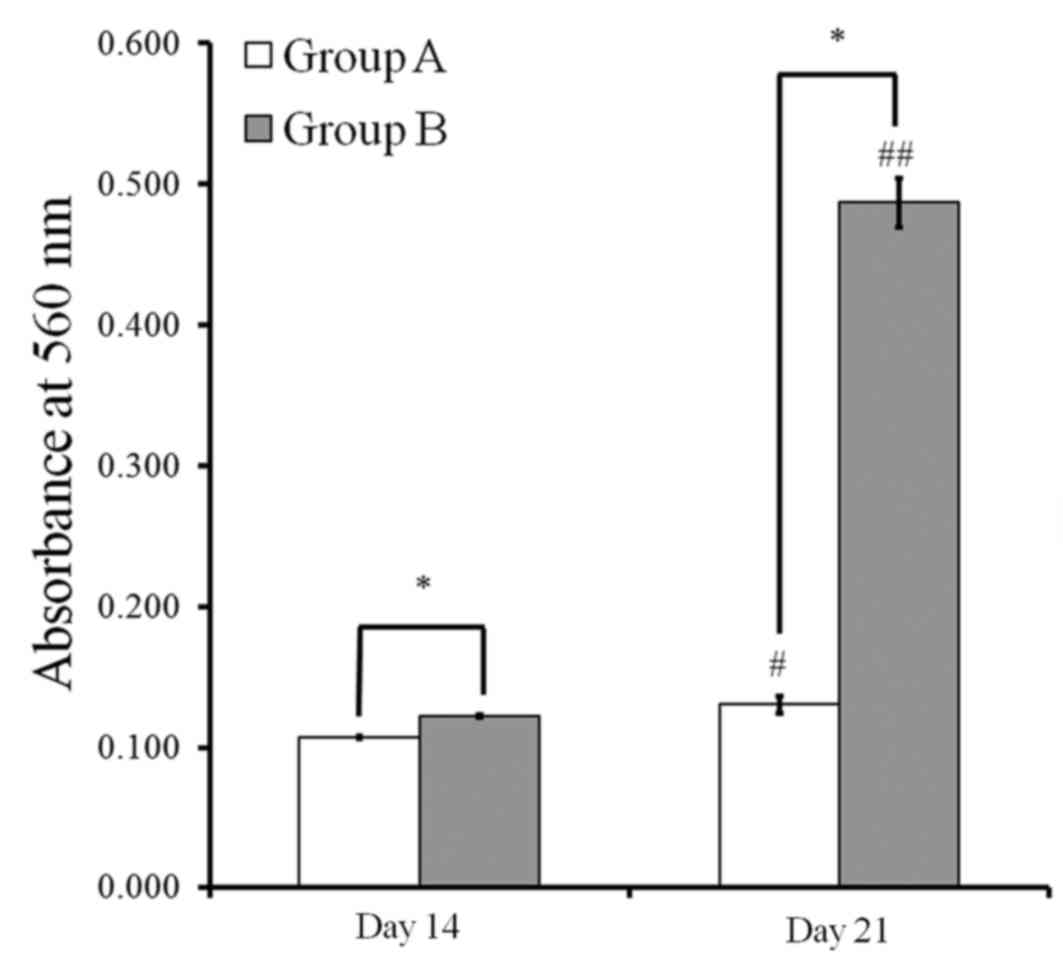
quantitative results regarding bound dye were presented in Fig. 8. Quantitative values at day 14 were
0.107±0.001 and 0.122±0.001 for groups A and B, respectively. The
values at day 21 were 0.130±0.006 and 0.487±0.018 for groups A and
B, respectively. Values for group B were significantly higher than
in group A at each point (P<0.05).
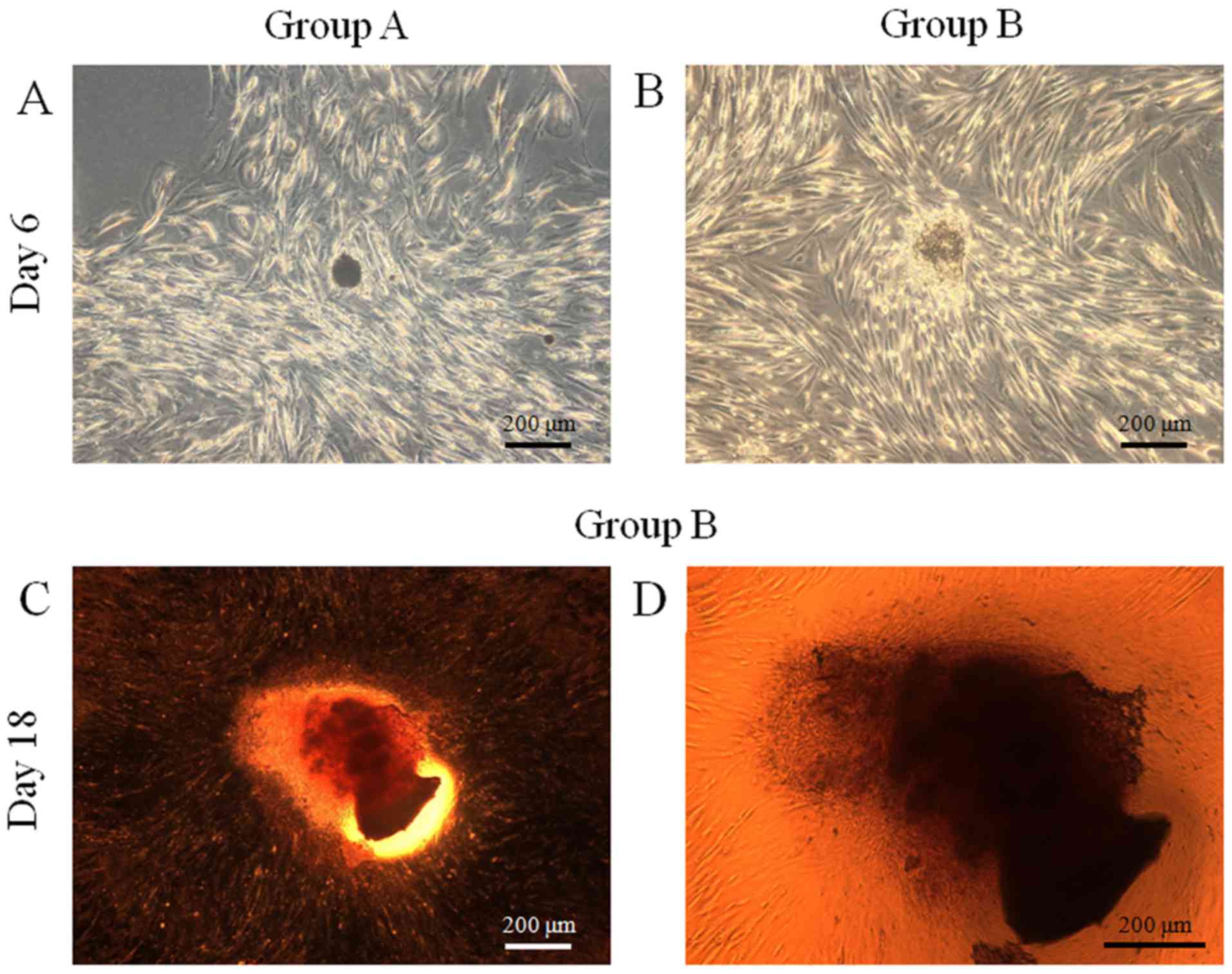
Adipogenic differentiation
The results of adipogenic differentiation are shown
in Fig. 9. Oil globules were
increased in both groups at day 18 compared with day 6.
Chondrogenic differentiation
The results of chondrogenic differentiation are
shown in Fig. 10. The spheroids
were stained with Alcian blue, and staining was more evident at day
18 when compared with day 6 in both groups.
Discussion
In the present study, the stemness, viability, and
differentiation potential of gingiva-derived stem-cell spheroids
were maintained during the experimental periods.
Spontaneous cell aggregation only occurs in a fairly
limited number of cell lines, and stem cells with self-renewing
capacity typically possess spheroid-forming capacity (7). It is possible to generate spheroids in
several ways, such as hanging-drop culture, pellet culture, dynamic
culture including spinner flask, or rotary cell-culture systems
(4,11,12).
Microwell culture systems have been developed to generate
homogenous stem-cell colonies of defined sizes and shapes, and to
study how colony morphology may affect cell fate (13). The authors of the present study have
recently demonstrated that stem-cell spheroids may be produced with
gingiva-derived stem cells using microwells (14). This method seems convenient for
generating cell aggregates without causing shear stress damage
(4). The decrease of diameter in
spheroids may be explained by the increased cell-cell contact from
neighboring cells in three-dimensional culture (5,15).
Accumulating evidence has suggested that the
cellular microenvironment has an important role in determining
stemness properties (4,16,17). The
present study demonstrated that the spheroids maintained viability
and stemness during the experimental period using
polydimethylsiloxane-based concave micromolds. It has also been
demonstrated in previous that the aggregation of adult human
mesenchymal stem cells to produce three-dimensional cellular
spheroids helped to maintain the expression of stemness marker
genes in cells, which was reiterated by the present findings
(4,6). A recent study has also indicated that
rabbit corneal stromal-cell-derived spheroids positively expressed
mesenchymal and stem-cell phenotypes, which were immunopositive for
vimentin and cluster of differentiation 34 (a mesenchymal cell
marker and a stem cell marker, respectively), as well as the mRNA
expression of nestin and Nanog (a neural stem cell marker and a
stem cell marker, respectively) (7).
The present study study also demonstrated that
viability was maintained during the experimental period. It was
recently suggested that a spheroid culture of mesenchymal stem
cells may meet the requirement of adherent growth and improve
culture efficiency (3). A recent
report showed that compact cellular spheroids were formed with
adipose-derived stem cells, and spheroids remained viable when
cultured in a microgravity bioreactor (4).
The present study clearly showed that osteogenic,
adipogenic, and chondrogenic differentiation were present within
the spheroids. The increase of differentiation was noted with an
increase in the number of cells. Enhanced chondrogenic
differentiation potential of human gingival fibroblasts was
previously evaluated via spheroid formation on chitosan membranes
(18).
Conclusively, stem-cell spheroids were demonstrated
to form gingival cells which maintained stemness, viability, and
differentiation potential during the experimental period. These
findings suggest that this convenient method may be applied as a
promising strategy for stem-cell therapy.
Acknowledgements
The present study was supported by Basic Science
Research Program through the National Research Foundation of Korea,
funded by the Ministry of Science, Information and Communication
Technology and Future Planning (grant no.
NRF-2014R1A1A1003106).
References
|
1
|
Park JB, Bae SS, Lee PW, Lee W, Park YH,
Kim H, Lee K and Kim I: Comparison of stem cells derived from
periosteum and bone marrow of jaw bone and long bone in rabbit
models. Tissue Eng Regen Med. 9:224–230. 2012. View Article : Google Scholar
|
|
2
|
Park JB, Lee KS, Lee W, Kim HS, Lee KH and
Kim IS: Establishment of the chronic bone defect model in
experimental model mandible and evaluation of the efficacy of the
mesenchymal stem cells in enhancing bone regeneration. Tissue Eng
Regen Med. 10:18–24. 2013. View Article : Google Scholar
|
|
3
|
Li Y, Guo G, Li L, Chen F, Bao J, Shi YJ
and Bu H: Three-dimensional spheroid culture of human umbilical
cord mesenchymal stem cells promotes cell yield and stemness
maintenance. Cell Tissue Res. 360:297–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang S, Liu P, Chen L, Wang Y, Wang Z and
Zhang B: The effects of spheroid formation of adipose-derived stem
cells in a microgravity bioreactor on stemness properties and
therapeutic potential. Biomaterials. 41:15–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsiao C, Tomai M, Glynn J and Palecek SP:
Effects of 3D microwell culture on initial fate specification in
human embryonic stem cells. AIChE J. 60:1225–1235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang CF, Hsu SH, Tsai KP and Tsai MH:
Segmentation and tracking of stem cells in time lapse microscopy to
quantify dynamic behavioral changes during spheroid formation.
Cytometry A. 87:491–502. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li H, Dai Y, Shu J, Yu R, Guo Y and Chen
J: Spheroid cultures promote the stemness of corneal stromal cells.
Tissue Cell. 47:39–48. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin SH, Kweon H, Park JB and Kim CH: The
effects of tetracycline-loaded silk fibroin membrane on
proliferation and osteogenic potential of mesenchymal stem cells. J
Surg Res. 192:e1–e9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park JB, Kim YS, Lee G, Yun BG and Kim CH:
The effect of surface treatment of titanium with
sand-blasting/acid-etching or hydroxyapatite-coating and
application of bone morphogenetic protein-2 on attachment,
proliferation, and differentiation of stem cells derived from
buccal fat pad. Tissue Eng Regen Med. 10:115–121. 2013. View Article : Google Scholar
|
|
11
|
Mehta G, Hsiao AY, Ingram M, Luker GD and
Takayama S: Opportunities and challenges for use of tumor spheroids
as models to test drug delivery and efficacy. J Control Release.
164:192–204. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Page H, Flood P and Reynaud EG:
Three-dimensional tissue cultures: Current trends and beyond. Cell
Tissue Res. 352:123–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hsiao C and Palecek SP: Microwell
regulation of pluripotent stem cell self-renewal and
differentiation. Bionanoscience. 2:266–276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SI, Yeo SI, Kim BB, Ko Y and Park JB:
Formation of size-controllable spheroids using gingiva-derived stem
cells and concave microwells: Morphology and viability tests.
Biomed Rep. 4:97–101. 2016.PubMed/NCBI
|
|
15
|
Azarin SM, Larson EA, Almodovar-Cruz JM,
de Pablo JJ and Palecek SP: Effects of 3D microwell culture on
growth kinetics and metabolism of human embryonic stem cells.
Biotechnol Appl Biochem. 59:88–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gattazzo F, Urciuolo A and Bonaldo P:
Extracellular matrix: A dynamic microenvironment for stem cell
niche. Biochim Biophys Acta. 1840:2506–2519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wan PX, Wang BW and Wang ZC: Importance of
the stem cell microenvironment for ophthalmological cell-based
therapy. World J Stem Cells. 7:448–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsu SH, Huang GS, Lin SY, Feng F, Ho TT
and Liao YC: Enhanced chondrogenic differentiation potential of
human gingival fibroblasts by spheroid formation on chitosan
membranes. Tissue Eng Part A. 18:67–79. 2012. View Article : Google Scholar : PubMed/NCBI
|