Introduction
Mesenchymal stem cells (MSCs) are mesodermal cells
derived from bone marrow, which may differentiate into osteoblasts,
chondrocytes, myoblasts, osteoblasts, chondrocytes, myoblasts,
stromal cells, adipocytes and other cells (1). Dysregulation of the differentiation of
MSCs into osteoblasts is involved in several bone-related diseases,
including osteoporosis (2). MSCs
have the potential to be used as therapeutic agents since they are
easily isolated and extracted from patients, without ethical or
technical problems. The inherent potential of MSCs makes them ideal
seed cells for cell transplantation therapy, which is expected to
become an effective treatment of intractable orthopedic disease
(3,4). Although MSCs are promising targets for
the treatment of a number of bone diseases, the outcome of MSCs
must be controlled before their therapeutic potential can be
realized. Therefore, an improved understanding of the signaling
pathways that control the differentiation of MSCs is required.
Nuclear receptors are ligand-activated transcription
factors that perform a central function in the differentiation of
stem cells (5). Among them, vitamin
D receptor (VDR) has a critical role in the control of osteogenic
differentiation of MSCs (6). VDR is
composed of three distinct regions: An N-terminal region,
containing a ligand-independent activation function-1; a highly
conserved central region, containing the DNA binding domain; and
the C-terminal region of the receptor, containing a multifunctional
domain that harbors the ligand binding domain, the retinoid X
receptor heterodimerization motif and ligand-dependent activation
function-2 (7). VDR response
elements (VDREs) have been identified in the promoters of vitamin
D3-responsive genes, such as osteopontin (8). Previous studies have characterized MSCs
as a target of VDR functions (9).
Furthermore, the epigenetic regulation of the VDR may be key to
rejuvenating osteoblastogenesis in MSCs from elders (10). The active form of vitamin D, 1α,
25-dihydroxyvitamin D3 [1,25(OH)2D3; VD3], is the ligand
for VDR and has been revealed to enhance osteoblast-mediated
mineralization (11). Moreover,
vitamin D induced transcriptional networks in the osteoblast
differentiation in a previous study (12). Thus, VDR is proposed as an important
pharmacological target for signaling pathways involved in
osteogenic differentiation of MSCs. Therefore, VDR can effectively
screen for novel drugs from medicinal plants, particularly
traditional Chinese herbs. We previously reported that steroids
found in traditional Chinese herb were able to control the fate of
MSCs (13,14).
In the present study, an in vitro model was
established using a VDRE reporter gene assay, which constructed a
VDRE promoter reporter and was transfected into MSCs. Then MSCs
were treated with steroids to identify VDR signaling activators
that may be involved in the osteogenic differentiation.
Furthermore, based on the previous finding that steroids are able
to enhance osteogenic differentiation of MSCs (13), various structural analogs of steroids
were investigated. The analogs included VD3, (+)-cholesten-3-one
(CN), steraric acid ethyl ester (SE), cholesterol (CL), cholesterol
myristate (CM), cyasterone (CE), β-sitosterol (SL) and oleanolic
acid (OA). Among them, CN was the specific compound that most
markedly promoted osteogenic differentiation of MSCs.
Materials and methods
Animal grouping and drug
administration
A total of 10 male specific pathogen free
Sprague-Dawley rats, aged 4 weeks (180–200 g), were acquired from
the Animal Centre of Guangzhou University of Chinese Medicine
(Guangzhou, China). All animals received humane care in accordance
with the guidelines set out by the Care of Experimental Animals
Committee of Guangzhou University of Chinese Medicine. Dulbecco's
modified Eagle medium (DMEM) and fetal bovine serum (FBS) were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA); VDRE, VD3, dimethyl sulphoxide (DMSO) and other chemical
reagents were purchased from Sigma-Aldrich (Merck Millipore,
Darmstadt, Germany); alizarin red stain solution, alkaline
phosphatase (ALP; BA0632), osteopontin (OPN; PB0589), Runt-related
transcription factor 2 (RUNX2; BA3613-2), VDR (PB0479) and collagen
II (BA0533) antibody were provided by Wuhan Boster Biological
Technology, Ltd., (Wuhan, China); and SE, CL, CM, CN, CE, SL and OA
were provided by Tokyo Chemical Industry Co., Ltd., (Tokyo,
Japan).
Culture of MSCs
The femur and tibia were harvested from rats,
anaesthetized by chloral hydrate (330 mg/kg; Wuhan Boster
Biological Technology, Ltd.) and sacrificed by cervical
dislocation, in order to collect fresh marrow. The marrow was mixed
with complete medium (low glucose DMEM supplemented with 10% FBS)
and gradient centrifuged at 900 × g for 30 min at room temperature
with Percoll at a density of 1.073 g•ml−1. Cells of the
appropriate density were collected, washed with PBS three times,
manually counted using a light microscope and cultured at a density
of 1×106 cm−2 on dishes supplemented with
complete medium, in a humidified atmosphere at 37°C and 5%
CO2, using an incubator. The medium was refreshed and
the suspension cells were removed every three days. Isolated MSCs
were confluent after nine days, and were digested using 0.25%
trypsin in order to promote separation. Cells were subsequently
passaged at a density of 1×104 cm−2 onto
dishes. MSCs that had been passaged three times were used for
subsequent experiments. The surface antigen identification and
differentiation ability of MSCs were verified by our previous study
(13,14).
Plasmids, small interfering (si) RNA
cell transfection and assay for luciferase activity
The purchased pGL3-basic vector and VDRE were
digested using purchased NheI and HindIII (Takara
Biotechnology Co., Ltd., Dalian, China), and both were combined to
generate the VDRE promoter-Luc vector. Plasmid for cytomegalovirus
(CMV) was cotransfected to normalize the variations in transfection
efficiency. The pGL3-basic vector, VDRE vector, and pCMV-Myc-basic,
pCMV-Myc-VDR, pCMV-Myc-VDR-N and pCMV-Myc-VDR-C plasmids were
provided by the National Laboratory of Medical Molecular Biology
(Beijing, China).
The sequence of the siRNA oligonucleotide that was
used to silence the VDR was 955-GUGCCAUUGAGGUCAUCAUTT-975. The
scramble siRNA with random nucleotides was
5′-UUCUCCGAACGUGUCACGUTT-3′ (negative control siRNA). Both
oligonucleotides were synthesized by Genepharma Corp., (Shanghai,
China). siRNA were transfected into cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocols.
Dual luciferase assay was performed to study the
effects of VD3, SE, CL, CM, CN, CE, SL and OA on VDRE promoter
activity. VD3 (30 ng·ml−1), SE, CL, CM, CN, CE, SL and
OA were dissolved in DMSO, respectively, and subsequently added to
MSCs with an increasing concentration of 0–100 µg·ml−1.
MSCs seeded in complete medium treated with an equal volume of DMSO
without steroids served as the control group. Luciferase assays
were performed using a Dual Luciferase Assay kit (Promega Corp.,
Madison, WI, USA) and were used to detect the luciferase activity.
A total of 10 µl of cell lysate was added to MSCs to detect firefly
luciferase activity. Following the standardization of
Renilla luciferase activity, the activity of Renilla
luciferase was detected.
Immunocytochemistry
Following treatment with CN, MSCs were fixed with 4%
polyoxymethylene for 20 min at room temperature and permeabilized
with 0.25% Triton X-100 (Sigma-Aldrich; Merck Millipore) and
blocked with 0.1% bovine serum albumin (BSA; Roche Diagnostics,
Basel, Switzerland). Subsequently, MSCs were incubated overnight at
4°C with the following primary antibodies at dilutions of 1:200:
ALP, OPN, RUNX2 and collagen II. After washing three times with
PBS, the cells were reacted with the horseradish
peroxidase-conjugated secondary antibody (ab6721; 1:2,000; Abcam,
Cambridge, UK) for 30 min at 37°C. The cell nuclei were stained
with hematoxylin (Wuhan Boster Biological Technology, Ltd.) and
visualized via light microscopy (Leica Camera AG, Wetzlar,
Germany). Percentage of positive cells vs. total cells in 4 fields
was calculated. Rat ALP, OPN, RUNX2 and collagen II primary
antibodies (all 1:200), mixed with non-immune serum, were used to
immunostain MSCs at 4°C overnight, respectively. Secondary antibody
was used to incubate for 30 min. The expression of ALP, OPN, RUNX2
and collagen II was detected after diaminobenzidine staining,
followed by hematoxylin as the counterstain. Cytoplasm of positive
cells were detected as deep brown. The same method was applied to
the control group, except that the primary antibody with non-immune
serum.
Calcium mineral deposition
MSCs were cultured for 14 days, as described. The
level of calcium mineral deposition was revealed using alizarin-red
staining (AR-S). Following three weeks in culture, cells were fixed
with 70% ethanol, washed five times with deionized water and
treated with 40 mM alizarin-red solution for 10 min at pH 4.2.
Subsequently, cells were washed with PBS for 15 min before being
treated with 10% cetylpyridinium chloride in 10 mM sodium phosphate
for 15 min at room temperature. An AR-S standard curve, at an
optical density of 540 nm, was used to calculate the AR-S
concentration.
Western blot analysis
Total protein was extracted from MSCs with cell
lysis buffer (Invitrogen, Thermo Fisher Scientific, Inc.) and the
protein concentration was determined using a Bradford protein assay
(bicinchoninic acid protein assay kit; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). SDS-PAGE (6–15%; Guge Biological Technology
Co.) was created and separated proteins (20 µg) were transferred to
a membrane. The membrane was blocked in 5% BSA at 24°C for 1 h,
incubated with primary polyclonal antibodies against ALP, OPN,
RUNX2, VDR, collagen II (all 1:200) and β-catenin (ab32572;
1:5,000; Abcam, Cambridge, UK) at 4°C overnight, followed by three
washes in TBST for 5 min each. The membrane was then incubated with
Alexa Fluor-conjugated goat anti-rabbit IgG secondary antibody
(ab150077; 1:2,000; Abcam) for 30 min at 24°C, washed with TBST
three times for 5 min each and protein bands were detected with
DAB. The blot was scanned and optical density values of the
targeted bands were analyzed with Image J software (1.48U; National
Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All data were expressed as mean ± standard
deviation. SPSS 20.0 software (IBM SPSS, Armonk, NY, USA) was used
to analyze all data. P<0.05 was considered to indicate a
statistically significant difference.
Results
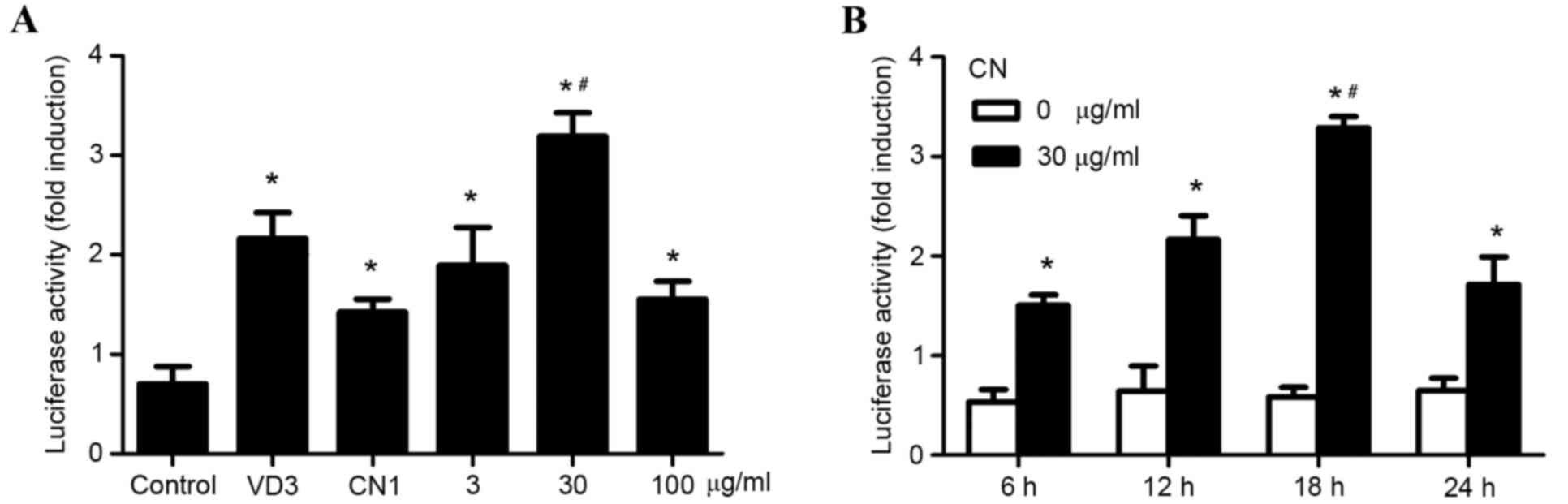
CN increases VDRE promoter
activity
Induced expression of VDR provoked by CN stimulation
of MSCs had been confirmed in our previous study (13,14). The
present study examined whether CN increased VDRE promoter activity.
VDRE promoter reporter construct was transfected into MSCs and
cells were treated with 1, 3, 30 or 100 µg·ml−1 of CN,
respectively. MSCs that did not receive treatment were considered
the control group, while VD3 was used for positive control, due to
its effective promotion in VDRE promoter activity, as verified by a
previous study. The highest VDRE promoter activity was observed at
a CN concentration of 30 µg·ml−1, which was
statistically significant when compared to the other groups
(P<0.05; Fig. 1A). MSCs
transfected with VDRE promoter reporter construct were treated with
30 µg·ml−1 of CN and compared to MSCs that did not
receive CN treatment. VDRE promoter activity was measured after 6,
12, 18 and 24 h of exposure. The results indicated that the optimal
time point of VDRE promoter activity of MSCs treated with 30 µg/ml
of CN was 18 h, as revealed by the significantly increased fold
induction of luciferase activity (P<0.05; Fig. 1B).
CN showed the highest activity on the
VDRE, which may be associated with its ketone group
In our previous studies (13,14), it
was revealed that steroids had a stimulating effect on VDRE
promoter activity; however, the specific compound responsible for
achieving this effect is still unclear. The present study aimed to
investigate MSCs that were transfected with VDRE promoter reporter
construct, followed by either no treatment with the analogs of
steroid (negative control), treatment with either VD3 (30
ng·ml−1, positive control), or with SE, CL, CM, CN, CE,
SL, and OA (30 µg/ml) for 18 h (Fig.
2A). A significantly increased activity level was observed in
CN-treated MSCs when compared with MSCs treated with the other
steroids (P<0.05; Fig. 2B). This
indicated that CN was the optimal compound in promoting the highest
VDRE promoter activity, even higher than VD3, which was the
positive control (P<0.05). Compared with the other compounds,
especially CL and SL with high similarity with CN, the highest
activity was observed in CN-treated MSCs; however, the only
structural difference between CN and CL is the presence of a ketone
group and OH group, respectively, which suggested that the ketone
group of CN may be involved in promoting VDRE promoter
activity.
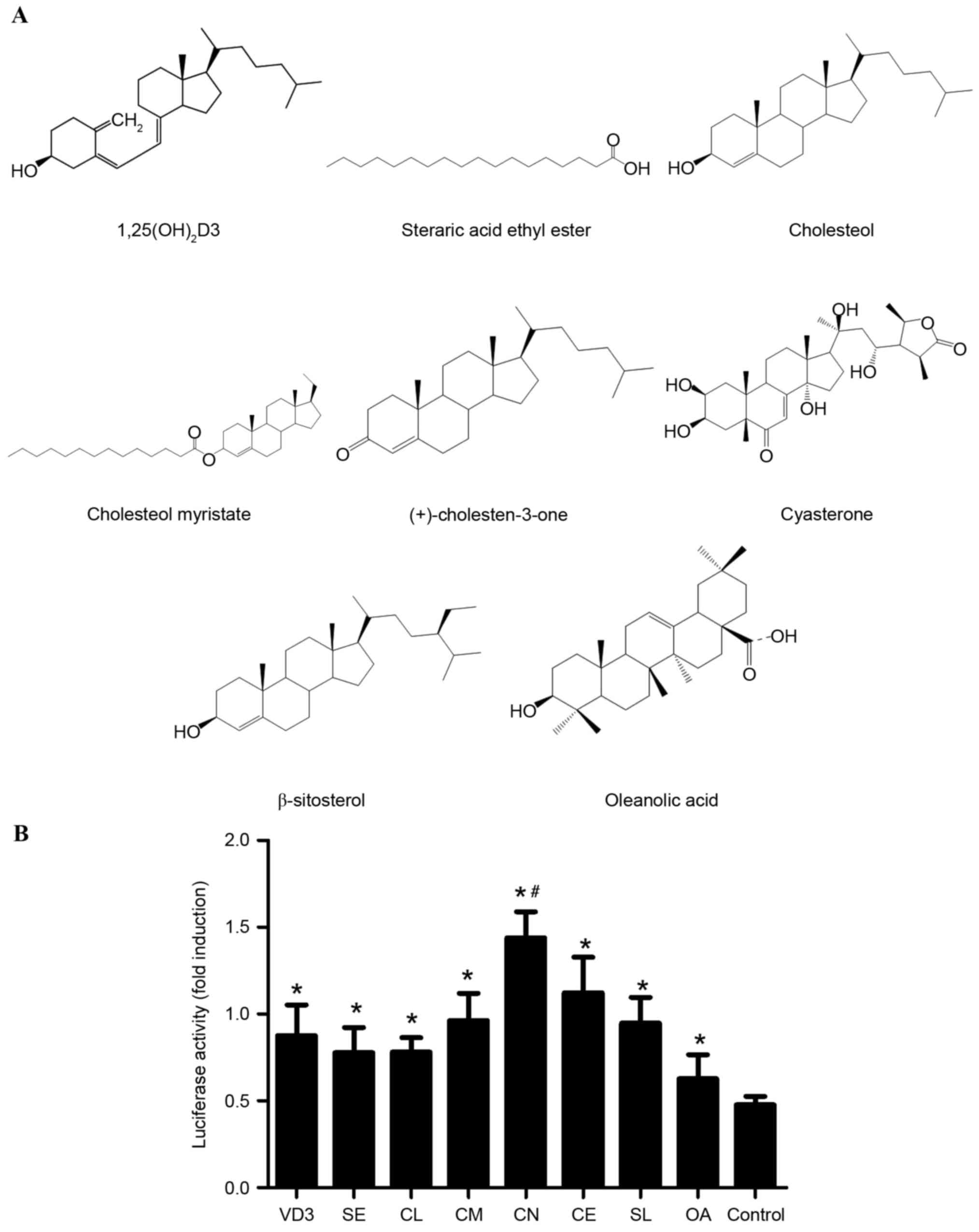 | Figure 2.Effective activity of VDRE promoter is
associated with the ketone group of CN that promotes the effective
activity of the VDRE promoter. (A) Structure of VD3, SE, CL, CM,
CN, CE, SL and OA. (B) Comparative effects of VD3, SE, CL, CM, CN,
CE, SL and OA. All data were expressed as mean ± standard
deviation. *P<0.05 vs. control; #P<0.05 vs. all
other groups. CN, VD3, 1α, 25-dihydroxyvitamin D3; SE, steraric
acid ethyl ester; CL, cholesterol; CM, cholesterol myristate; CE,
cyasterone; SL, β-sitosterol; OA, oleanolic acid. |
CN promotes osteogenic differentiation
of MSCs
MSCs were stimulated by CN (30 µg/ml) for seven
days, immunocytochemically stained using osteogenesis markers and
AR-S and further analyzed using western blotting.
Immunocytochemical staining for osteogenesis markers containing
ALP, OPN, RUNX2 and collagen II indicated that CN promoted
osteogenic differentiation of MSCs as CN-treated MSCs exhibited a
significantly increased number of osteogenisis-positive cells
compared with MSCs that did not receive CN treatment (P<0.05;
Fig. 3A). AR-S demonstrated that
CN-treated MSCs exhibited an increased number of calcium mineral
deposition derived from osteoblasts when compared with MSCs that
were not treated with CN (Fig. 3B).
Furthermore, western blotting revealed significantly higher
expression of ALP, OPN, RUNX2 and collagen II in CN-treated MSCs
when compared to MSCs that did not receive CN treatment, indicating
that CN-treated MSCs exhibited significantly increased osteogenic
cell differentiation (P<0.05; Fig.
3C).
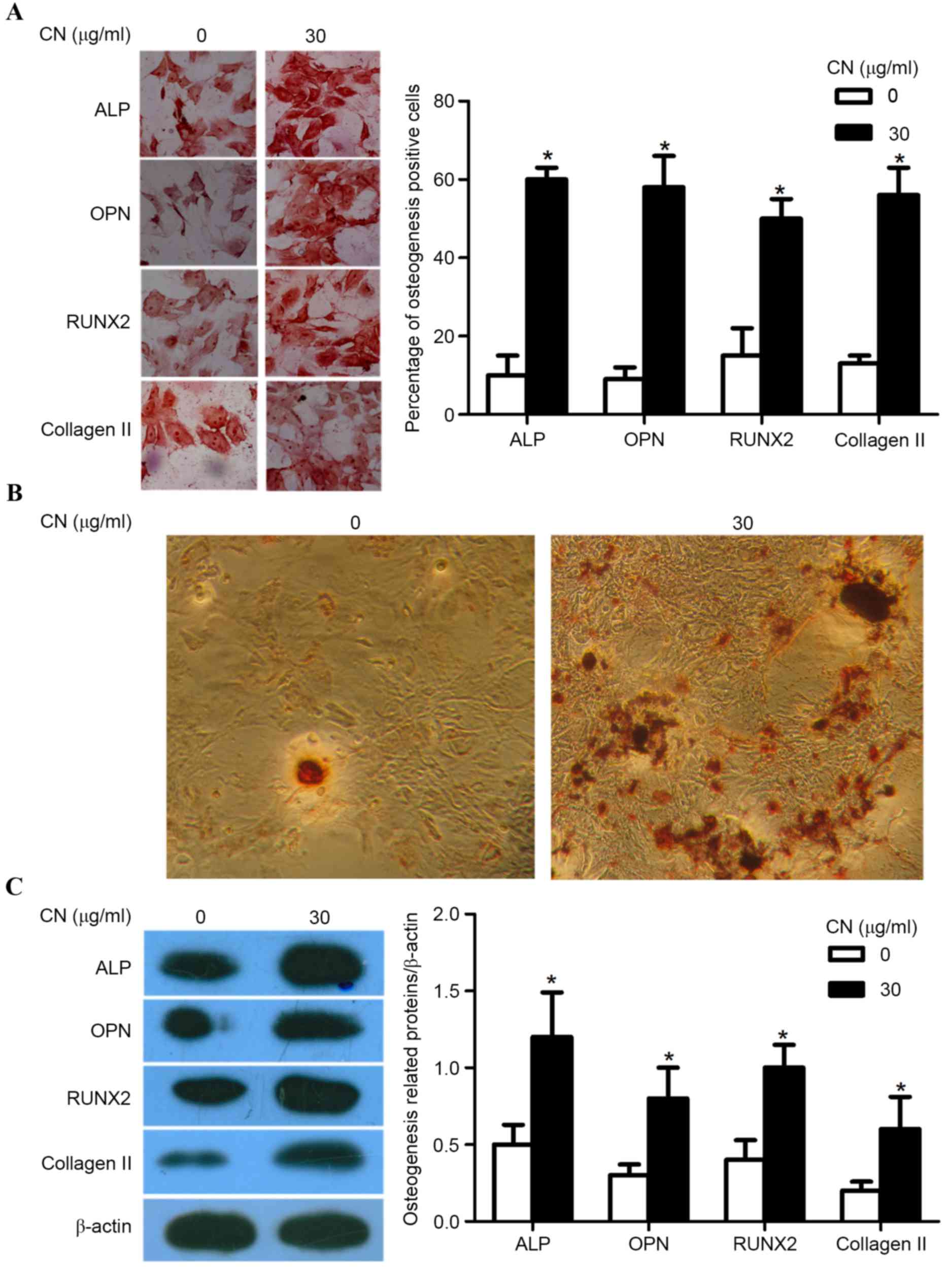 | Figure 3.CN promotes the osteogenic
differentiation of MSCs. (A) Photomicrographs were captured at a
magnification of ×200 and demonstrated the levels of ALP, OPN,
RUNX2 and collagen II expressed in MSCs, where the cytoplasm was
stained dark brown (left panel). Comparison of the percentage of
ALP, OPN, RUNX2 and collagen II-positive cells after CN treatment
for seven days (right panel). (B) Alizarin-red staining revealed
the calcium mineral deposition of CN-treated MSCs compared with
MSCs that were not treated with CN dyed by alizarin-red staining.
(C) Western blot analysis indicated the expression of ALP, OPN,
RUNX2 and collagen II (left panel) by comparing the relative
density of ALP/β-actin, OPN/β-actin, RUNX2/β-actin and collagen
II/β-actin band (right panel). All data were expressed as mean ±
standard deviation. *P<0.05 vs. 0 µg/ml. MSCs, mesenchymal stem
cells; ALP, alkaline phosphatase; OPN, osteopontin; RUNTX2,
runt-related transcription factor 2; CN, (+)-cholesten-3-one. |
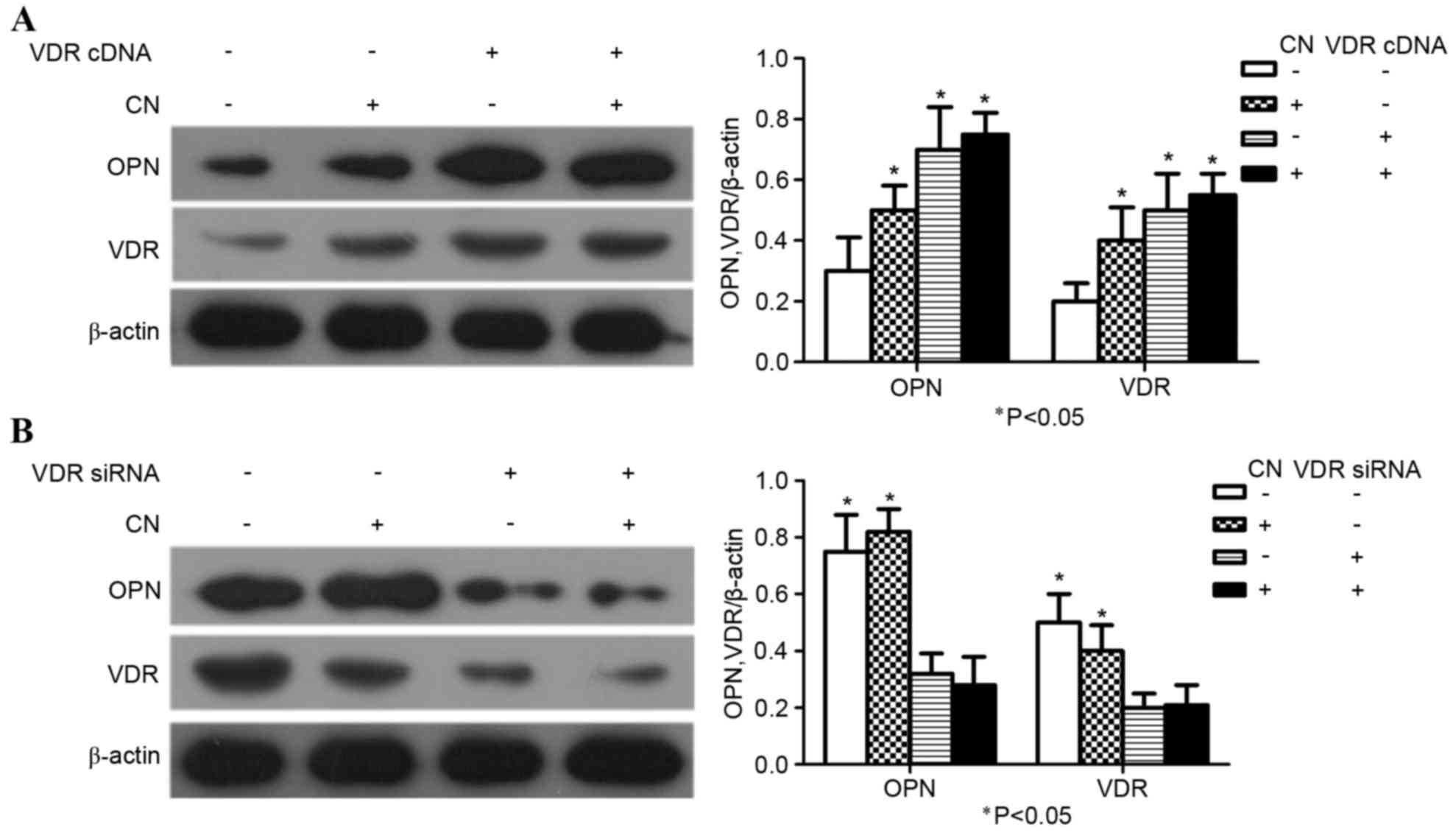
CN promotes osteogenic differentiation
of MSCs and requires VDR
MSCs transfected with VDR cDNA construct were
treated with optimal concentrations of CN. The expression of OPN
and VDR were significantly increased in the group that was
comprised of the combination of VDR cDNA and CN compared with the
group where VDR cDNA and CN were both absent. (P<0.05; Fig. 4A). MSCs were also transfected with a
VDR siRNA construct and treated with optimal concentrations of CN.
Furthermore, VDR siRNA inhibited the expression of OPN and VDR in
MSCs compared with the groups where VDR siRNA was present and CN
was absent (P<0.05; Fig. 4B).
These experiments indicated that CN-induced differentiation of MSCs
required VDR.
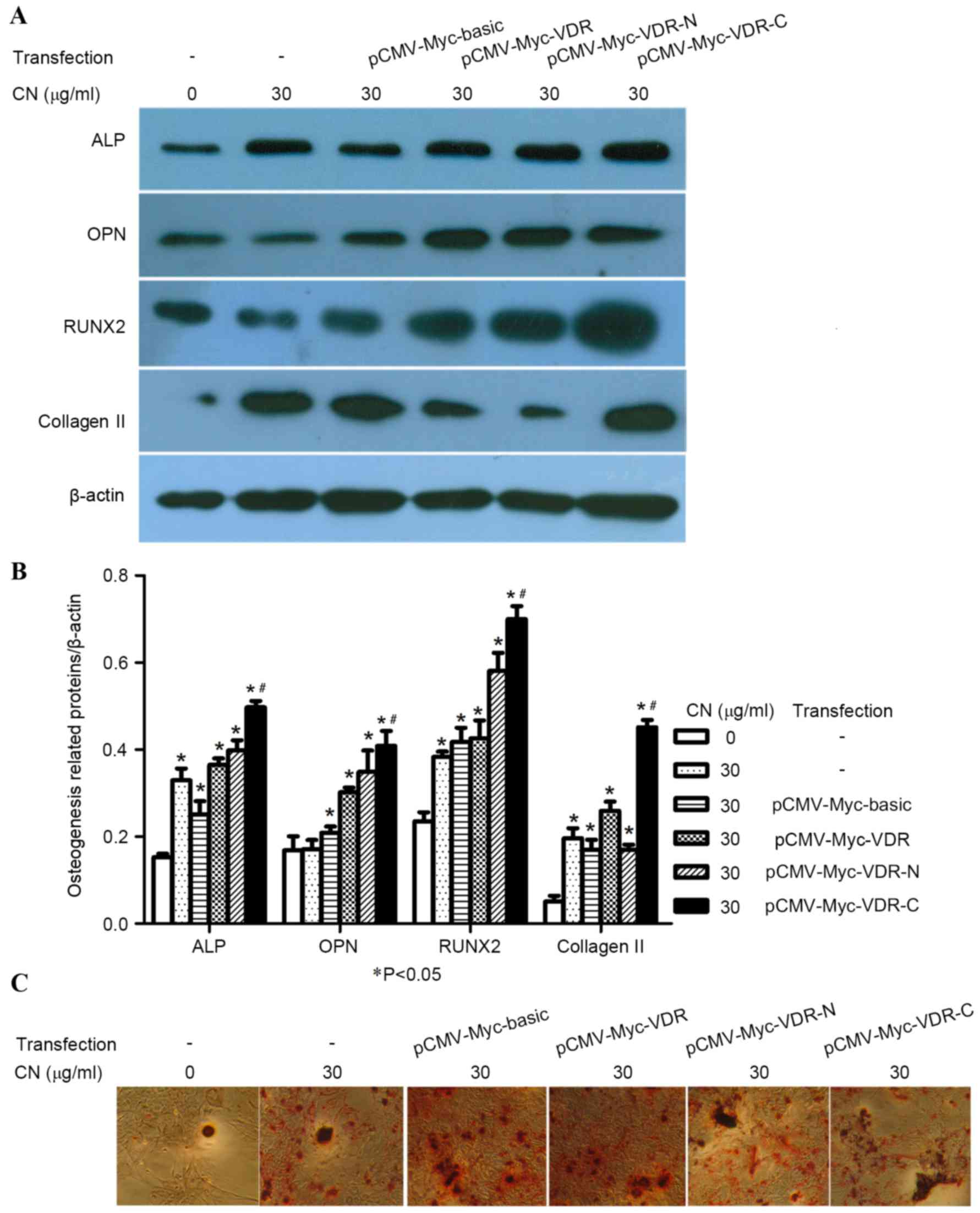
C-terminal region of VDR is
responsible for the action of CN
VDR fusion proteins included the following: VDR
1–91, an N-terminal region containing activation function-1 (AF-1)
domain and a DNA binding domain (DBD) and VDR 115–427, a C-terminal
region with a ligand binding domain, and a ligand-dependent AF-2
domain (Fig. 5A). To confirm whether
CN interaction with the binding domains of VDR affected the
differentiation of MSCs into osteogenic cells, MSCs were stimulated
by CN and transfected with either pCMV-Myc-basic, pCMV-Myc-VDR,
pCMV-Myc-VDR-N or pCMV-Myc-VDR-C plasmids. After seven days,
immunocytochemical staining demonstrated that groups transfected
with pCMV-Myc-VDR-C plasmids exhibited significantly increased
osteogenic-positive cells (P<0.05; Fig. 5B and C). Western blot analysis
indicated the expression of ALP, OPN and RUNX2 increased when MSCs
were treated with CN and transfected with pCMV-Myc-VDR-C plasmids
(Fig. 6A and B). Moreover, AR-S
identified that CN-treated MSCs transfected with pCMV-Myc-VDR-C
plasmids exhibited increased numbers of osteogenic-positive cells
when compared with the positive and negative control groups
(Fig. 6C).
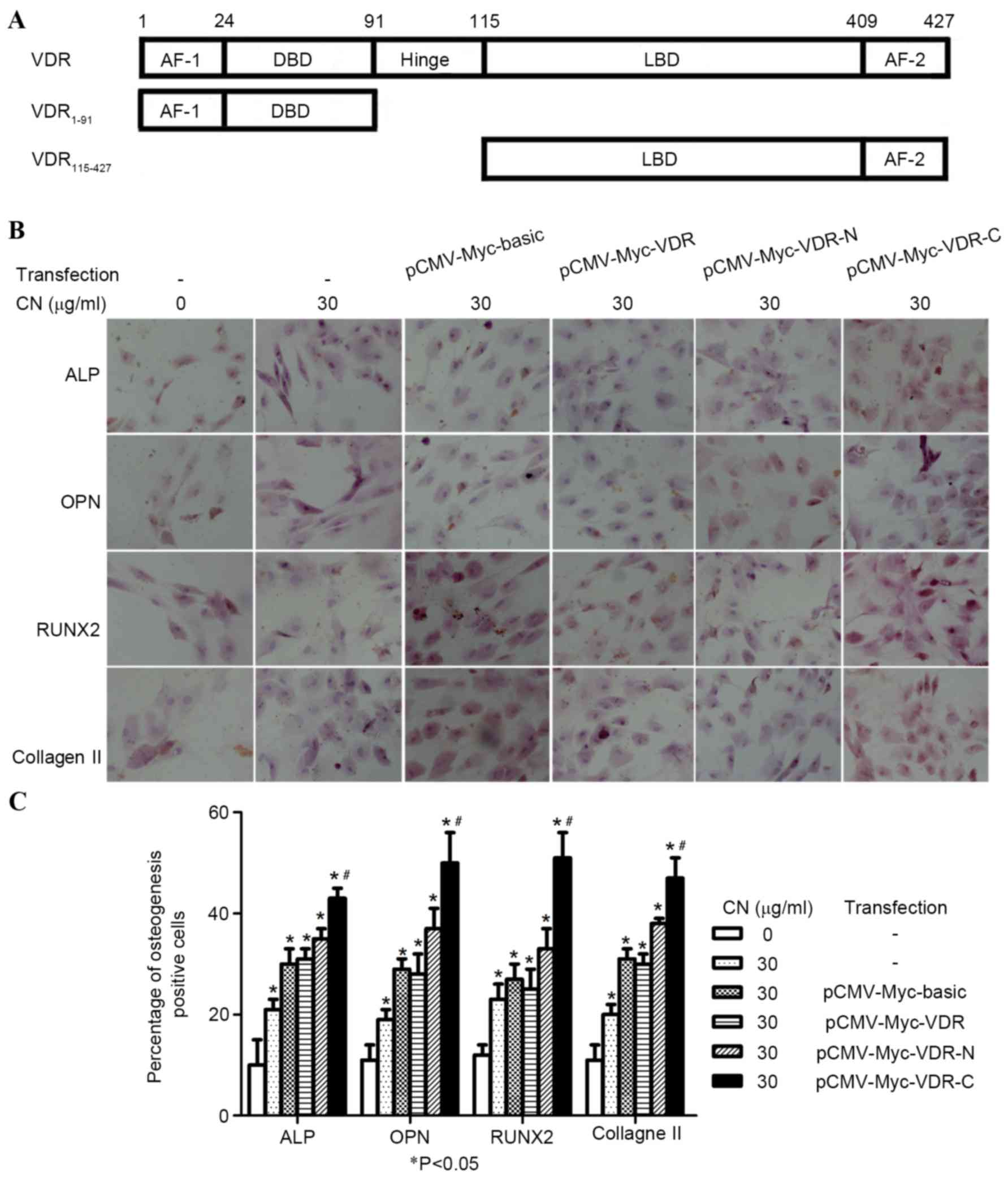 | Figure 5.C-terminal region of the VDR is
responsible for the action of CN. (A) Structure of VDR. (B)
Photomicrographs showing ALP, OPN, RUNX2 and collagen II-positive
cells in cytoplasm, which were dark brown (magnification, ×200).
(C) Comparison of the percentage of ALP, OPN, RUNX2 and collagen
II-positive cells after CN treatment and transfection for seven
days. All data were expressed as mean ± standard deviation.
*P<0.05 vs. CN and transfection were absent;
#P<0.05 vs. all other groups. VDR, vitamin D
receptor; OPN, osteopontin; CN, (+)-cholesten-3-one; ALP, alkaline
phosphatase; RUNX2, runt-related transcription factor 2; CMV,
cytomegalovirus. |
Discussion
MSCs are capable of self-renewal and differentiation
into osteogenic cells; however, the efficiency of these functions
is typically low (15) and the
process is not fully understood. Thus, the present study aimed to
elucidate the process of MSC differentiation into osteogenic cells
by exploring its physiological mechanism and therapeutic potential.
In the present study, a functional stem cell assay was used based
on the VDR pathway as this facilitated improved understanding of
the identified compounds involved in this pathway. The major
findings of the present study were: i) CN exhibited significantly
higher VDRE promoter activity when compared with SE, CL, CM, CE, SL
and OA; ii) CN-promoted osteogenic differentiation of MSCs that
required VDR; and iii) the C-terminal region of the VDR is involved
in the action of CN. The present study demonstrated a model that
revealed structure-function relationships between steroids and VDR.
The findings of the present study provide the incentive to develop
a pioneering strategy to screen novel drugs for orthopedic
disorders and to study a series of similar compounds involved in
the regulation of stem cells.
In the present study, CN was identified as an
inducer of MSC differentiation into osteoblasts, through cell
phenotypic analysis. MSCs differentiate into all mesodermal cell
types, including osteoblasts, chondrocytes, myoblasts, stromal
cells and skeletal muscle cells, under appropriate conditions.
Therefore, MSCs were chosen as a cell model in the present study.
There is growing interest in the use of MSCs for the repair of bone
damage; however, this requires efficient protocols for directing
the differentiation of MSCs into the osteoblast lineage. Several
studies have explored the use of bone morphogenetic proteins to
induce MSCs toward the osteoblast lineage (16,17).
Furthermore, these reports have described the positive effect of
large molecules on the osteogenesis of MSCs; however, small
molecules that may promote the osteogenic differentiation of MSCs
have not been identified (16,17).
Previous findings have also indicated that dexamethasone and VD3
were used to promote MSCs differentiation in vitro (18). In the present study,
immunohistochemical analysis and western blot analysis established
that CN induced MSCs to differentiate into osteoblasts. This result
is consistent with our previous findings, which provided evidence
that CN promotes the differentiation of neural stem cells (19). Therefore, CN may be a potential
therapeutic molecule for treating differentiation-related bone
diseases.
Another notable finding of the present study was
that CN functions as an activator of VDR. VDR, which consists of an
N-terminal DNA-binding domain, a C-terminal ligand-binding domain
and an intervening hinge region, is the nuclear hormone receptor of
the vitamin D endocrine system that is predominantly involved in
the maintenance of calcium and phosphate homeostasis and bone
development (20–22). Furthermore, VDR is a ligand-induced
nuclear transcription factor that regulates the expression of genes
in critical mineral-regulating target tissues, such as bone, in
order to maintain appropriate mineral homeostasis. Humans and mouse
models have indicated that a lack of functional VDR is associated
with severe bone diseases (20–22).
Furthermore, it has also been suggested that VDR has a direct role
in promoting osteoblast differentiation (23). Additionally, prior reports have
identified that VDR-null mice exhibited a reduction in osteoblasts,
accompanied by a decrease in trabecular bone volume (24), and MSC differentiation was impaired
in VDR mice that presented with haploinsufficiency (25). Thus, strategies targeting VDR
pathways for the stimulation of MSC differentiation are highly
desirable.
We hypothesized that VDR may be one of the direct
mediators of CN actions. Several experiments were performed to test
this hypothesis, including the use of transcription assays, which
employed a VDRE-firefly luciferase reporter plasmid and
immunoblotting of CN-mediated induction of VDR target genes.
Furthermore, over-expression of VDR and knockdown studies with
VDR-siRNA were performed, in addition to a series of deletion
mutants of the VDR and a MSCs differentiation assay. Notably,
compared with the other investigated compounds, CN showed the
highest promotion for the activity of VDRE promoter; but CL and SL
with the higher similarity with CN, exhibited reduced VDRE promoter
activity. This indicated that the ketone group of CN may be a
functional group that is associated with the activity of VDRE. The
present study also revealed that CN induced the expression of the
VDR target genes, such as osteopontin, which suggested that the VDR
pathway was activated by CN. This result is consistent with
observations on the activity of the VDRE promoter from the present
study. siRNA-mediated knockdown of VDR in the MSCs differentiation
model system revealed that the pro-differentiation effects of CN
required VDR. Furthermore, a series of deletion mutants of the VDR
were generated and used to identify that the C-terminal region of
the VDR is responsible for the action of CN. These results are
consistent with previous reports that nuclear receptors are
activated by small lipophilic ligands (5,7,9).
The present findings have promising clinical
applications. With the prevalent-use of CN, it may be possible to
improve the quality of engineered bone via MSC-mediated
osteogenesis. This is an example of pharmacological control of
MSC-mediated osteogenesis and highlights the need for an extensive
screen of other families of small molecules both for bone formation
and to regulate other differentiation pathways. Manipulation of
MSC-mediated osteogenesis is an interesting alternative approach as
the agents used in vitro were cleared from the graft before
implantation.
VDR is an important target for osteoporosis and
targeting VDR pharmacologically for the purpose of modulating stem
cell function is a promising area in regenerative medicine. By
identifying the role of VDR in MSCs and developing corresponding
specific ligands as modulators, the therapeutic delivery of stem
cells may become more controlled and efficient. Hence, the
activation of vitamin D receptor by CN may provide a novel concept
for the treatment of bone diseases, such as fracture repair and
osteoporosis. Finally, systemic administration of CN may be used to
treat patients by targeting either their endogenous or donated
MSCs.
In conclusion, the present study demonstrated that a
stem cell analysis system based on VDR signaling may be used to
identify molecules that induce the osteogenic differentiation of
MSCs and specifically revealed that CN significantly increased the
efficiency of osteogenic differentiation of MSCs by activating the
vitamin D receptor. These findings may have various applications in
the field of bone and joint repair and raise interesting questions
about the role of CN in osteogenesis.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81273896, 81273783
and 81473699), Guangdong Technology Projects of Self-financing
Category (grant no. 2014807), Guangxi Scientific and Technological
Issues (grant no. 2015AD09631) and Research Projects of
Construction of Chinese Medicine province of Bureau of Traditional
Chinese Medicine of Guangdong Province (grant no. 20141084).
References
|
1
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olsen BR, Reginato AM and Wang W: Bone
development. Annu Rev Cell Dev Biol. 16:191–220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cavallo C, Desando G, Cattini L, Cavallo
M, Buda R, Giannini S, Facchini A and Grigolo B: Bone marrow
concentrated cell transplantation: Rationale for its use in the
treatment of human osteochondral lesions. J Biol Regul Homeost
Agents. 27:165–175. 2013.PubMed/NCBI
|
|
4
|
Gao C, Seuntjens J, Kaufman GN, Tran-Khanh
N, Butler A, Li A, Wang H, Buschmann MD, Harvey EJ and Henderson
JE: Mesenchymal stem cell transplantation to promote bone healing.
J Orthop Res. 30:1183–1189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gronemeyer H, Gustafsson JA and Laudet V:
Principles for modulation of the nuclear receptor superfamily. Nat
Rev Drug Discov. 3:950–964. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Olivares-Navarrete R, Sutha K, Hyzy SL,
Hutton DL, Schwartz Z, McDevitt T and Boyan BD: Osteogenic
differentiation of stem cells alters vitamin D receptor expression.
Stem Cells Dev. 21:1726–1735. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Giguere V: Orphan nuclear receptors: From
gene to function. Endocr Rev. 20:689–725. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Noda M, Vogel RL, Craig AM, Prahl J,
DeLuca HF and Denhardt DT: Identification of a DNA sequence
responsible for binding of the 1,25-dihydroxyvitamin D3 receptor
and 1,25-dihydroxyvitamin D3 enhancement of mouse secreted
phosphoprotein 1 (SPP-1 or osteopontin) gene expression. Proc Natl
Acad Sci USA. 87:9995–9999. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeong Y and Mangelsdorf DJ: Nuclear
receptor regulation of stemness and stem cell differentiation. Exp
Mol Med. 41:525–537. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou S, Geng S and Glowacki J: Histone
deacetylation mediates the rejuvenation of osteoblastogenesis by
the combination of 25 (OH)D3 and parathyroid hormone in MSCs from
elders. J Steroid Biochem Mol Biol. 136:156–159. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woeckel VJ, Bruedigam C, Koedam M, Chiba
H, van der Eerden BC and van Leeuwen JP: 1α,25-dihydroxyvitamin D3
and rosiglitazone synergistically enhance osteoblast-mediated
mineralization. Gene. 512:438–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van de Peppel J and van Leeuwen JP:
Vitamin D and gene networks in human osteoblasts. Front Physiol.
5:1372014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen DF, Zeng HP, Du SH, Li H, Zhou JH, Li
YW, Wang TT and Hua ZC: Extracts from Plastrum testudinis promotes
proliferation of rat bone-marrow derived mesenchymal stem cells.
Cell Prolif. 40:196–212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen DF, Zhang HL, Du SH, Li H, Zhou JH,
Li YW, Zeng HP and Hua ZC: Cholesterol myristate suppresses the
apoptosis of mesenchymal stem cells via upregulation of inhibitor
of differentiation. Steroids. 75:1119–1126. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Meijer GJ, de Bruijn JD, Koole R and van
Blitterswijk CA: Cell-based bone tissue engineering. PLoS Med.
4:e92007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X and Cao X: BMP signaling and
skeletogenesis. Ann N Y Acad Sci. 1068:26–40. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ryoo HM, Lee MH and Kim YJ: Critical
molecular switches involved in BMP-2-induced osteogenic
differentiation of mesenchymal cells. Gene. 366:51–57. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siddappa R, Fernandes H, Liu J, van
Blitterswijk C and de Boer J: The response of human mesenchymal
stem cells to osteogenic signals and its impact on bone tissue
engineering. Curr Stem Cell Res Ther. 2:209–220. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen DF, Meng LJ, Du SH, Zhang HL, Li H,
Zhou JH, Li YW, Zeng HP and Hua ZC: (+)-Cholesten-3-one induces
differentiation of neural stem cells into dopaminergic neurons
through BMP signaling. Neurosci Res. 68:176–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bouillon R, Carmeliet G, Verlinden L, van
Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C and Demay M:
Vitamin D and human health: Lessons from vitamin D receptor null
mice. Endocr Rev. 29:726–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verstuyf A, Carmeliet G, Bouillon R and
Mathieu C: Vitamin D: A pleiotropic hormone. Kidney Int.
78:140–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gallagher JC and Sai AJ: Vitamin D
insufficiency, deficiency, and bone health. J Clin Endocrinol
Metab. 95:2630–2633. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou S, Glowacki J, Kim SW, Hahne J, Geng
S, Mueller SM, Shen L, Bleiberg I and LeBoff MS: Clinical
characteristics influence in vitro action of 1,25-dihydroxyvitamin
D(3) in human marrow stromal cells. J Bone Miner Res. 27:1992–2000.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Panda DK, Miao D, Bolivar I, Li J, Huo R,
Hendy GN and Goltzman D: Inactivation of the 25-hydroxyvitamin D
1alpha-hydroxylase and vitamin D receptor demonstrates independent
and interdependent effects of calcium and vitamin D on skeletal and
mineral homeostasis. J Biol Chem. 279:16754–16766. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Paula FJ, Dick-de-Paula I, Bornstein S,
Rostama B, Le P, Lotinun S, Baron R and Rosen CJ: VDR
haploinsufficiency impacts body composition and skeletal
acquisition in a gender-specific manner. Calcif Tissue Int.
89:179–191. 2011. View Article : Google Scholar : PubMed/NCBI
|