Introduction
Chronic obstructive lesions of the proximal
subclavian artery (SCA) lead to retrograde blood flow in the
ipsilateral vertebral artery, which results in symptoms such as
vertebrobasilar insufficiency, upper limb claudication, and
transient ischemic attacks (1). The
most common cause of such obstructive lesions is atherosclerosis
(2).
The present treatment methods for chronic
obstructive lesions of the SCA include extra-anatomic
carotid-subclavian bypass, percutaneous endovascular angioplasty
and stenting (1). Although open
surgery has a better patency compared with endovascular treatment
(3), experienced physicians
generally prefer endovascular treatment as the first-line
treatment, reserving open surgical treatment for patients with
total occlusive lesions or stenotic lesions that are anatomically
unsuitable for endovascular repair (2).
SCA lesions are classified as stenosis or occlusion,
according to the severity of obstruction (4). Chronic total occlusive lesions,
particularly those located at the ostium of the SCA, are more
complicated and difficult to operate on (5). Therefore, the technical success of
surgery is excellent for stenosis, whereas for occlusions it varies
largely (1). The primary reason for
this is that in the ostium of the SCA the guiding catheter cannot
be stably fixed, and the guide wire struggles to cross the proximal
fibrous cap of the occlusive lesions (5).
Noguchi et al (6) demonstrated that the presence of
calcification, the length of the occlusion and the presence of
multi-vessel disease were independent predictors of the procedural
success rate for percutaneous transluminal coronary angioplasty of
chronic total occlusions.
The aim of the present study was to classify ostial
occlusions of the SCA according to their angiographic morphology in
order to determine the role of angiographic morphology in SCA
occlusions and investigate the interventional operation strategy
and success rate.
Patients and methods
Patients
The inclusion criteria for the present study were as
follows: Total occlusion of the SCA, occlusions located in the
proximal segment (from the ostium of the SCA to the vertebral
artery) (7), and attempted
percutaneous transluminal angioplasty or stenting for the
occlusions. The exclusion criteria were: SCA hemodynamically
irrelevant stenosis, >2 SCA occlusions or occlusions developed
with collateral circulation.
Eventually, the present study included seven
patients with total occlusion of the left SCA (LSCA) and one
patient with total occlusion of the right SCA (RSCA), who were
treated at the Southwest Hospital or Xinqiao Hospital (Chongqing,
China) between August 2010 and April 2014. A total of 6 male and 1
female patients with a mean age of 65.6 (range, 60–72) years were
successfully treated with percutaneous transluminal angioplasty and
stenting. One 71-year-old female patient with RSCA occlusion did
not respond to endovascular treatment and therefore underwent
medical therapy instead of stenting. Patient demographics and
clinical characteristics, including the presence of co-morbidities,
are summarized in Table I. Total
occlusion of the SCA was diagnosed in all patients via duplex
ultrasound evaluation, computed tomography angiography (CTA) or
conventional angiography. The lesions present in 7 patients were
caused by atherosclerosis resulting from their history of
hypertension in conjunction with dyslipidemia or diabetes. The RSCA
occlusive lesion in the female patient treated with stenting was
attributed to her history of Takayasu arteritis and hydrocortisone
therapy. The present study was approved by the review committee of
Third Military Medical University (Chongqing, China) and written
informed consent was obtained from all patients prior to
participation in the current study. All patients and their families
were informed about the intended use of self-expanding or
balloon-expandable stents in the SCA and the investigative nature
of this procedure.
 | Table I.Demographics and clinical
characteristics of patients. |
Table I.
Demographics and clinical
characteristics of patients.
| Patient no./age
(y)/Sex | Symptoms | Comorbidities |
|---|
| 1/60/M | Asymptomatic | CI |
| 2/70/M | Upper limb
claudication | CI, HTN, DM, CHD |
| 3/62/M | Asymptomatic | CHD, HL |
| 4/68/M | Upper limb
claudication | HTN, DM, HL |
| 5/62/F | Dizziness, upper
limb claudication | TIA |
| 6/65/M | Dizziness, upper
limb claudication | CI, DM |
| 7/71/F | Drop attack,
dizziness | HTN, DM, CHD |
| 8/72/M | Vertigo, upper limb
claudication | HTN, DM, HL |
Percutaneous transluminal
angioplasty
All patients were premedicated with aspirin (100
mg/day; Bayer AG, Leverkusen, Germany) and clopidogrel (75 mg/day;
Sanofi S.A., Paris, France) ommencing 3 days prior to the
operation. All procedures were performed under local anesthesia of
100 mg Lidocaine (China Otsuka Pharmaceutical Co. Ltd., Tianjin,
China). Right femoral access was obtained via a standard Seldinger
puncture (8), followed by the
placement of an 8 Fr introducer sheath. Anticoagulation was
preliminarily achieved with an initial bolus of 6,000 IU
intravenous heparin (Shanghai No. 1 Biochemical &
Pharmaceutical Co. Ltd., Shanghai, China). An 8 Fr guiding catheter
was then advanced over the wire into the ostium of the LSCA, and an
angiogram was obtained in the anteroposterior and left anterior
oblique (45 degree) views to confirm the location, length and
degree of the lesion. According to the angiographic morphology, the
occlusions of the 8 patients were classified as: Rat-tail, peak,
hilly or plain types (Fig. 1). All
angiographic morphologies, the treatment techniques used and
follow-ups are summarized in Table
II.
 | Table II.Angiographic morphologies, treatment
techniques, follow-up duration and results. |
Table II.
Angiographic morphologies, treatment
techniques, follow-up duration and results.
| Patient no. |
Location/Degree/Type of lesion | Access of
approach | Stent-craft size
(mm) | Follow-up duration
(m) | Result |
|---|
| 1 | LSCA
Proximal/Subtotal occlusive/Rat-tail Type | Femoral (R) | 8×30, 9×20
(SES) | 19 | Patent;
Recovered |
| 2 | LSCA Proximal/Total
occlusive/Peak Type | Femoral (R) | 9×30 (BAS) | 6 | Patent;
Recovered |
| 3 | LSCA Proximal/Total
occlusive/Peak Type | Femoral (R) | 8×30 (SES) | 1 | Patent;
Recovered |
| 4 | LSCA Proximal/Total
occlusive/Peak Type | Femoral (R) | 9×30 (SES) | 36 | Patent;
Recovered |
| 5 | RSCA Proximal/Total
occlusive/Peak Type | Femoral (R) and
radial (R) | 8×30 (SES) | 6 | Patent;
Recovered |
| 6 | LSCA Proximal/Total
occlusive/Hilly Type | Femoral (R) and
radial (L) | 12×40, 10×20
(SES) | 17 | Patent;
Recovered |
| 7 | LSCA Proximal/Total
occlusive/Hilly Type | Femoral (R) |
| 3 | Non-patent;
TIA |
| 8 | LSCA Proximal/Total
occlusive/Plain Type | Femoral (R) and
radial (L) | 9×30,8×30
(SES) | 25 | Patent;
Recovered |
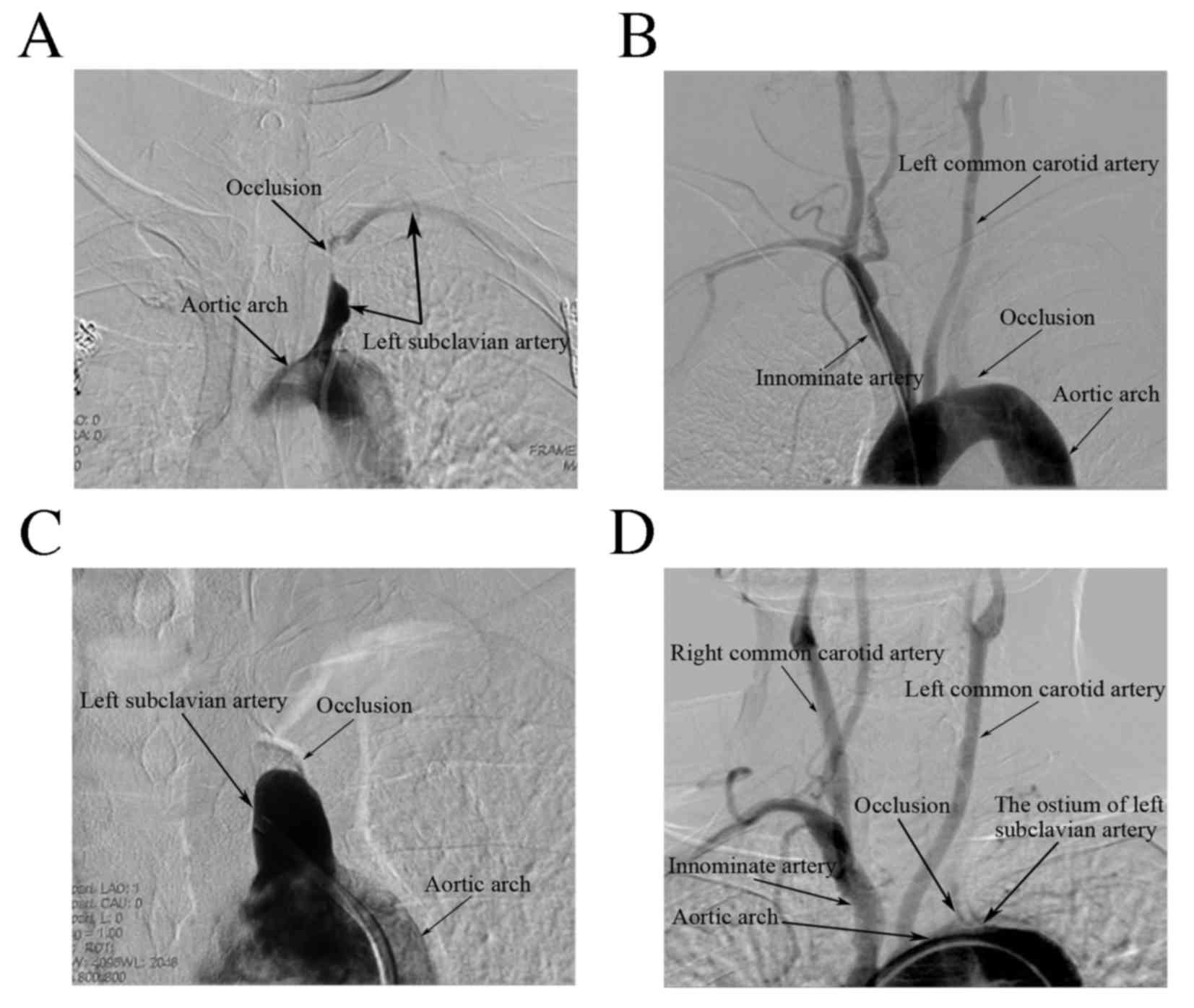
Rat-tail type occlusion
The angiography of Patient 1 revealed subtotal
occlusion of the LSCA with a very narrow eccentric lumen, the
outline of which resembled a rat's tail (Fig. 1A). Using digital road mapping, a
0.014-inch Pilot 150 guiding wire (Abbott Vascular; Abbott, Abbott
Park, IL, USA) was used to gently penetrate the lesion across the
lumen, and a 4×20 mm Sprinter Legend balloon catheter (Medtronic,
Minneapolis, MN, USA) was inflated to dilate the lesion over the
wire. An 8×30 mm Precise Self-Expanding Stent (Cordis Corporation,
Hialeah, FL, USA) and a 9×20 mm Protégé Self-Expanding Stent
(Medtronic) were procedurally navigated over the exchange wire and
mutually overlapped across the occlusive segment of the LSCA.
Extreme care was taken to ensure that the stent covered the entire
atherosclerotic lesion.
Peak type occlusion
The angiographies of 4 patients revealed totally
occlusive SCA with Thrombosis in Myocardial Infarction (TIMI) grade
0 antegrade flow. This type of lesion was classified as peak type
occlusion (Fig. 1B). The occlusion
was crossed by a 0.014-inch guiding wire, with the wire tip
repeatedly stabbing the top of the ‘peak’. The lesion was
subsequently dilated using a Sprinter Legend or percutaneous
transluminal angioplasty (PTA) balloon catheter (Invatec S.p.A.,
Roncadelle, Italy) and an appropriate stent was applied to the
lesion. In Patient 5, 1.5×20 mm and 2×20 mm Sprinter Legend balloon
catheters were introduced to dilate the occlusion once the guiding
wire had crossed the RSCA occlusion via the femoral access;
however, the angiography revealed ~40% residual stenosis. A 5×30 mm
EV3 balloon catheter was therefore inflated to dilate the stenosis,
and a subsequent angiography demonstrated satisfactory
recanalization of the RSCA with <10% residual stenosis. An 8×30
mm Protégé Self-Expanding Stent was subsequently deployed at the
ostium of the LSCA.
Hilly type occlusion
The angiographies of Patient 6 and Patient 7
revealed totally occlusive ostia of the LSCAs, with TIMI grade 0
antegrade flow. As an occlusion of this type resembles a hill, it
is referred to as a ‘hilly’ type occlusion (Fig. 1C). A combined femoral and radial
approach was used in patients with this type of occlusion. Both the
0.018-inch guiding wire and the 0.035-inch guiding wire failed to
cross the lesions via femoral access in Patient 6. A 0.014-inch
Pilot 150 wire eventually succeeded in crossing the occlusion
following repeated gentle attempts from the left radial side.
Subsequently, a PTA balloon catheter was used to dilate the lesion,
and 12×40 mm and 10×20 mm Protégé Self-Expanding stents were
procedurally advanced coaxially over the wire and deployed across
the occlusion. A 0.014-inch stiff ChoICE PT Extra Support wire
(Boston Scientific, Marlborough, MA, USA) was inserted into the
LSCA ostium via the femoral access in Patient 7. Repeated attempts
to pass the wire through the occlusion with the support of a
Sprinter Legend balloon catheter were unsuccessful. Another
0.014-inch Pilot 150 guiding wire was then selected. An angiography
following approximately 2-cm advancement of the Pilot 150 guiding
wire revealed that the distal artery exhibited an antegrade flow;
however, the wire tip was not in the lumen with the arterial
dissection forming at the lesion. The patient and her family were
informed about the situation and the procedure was immediately
aborted.
Plain type occlusion
The angiography of Patient 8 revealed an occlusive
lesion with TIMI grade 0 antegrade flow in the ostium of the LSCA.
Occlusions of this type, with a flat bottom, are termed as ‘Plain’
type occlusions (Fig. 1D). A similar
combined femoral and radial approach is used in patients with this
type of occlusion. Both the 0.018-inch guiding wire and the
0.035-inch guiding wire failed to cross the lesions even via the
femoral access. A 0.014-inch Pilot 150 wire successfully crossed
the occlusion from the left radial side. Subsequently, a PTA
balloon catheter was used to dilate the lesion and 9×30 mm and 8×30
mm Precise Self-Expanding stents were procedurally advanced
coaxially over the wire and deployed across the occlusion.
A final angiography was performed to evaluate the
results of stent surgery and patency of the SCA as well as its
normal antegrade flow.
Follow-up
All 8 patients were transferred to the
cardiovascular intensive care unit following the procedure and
subjected to electrocardiograph monitoring for 24 h prior to being
moved to an ordinary ward. Patients were discharged 3 days later
with a prescription of 100 mg/day aspirin for life and 75 mg/day
clopidogrel for 3 months, unless otherwise indicated. All patients
were evaluated by experienced physicians post-surgery and then
periodically during the follow-up period. Follow-up Doppler
ultrasound, CTA or angiography was performed every 3 months for the
first 6 months, and every 6 months thereafter.
Results
Stenting and typing
A total of 9 self-expanding and 1 balloon-expandable
stent were implanted in the SCA of 7 patients with totally
occlusive lesions. In 3 patients, 2 stents were used, whereas a
single stent was used in a further four patients. Final angiograms
obtained at the end of the procedure confirmed satisfactory
recanalization of the SCA with <10% residual stenosis and good
patency of stents in all cases. The endovascular procedure for
arterial dissection via femoral access failed in one female
patient. The lesions of the eight patients were categorized as
follows according to their angiographic appearance: Rat-tail, 1/8
(12.5%); peak, 4/8 (50%); hilly type, 2/8 (25%); and plain, 1/8
(12.5%; Table II).
Complications
No major strokes or mortalities occurred within 30
days of the procedure. However, minor bleeding at the femoral
access site was noted in 2 patients, which was controlled by
applying gauze pressure. A post-procedure CTA of the female patient
with arterial dissection revealed a tear between the intima and the
media layer at the ostium of the LSCA. The patient was kept under
close and continuous clinical observation. During the first 3
months, the patient still presented symptoms of transient ischemic
attacks, such as vertebrobasilar insufficiency. However, she was
lost to follow-up after 3-months as she gave no response to
attempts at contact.
Follow-up
The 7 remaining patients attended a post-procedural
follow-up following discharge from hospital. The mean follow-up
time was 15.7 months (range, 1–36 months), whereas the total
follow-up time varied among patients according to the date of their
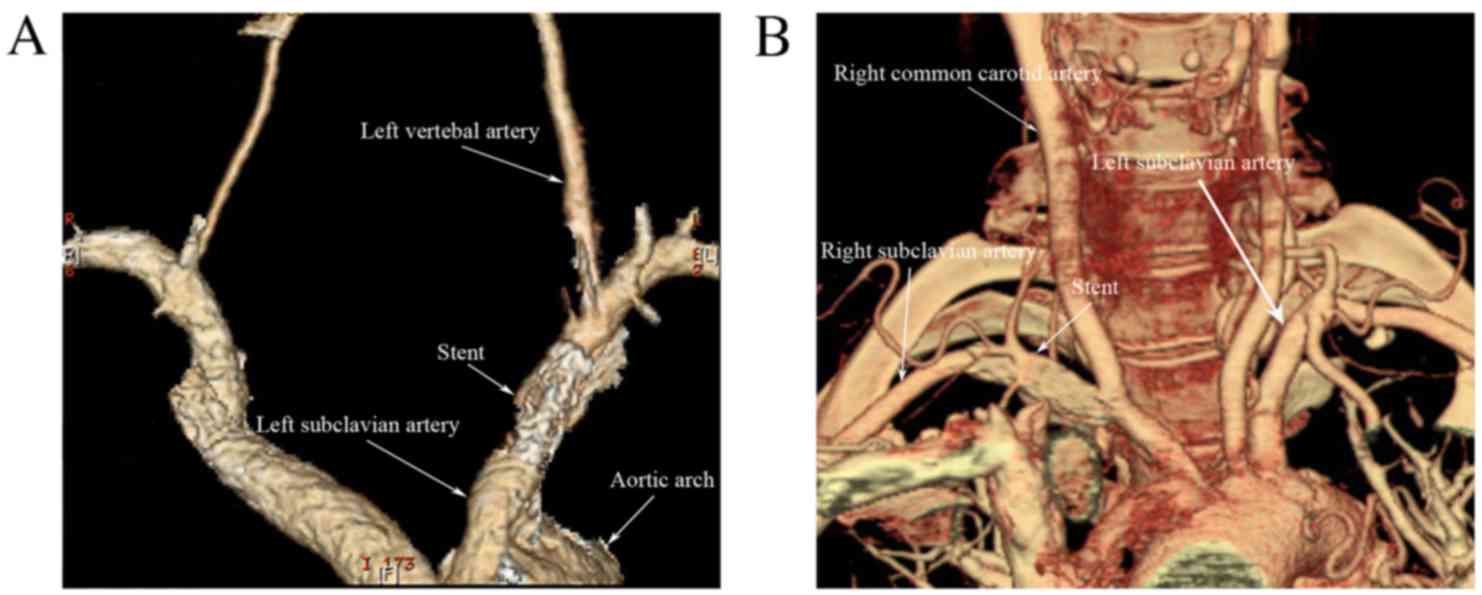
most recent Doppler ultrasound, CTA (Fig. 2), or angiography. No ischemic
symptoms, including vertebrobasilar insufficiency, upper extremity
ischemia, or transient ischemic attacks attributable to the
procedure were observed during the follow-up period, and no
evidence of neointimal hyperplasia or restenosis was observed on
follow-up angiography or color duplex Doppler.
Discussion
The present treatment methods for chronic
obstructive lesions of the SCA consist of endovascular or surgical
techniques (2). In 1980, Bachman and
Kim (9), and Mathias et al
(10) separately reported
percutaneous transluminal angioplasty as a method for the treatment
of SCA stenosis for the first time. Thereafter, multiple studies
have confirmed the safety and effectiveness of endovascular
treatment for this purpose (7,11–23). The
prevalence of endovascular treatment for SCA lesions and the
advancement of interventional techniques during the last 3 decades
has led to 91–100% technical success of treatment for SCA lesions
(1). Endovascular repair has been
regarded as the primary choice for the treatment of SCA lesions as
it is less invasive, has lower intraoperative and postoperative
complication rates, better patient comfort and decreased hospital
stay, particularly among patients at high risk of surgical
complications (1,2,24).
SCA lesions are classified as stenosis or occlusion
according to the severity of obstruction (4). Multiple studies have demonstrated that
the endovascular technical success for stenosis is excellent,
whereas the occlusions are more challenging to treat. The
endovascular treatment success rate with interventional techniques
for stenotic lesions is 94–100% (7,12,18,23,25–33),
whereas for totally occlusive lesions it is 47–100% (7,12,18,19,23,25–34)
(Table III). It may therefore be
concluded that the endovascular treatment technique for stenotic
lesions is well established, whereas that for occlusive lesion is
more difficult and controversial, particularly when the occlusion
is located at the ostium of the SCA.
 | Table III.Summary of the published series of
PTA or stenting-PTA of total occlusions of the subclavian artery
(7,12,18,19,23,25–34). |
Table III.
Summary of the published series of
PTA or stenting-PTA of total occlusions of the subclavian artery
(7,12,18,19,23,25–34).
| Author | Technical success
of stenotic lesion (%) | Technical success
of occlusive lesion (%) | Number of
patients | Number of
operations | Year | (Refs.) |
|---|
| Miyakoshi | 100 | 80 | 36 | 36 | 2012 | (25) |
| Aziz | 94 | 64 | 1300 |
| 2011 | (26) |
| Sixt | 100 | 87 | 107 | 108 | 2009 | (27) |
| Palchik | 100 | 54 |
| 67 | 2008 | (7) |
| AbuRahma | 100 | 92 | 121 | 121 | 2007 | (28) |
| Przewlocki | 100 | 72 | 75 | 76 | 2006 | (29) |
| Woo | 100 | 57 | 25 | 27 | 2006 | (23) |
| De Vries | 100 | 65 | 110 | 109 | 2005 | (12) |
| Brountzos | 98 | 85 | 47 | 49 | 2004 | (18) |
| González |
| 100 | 9 | 9 | 2002 | (19) |
| Henry | 100 | 47 | 113 | 113 | 1999 | (30) |
| Sueoka | 100 | 100 | 7 |
| 1996 | (31) |
| Criado | 100 | <90 | 26 | 30 | 1995 | (32) |
| Kumar | 100 | 100 | 27 | 31 | 1995 | (23) |
| Mathias |
| 83 | 46 |
| 1993 | (34) |
Chronically occlusive lesions in the SCA may be
caused by atherosclerosis, Takayasu arteritis, giant cell
arteritis, FMD, and radiation-induced arteriopathy; of these,
atherosclerosis is the primary cause (2). A previous study (35) investigating the chronically occluded
coronary artery reported that the typical chronic occlusion may be
classified as ‘soft’, ‘hard’, or a mixture of both. Soft plaque
consists of cholesterol-laden cells and foam cells with loose
fibrous tissue and neovascular channels. Older occlusions, defined
as ‘hard plaques’, have higher concentrations of calcium and
collagen components.
The typical atherosclerotic plaque of chronic
occlusions is primarily characterized by a collagen-rich
extracellular matrix, intra- and extracellular lipids, smooth
muscle cells and mixed components, including a small quantity of
cholesterol, dense collagen and calcium deposits (35). The collagen-rich fibrous tissue is
particularly dense at proximal and distal ends of the lesion,
referred to as proximal and distal fibrous caps, respectively
(36). The distal fibrous cap of a
chronic occlusion is considered to be less resistant compared with
the proximal one (37,38).
Fibrous caps of the occlusion lesions caused by
atherosclerosis are typically solid following the long-term process
of formation and calcification (35). Therefore, the present authors suggest
that successful passage of the wire through the occlusive lesion is
the key step of recanalization. In the retrograde approach, the
catheter could be delivered over a shorter distance and remain in
the distal SCA, gaining more stability and axial support and
avoiding the more rigid proximal fibrous cap (5). When traversing the occlusion via the
femoral artery is unsuccessful, the guiding wire may succeed using
a retrograde approach via the radial access.
On the basis of the above analysis and the findings
of the present study, chronic total occlusions at the ostia of the
SCA were classified into 4 types according to the angiographic
morphology of the proximal vessel (Fig.
1), which is important for selecting the optimal interventional
operation strategy.
Rat-tail type lesions are not completely occlusive,
exhibiting TIMI grade I antegrade flow in the angiographic
appearance (Fig. 1A). This suggests
that such lesions are formed over a short period of time, as they
consist of cholesterol-laden cells and foam cells with loose
fibrous tissue and neovascular channels (35). This suggests that the true lumen, to
which the tip of the Rat-tail points, has not been totally
occluded, and the proximal fibrous cap is thin and soft, allowing
the guide wire to cross with relative ease (38).
Peak type lesions are completely occlusive and
exhibit TIMI grade 0 antegrade flow in angiographic appearance
(Fig. 1B). This type of occlusion
forms later and mainly consists of loose fibrous tissue with slight
calcification (35). The lumen
tapers gradually from the distal end and becomes occlusive at the
proximal end (38), suggesting that
the tip of the peak points to the true lumen. When the guiding wire
pierces the dissection, it is feasible to perform PTA and stenting
as long as the guiding wire reaches the true lumen at the distal
end prior to the vertebral artery ostium.
The transfemoral artery operation approach is
preferred for rat-tail and peak type occlusive lesions as the
guiding wire is able to easily traverse to the distal lumen
following repeated gentle operative attempts.
Hilly type lesions are completely occlusive,
exhibiting TIMI grade 0 antegrade flow in angiographic appearance.
This type of occlusion takes a long time to form and consists of
highly calcified tissue and a dense fibrous cap (35). The concave proximal fibrous cap does
not have a specific point (Fig. 1C).
The guiding wire did not stabilize on insertion via the femoral
artery and the puncture point may not be determined; the wire
always entered the dissection site instead of the true lumen, even
with repeated insertion attempts.
Plain type lesions are completely occlusive,
exhibiting TIMI grade 0 antegrade flow in angiographic appearance
(Fig. 1D). They have a lengthy
formation time, and consist of a dense fibrous cap with severe
calcification (35). The guiding
wire did not stabilize on insertion via the femoral artery, and the
puncture point may not be determined.
In patients with hilly and plain type occlusions,
both the 0.018-inch V-18 guiding wire and the 0.035-inch Amplatz
Super Stiff guiding wire were unsuccessful in traversing the
lesions via the femoral access. The occlusion was finally traversed
successfully by a 0.014-inch Pilot 150 wire via the radial
artery.
The dual approach, which includes the femoral and
radial artery, is preferred in hilly and plain type occlusive
lesions. The catheter is inserted via the femoral artery to observe
the location, length and degree of the lesion. The guiding wire is
delivered via the radial artery to cross the occlusion and deploy
the stent (5,38). The hilly type occlusion, which is
treatable using the dual approach, was observed in Patient 6.
Attempts to traverse the occlusion via the femoral artery failed
and ultimately led to dissection, therefore the operation was
aborted. The retrograde approach via the radial artery should be
attempted to traverse the occlusion and deploy the stent covering
the dissection.
The present study had some limitations, due to the
small number of patients and the fact that all of the occlusions
included were caused by atherosclerosis.
In conclusion, the findings of the present study
demonstrate that the transfemoral artery operation approach is
preferred in rat-tail and peak type occlusions, whereas the dual
approach, including both femoral and radial arteries, is preferred
for the treatment of hilly and plain type occlusions. The
angiographic morphology typing used in the present study serves as
a reference for selecting the interventional operation strategy to
be used to decrease complications and improve the technical success
rate. The endovascular treatment technique for ostial occlusions of
the SCA is challenging and warrants further research to improve
current operative methods. Further studies involving more patients
are required to confirm the advantages and effectiveness of the
angiographic morphology typing.
Acknowledgements
The authors of the present study would like to thank
Dr Shuai Shao (Department of Dermatology, Xijing Hospital, Fourth
Military Medical University, Xi'an, China) for her comprehensive
help, as well as Professor Qing-Wu Yang and Associate Professor
Yong Liu (Department of Neurology, Xinqiao Hospital, Third Military
Medical University, Chongqing, China) for their case data. This
work was supported by a grant from the National Natural Science
Foundation of China (grant no. 81270406).
References
|
1
|
Aiello F and Morrissey NJ: Open and
endovascular management of subclavian and innominate arterial
pathology. Semin Vasc Surg. 24:31–35. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brott TG, Halperin JL, Abbara S, Bacharach
JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, et
al: 2011
ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS
guideline on the management of patients with extracranial carotid
and vertebral artery disease. A report of the American college of
cardiology Foundation/American heart association task force on
practice guidelines, and the American stroke association, American
association of neuroscience nurses, American association of
neurological surgeons, American college of radiology, American
society of neuroradiology, congress of neurological surgeons,
society of atherosclerosis imaging and prevention, society for
cardiovascular angiography and interventions, society of
interventional radiology, society of neurointerventional surgery,
society for vascular medicine, and society for vascular surgery.
Circulation. 124:e54–e130. 2011.PubMed/NCBI
|
|
3
|
Modarai B, Ali T, Dourado R, Reidy JF,
Taylor PR and Burnand KG: Comparison of extra-anatomic bypass
grafting with angioplasty for atherosclerotic disease of the
supra-aortic trunks. Br J Surg. 91:1453–1457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delaney CP, Couse NF, Mehigan D and
Keaveny TV: Investigation and management of subclavian steal
syndrome. Br J Surg. 81:1093–1095. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Satti SR, Golwala SN, Vance AZ and Tuerff
SN: Subclavian steal: Endovascular treatment of total occlusions of
the subclavian artery using a retrograde transradial subintimal
approach. Interv Neuroradiol. 22:340–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noguchi T, Miyazaki MDS, Morii I, Daikoku
S, Goto Y and Nonogi H: Percutaneous transluminal coronary
angioplasty of chronic total occlusions. Determinants of primary
success and long-term clinical outcome. Catheter Cardiovasc Interv.
49:258–264. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Palchik E, Bakken AM, Wolford HY, Saad WE
and Davies MG: Subclavian artery revascularization: an outcome
analysis based on mode of therapy and presenting symptoms. Ann Vasc
Surg. 22:70–78. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Seldinger SI: Catheter replacement of the
needle in percutaneous arteriography: A new technique. Acta Radiol.
39:368–376. 1953. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bachman DM and Kim RM: Transluminal
dilatation for subclavian steal syndrome. Am J Roentgenol.
135:995–996. 1980. View Article : Google Scholar
|
|
10
|
Mathias K, Staiger J, Thron A, Spillner G,
Heiss HW and Konrad-Graf S: Percutaneous transluminal dilatation of
the subclavian artery (author's transl). Dtsch Med Wochenschr.
105:16–18. 1980.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Westerband A, Rodriguez JA, Ramaiah VG and
Diethrich EB: Endovascular therapy in prevention and management of
coronary-subclavian steal. J Vasc Surg. 38:699–704. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
De Vries JP, Jager LC, Van den Berg JC,
Overtoom TC, Ackerstaff RG, Van de Pavoordt ED and Moll FL:
Durability of percutaneous transluminal angioplasty for obstructive
lesions of proximal subclavian artery: Long-term results. J Vasc
Surg. 41:19–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang KQ, Wang ZG, Yang BZ, Yuan C, Zhang
WD, Yuan B, Xing T, Song SH, Li T, Liao CJ and Zhang Y: Long-term
results of endovascular therapy for proximal subclavian arterial
obstructive lesions. Chin Med J (Engl). 123:45–50. 2010.PubMed/NCBI
|
|
14
|
Paukovits TM, Lukács L, Bérczi V,
Hirschberg K, Nemes B and Hüttl K: Percutaneous endovascular
treatment of innominate artery lesions: A single-centre experience
on 77 lesions. Eur J Vasc Endovasc Surg. 40:35–43. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higashimori A, Morioka N, Shiotani S,
Fujihara M, Fukuda K and Yokoi Y: Long-term results of primary
stenting for subclavian artery disease. Catheter Cardiovasc Interv.
82:696–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bates MC, Broce M, Lavigne PS and Stone P:
Subclavian artery stenting: Factors influencing long-term outcome.
Catheter Cardiovasc Interv. 61:5–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brountzos EN, Malagari K and Kelekis DA:
Endovascular treatment of occlusive lesions of the subclavian and
innominate arteries. Cardiovasc Intervent Radiol. 29:503–510. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brountzos EN, Petersen B, Binkert C,
Panagiotou I and Kaufman JA: Primary stenting of subclavian and
innominate artery occlusive disease: A single center's experience.
Cardiovasc Intervent Radiol. 27:616–623. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
González A, Gil-Peralta A, González-Marcos
JR and Mayol A: Angioplasty and stenting for total symptomatic
atherosclerotic occlusion of the subclavian or innominate arteries.
Cerebrovasc Dis. 13:107–113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Henry M, Henry I, Polydorou A, Polydorou A
and Hugel M: Percutaneous transluminal angioplasty of the
subclavian arteries. Int Angiol. 26:324–340. 2007.PubMed/NCBI
|
|
21
|
Martinez R, Rodriguez-Lopez J, Torruella
L, Ray L, Lopez-Galarza L and Diethrich EB: Stenting for occlusion
of the subclavian arteries. Technical aspects and follow-up
results. Texas Hear Inst J. 24:23–27. 1997.
|
|
22
|
Patel SN, White CJ, Collins TJ, Daniel GA,
Jenkins JS, Reilly JP, Morris RF and Ramee SR: Catheter-based
treatment of the subclavian and innominate arteries. Catheter
Cardiovasc Interv. 71:963–968. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woo EY, Fairman RM, Velazquez OC, Golden
MA, Karmacharya J and Carpenter JP: Endovascular therapy of
symptomatic innominate-subclavian arterial occlusive lesions. Vasc
Endovascular Surg. 40:27–33. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Karpenko A, Starodubtsev V, Ignatenko P
and Gostev A: Endovascular treatment of the subclavian artery
steno-occlusive disease. J Stroke Cerebrovasc Dis. 26:87–93. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyakoshi A, Hatano T, Tsukahara T,
Murakami M, Arai D and Yamaguchi S: Percutaneous transluminal
angioplasty for atherosclerotic stenosis of the subclavian or
innominate artery: Angiographic and clinical outcomes in 36
patients. Neurosurg Rev. 35:121–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aziz F, Gravett MH and Comerota AJ:
Endovascular and open surgical treatment of brachiocephalic
arteries. Ann Vasc Surg. 25:569–581. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sixt S, Rastan A, Schwarzwälder U,
Bürgelin K, Noory E, Schwarz T, Beschorner U, Frank U, Müller C,
Hauk M, et al: Results after balloon angioplasty or stenting of
atherosclerotic subclavian artery obstruction. Catheter Cardiovasc
Interv. 73:395–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
AbuRahma AF, Bates MC, Stone PA, Dyer B,
Armistead L, Dean Scott L and Scott Lavigne P: Angioplasty and
stenting versus carotid-subclavian bypass for the treatment of
isolated subclavian artery disease. J Endovasc Ther. 14:698–704.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Przewlocki T, Kablak-Ziembicka A,
Pieniazek P, Musialek P, Kadzielski A, Zalewski J, Kozanecki A and
Tracz W: Determinants of immediate and long-term results of
subclavian and innominate artery angioplasty. Catheter Cardiovasc
Interv. 67:519–526. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Henry M, Amor M, Henry I, Ethevenot G,
Tzvetanov K and Chati Z: Percutaneous transluminal angioplasty of
the subclavian arteries. J Endovasc Surg. 6:33–41. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sueoka BL: Percutaneous transluminal stent
placement to treat subclavian steal syndrome. J Vasc Interv Radiol.
7:351–356. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Criado FJ and Queral LA: The role of
angioplasty and stenting in the treatment of occlusive lesions of
supra-aortic trunks. J Mal Vasc. 21:(Suppl A). S132–S138. 1996.
|
|
33
|
Kumar K, Dorros G, Bates MC, Palmer L,
Mathiak L and Dufek C: Primary stent deployment in occlusive
subclavian artery disease. Cathet Cardiovasc Diagn. 34:281–285.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mathias KD, Lüth I and Haarmann P:
Percutaneous transluminal angioplasty of proximal subclavian artery
occlusions. Cardiovasc Intervent Radiol. 16:214–218. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stone GW, Kandzari DE, Mehran R, Colombo
A, Schwartz RS, Bailey S, Moussa I, Teirstein PS, Dangas G, Baim
DS, et al: Percutaneous recanalization of chronically occluded
coronary arteries: A consensus document: Part I. Circulation.
112:2364–2372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sumitsuji S, Inoue K, Ochiai M, Tsuchikane
E and Ikeno F: Fundamental wire technique and current standard
strategy of percutaneous intervention for chronic total occlusion
with histopathological insights. JACC Cardiovasc Interv. 4:941–951.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Surmely JF, Katoh O, Tsuchikane E, Nasu K
and Suzuki T: Coronary septal collaterals as an access for the
retrograde approach in the percutaneous treatment of coronary
chronic total occlusions. Catheter Cardiovasc Interv. 69:826–832.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ozawa N: A new understanding of chronic
total occlusion from a novel PCI technique that involves a
retrograde approach to the right coronary artery via a septal
branch and passing of the guidewire to a guiding catheter on the
other side of the lesion. Catheter Cardiovasc Interv. 68:907–913.
2006. View Article : Google Scholar : PubMed/NCBI
|