Introduction
Volatile anesthetics such as isoflurane and
sevoflurane are used in pediatric surgical procedures and in
imaging studies (1). Extended
exposure to volatile anesthetics causes widespread
neurodegeneration in the developing brains of animals, and also
results in persistent learning and memory deficits (2–7).
Children <4 years of age who have undergone surgery under
general anesthesia more than once have a greater likelihood of
developing cognitive disabilities and also experience difficulties
in reading and learning (8,9). Previous studies have raised serious
concerns regarding the safety of such anesthetics, and have
therefore necessitated protective strategies (2,3,8,9).
Isoflurane has been reported to induce
neuroapoptosis and degeneration by [Ca2+]i
overload (10,11), which subsequently activates the
mitochondrial pathways of apoptosis (12,13), and
has also been demonstrated to be associated with the activation of
c-Jun N-terminal kinase (JNK) (4).
JNK is a member of the mitogen activated protein kinases (MAPKs).
MAPKs are a family of serine-threonine protein kinases that consist
of three major members: Extracellular-signal-regulated kinases
(ERKs), p38 MAPK and JNK. MAPK signalling pathways serve important
roles in cell survival, development, neurodegeneration, pain and
inflammation under normal and pathological conditions (14–17). The
JNK signalling cascade involves mediating neuronal apoptosis
through the regulation of Bcl-2 family members and activation of
c-Jun (18), resulting in indirect
transcriptional regulation of the Bcl-2 family members (19), including downregulation of the
anti-apoptotic proteins (19,20).
Recent studies have revealed that the involvement of
MAPK cascades in anesthetics-induced neurotoxicity. Li et al
(7) reported the involvement of the
JNK pathway in isoflurane-induced neurodegeneration in the
hippocampi of neonatal rats. Furthermore, N-stearoyl-l-tyrosine was
reported to protect the developing brain against
sevoflurane-induced neurotoxicity via the ERK1/2 signalling pathway
(21). Finally, Sanders et al
(22) reported that dexmedetomidine
upregulated ERK1/2 in brain tissues of neonatal rats exposed to
isoflurane.
Rutin is a flavonoid glycoside found in numerous
plants including buckwheat, limes, oranges, grapefruit and berries.
Numerous pharmacological properties of rutin, including antioxidant
(23), anti-inflammatory (24) and hypolipidemic properties (25) have been reported. The present study
endeavoured to investigate the role of rutin in terms of providing
neuroprotection against isoflurane-induced neuroapoptosis in the
hippocampi of neonatal rats through the modulation of MAPK
cascades.
Materials and methods
Reagents and chemicals
Isoflurane (0.75%) and rutin were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Antibodies
against cleaved caspase-3, Bcl2-associated agonist of cell death
(Bad), phospho-Bad, Bcl-xL, Bax, β-actin, JNK, phospho-JNK,
phospho-c-Jun, ERK1/2, phospho-ERK1/2, p38 and phospho-p38 were
purchased from Cell Signalling Technology (Danvers, MA, USA). All
the chemicals used in the current study were of analytical grade
and purchased from Sigma-Aldrich, unless otherwise specified.
Animals
The present study was approved (approval no.
HAU24677244) by the Animal Care and Ethical Committee of Huazhong
Agriculture University (Wuhan, China) and was performed in
accordance with the National Institutes of Health Guide for the Use
of Laboratory Animals (26). A total
of 12 pregnant female Sprague-Dawley rats (Guangdong Medical
Laboratory Animal Center, Guandong, China) were used in this study,
and were maintained in an environment with a 12 h light/dark cycle
and a room temperature of 22±1°C (humidity, 55–60%). Animals were
provided with ad libitum access to water and food and were
housed individually in separate cages and monitored closely from
the day of birth [postnatal day 0 (P0)]. Pups were then carefully
maintained with their littermates in a standard environment, as
outlined, with a 12 h light/dark cycle and free access to water. A
total of 60 pups (n=12/group) weighing 18–28 g were used for the
experiments. Pups in the treatment groups were administered rutin
[10, 20 or 40 mg/kg body weight (b.wt)] orally, once every day from
P1, and treatment was continued until P15 in addition to a standard
diet. Control group pups did not receive rutin or isoflurane.
Separate group of rat pups that were exposed only to isoflurane
served as the anesthetic control.
Exposure to anesthesia
A total of 60 pups (n=12/group) were assigned into
groups as follows: Group 1, control pups, not treated with
anesthetic or rutin; group 2, anesthetic control, exposed to
isoflurane (0.75%) for 6 h (7,27) and
not treated with rutin; group 3, treated with 10 mg/kg rutin and
exposed to isoflurane on P7; group 4, treated with 20 mg/kg rutin
and exposed to isoflurane on P7; and group 5, treated with 40 mg/kg
rutin and exposed to isoflurane on P7. On P7, 1 h prior to the
delivery of isoflurane, rutin was administrated. At the end of the
6th hour of anesthetic exposure, six rat pups (n=6) from each
experimental group were sacrificed and their brains were excised.
For sacrifice, within 1 h of anesthesia, pups were perfused
transcardially with ice-cold saline followed by 4% paraformaldehyde
in 0.1 M phosphate buffer The hippocampi of the rat pups were then
used for protein expression analysis by western blotting and TUNEL
assay. The remaining rats (n=6) continued to receive rutin
supplementation until P15, and were then maintained according to
the standard experimental conditions until P31. Rats were subjected
to memory and learning studies via Morris Water Maze tests.
TUNEL assay
TUNEL studies were performed as described previously
by Li et al (7). Briefly, rat
pups that were anesthetized with isoflurane were perfused
transcardially with ice-cold saline, followed by 4%
paraformaldehyde in 0.1 M phosphate buffer. The brain tissues were
harvested and post-fixed in formaldehyde for 48 h at 4°C, embedded
in paraffin and sectioned (5 µm thickness). TUNEL positive cells
were determined using the Dead End fluorometric TUNEL System kit
(Promega Corporation, Madision, WI, USA) and positive cell counts
in the hippocampal CA1, CA3 and DG regions were analyzed with an
inverted Eclipse Ti2 microscope and NIS-Elements BR imaging
processing and analysis software (Nikon Corporation, Tokyo,
Japan).
Western blot analysis
Western blot analysis was performed to assess the
expression levels of various proteins following isoflurane exposure
and rutin treatment. Western blot analysis was performed as
previously described by Li et al (6,7).
Briefly, protein concentrations were determined using a BCA protein
assay (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each of the
protein samples (60 µg) was separated by 12% polyacrylamide gel
electrophoresis, blotted on a nitrocellulose membrane and
hybridized using the following antibodies: Anti-Bad (1:1,000;
9268), anti-phospho-Bad (1:1,000; 5284), anti-Bcl-xL (1:2,000;
2764), anti-cleaved caspase-3 (1:2,000; 9661), anti-Bax (1:1,000;
5023), anti-Bcl-2 (15071; 1:1,000), anti-ERK1/2 (1:1,000; 9102),
anti-phospho-ERK1/2 (1:1,000; 9101), anti-JNK (1:1,000),
anti-phospho-JNK (1:1,000; 9255), anti-phospho-c-Jun (1:1,000;
3270), anti-β-actin (1:2,000; 3700), anti-p38 (1:1,000; 8690) and
phospho-p38 (1:1,000; 4511). The immunoreactive bands were observed
using an ECL detection kit (RPN2232) and images were scanned using
Image Master 2D Platinum 7.0 scanner (both GE Healthcare,
Pittsburgh, PA USA). Images were analyzed by ImageQuant TL software
v2003.03 (version .8.3; GE Healthcare). The band signals of the
proteins were normalized relative to β-actin bands.
Memory and learning studies
Morris water maze test
To assess the effects of rutin on isoflurane-induced
alterations in memory and learning, spatial reference memory and
learning assessments, including the Morris water maze test, were
performed as previously described by Li et al (28).
The rat pups exposed to isoflurane and/or rutin were
trained in the Morris water maze for 4 days between P26 and P29. A
platform of ~10 cm in diameter was submerged in a circular pool
(200 cm in diameter, 60 cm depth) filled with warm water (23±2°C).
The rats were subjected to 2 sessions per day. Animals were allowed
60 sec to locate the hidden platform, and if unable to locate it
within 60 sec, they were gently guided. All trials and swim paths
were recorded with ANY-maze Video Tracking system (Stoelting Co.,
Wood Dale, IL, USA), which measured the time taken (latency) to
find the platform(s), as well as other behavioral information
obtained during the spatial reference memory test. Subsequent to
the trials, each rat was placed in a holding cage under an infrared
heat lamp prior to being returned to its regular cage.
Cued trials
Cued trials were conducted on P31 to assess any
non-cognitive performance impairments such as visual impairments
and/or swimming difficulties. The pool was covered with a white
cloth to conceal the visual cues. The rats undertook four trials
per day. In each trial, they were placed in a particular section of
the swimming pool towards the wall and were then allowed to swim to
a platform with a rod that served as a cue. The rod was placed 20
cm above water level in a random position in any of the four
quadrants of the swimming pool. The rat pups were given 60 sec to
locate the platform and 30 sec to sit on the platform, after which
they were removed from the pool. The rats that failed to locate a
platform within 60 sec were gently guided toward it and allowed to
remain there for 30 sec. The time taken by each rat to reach the
cued platform was recorded and analyzed.
Place trials
Subsequent to the cued trials, the white curtains
that surrounded the pool were removed and the same rats were
assessed in place trials to determine their ability to understand
the spatial relationship between distance cues and the escape
platform (no cue rod), that was placed in one of the four quadrants
and remained in the same position for all place trials. The
starting points were random for each rat. The time taken to reach
the platform was recorded.
Probe trials
In order to assess the memory of rat pups, probe
trials were conducted 24 h after place trials. The submerged
platform was removed and the rats were placed in the opposite
quadrant and allowed to swim for 60 sec. The time each rat spent in
each quadrant whilst attempting to locate the platform was
recorded. The data are expressed as the percentage of time spent by
the animals in each of the quadrants.
Statistical analysis
All values are presented as the mean ± standard
deviation of three or six individual experiments. Multiple group
comparisons were performed using one-way analysis of variance,
followed by Duncan's multiple range test. The analysis was
performed using SPSS statistical software (version 17.0; SPSS,
Inc., Chicago, IL, USA).P<0.05 was considered to indicate a
statistically significant difference.
Results
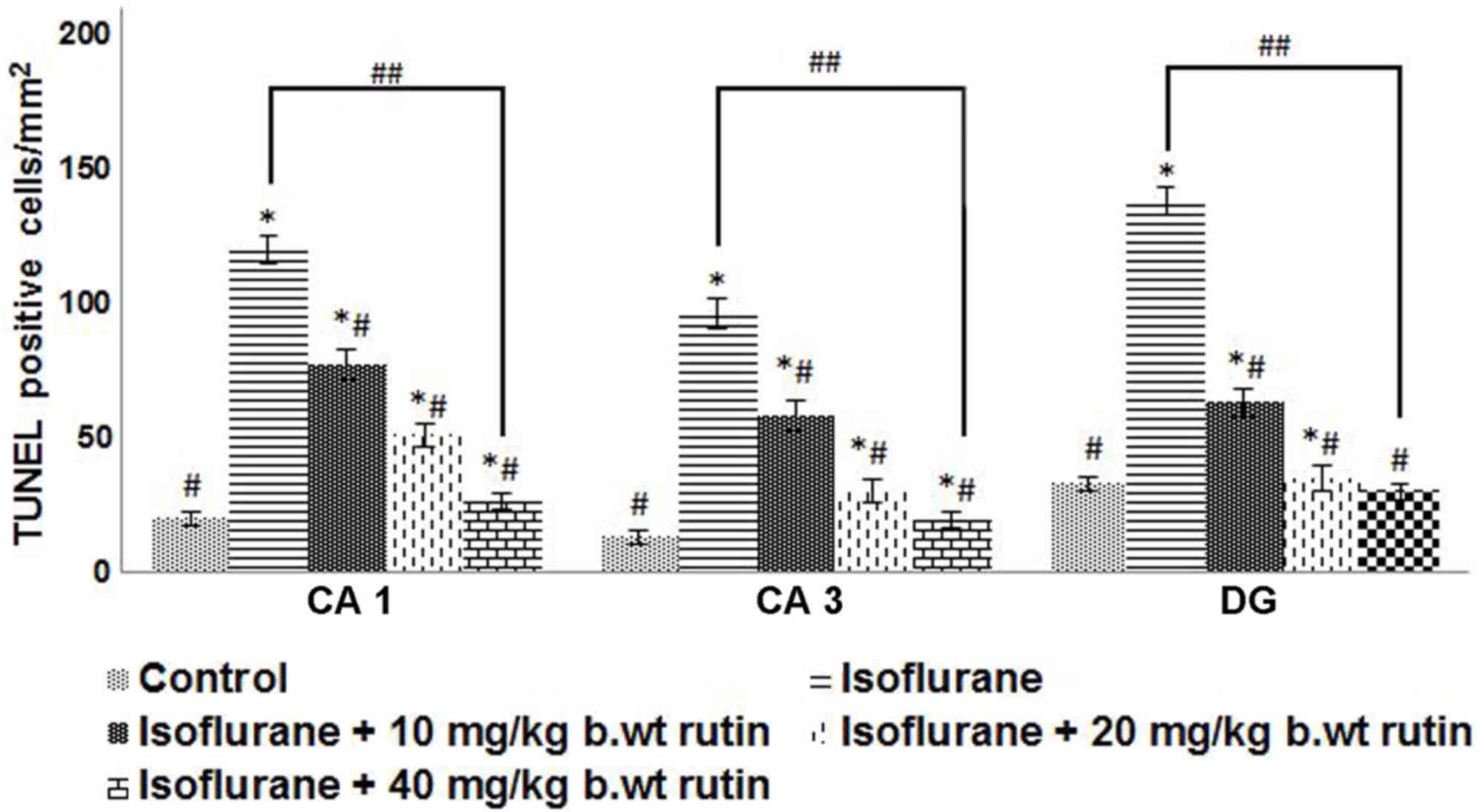
Rutin treatment reduces
isoflurane-induced neuroapoptosis
Isoflurane, a commonly used volatile anesthetic,
induces neuroapoptosis and long-term cognitive dysfunction in
developing animals (4,5,12). In
the present study, apoptosis was evaluated by TUNEL assay following
isoflurane exposure in P7 rat pups. The delivery of 6 h of
isoflurane exposure significantly raised the proportion of
TUNEL-positive cells in CA1, CA3 and in the dentate gyrus (DG)
regions (P<0.05). The increases were more pronounced in the DG
region when compared with the CA1 and CA3 regions. Rutin (10, 20 or
40 mg/kg b.wt) pre-treatment significantly reduced (P<0.05) the
TUNEL-positive cell counts in a dose-dependent manner, as compared
to the isoflurane-only group (Fig.
1).
Rutin regulates the expression levels
of apoptotic cascade proteins
To further assess apoptosis, quantification of
cleaved caspase-3 protein expression was performed by western blot
analysis. Endogenous levels of activated caspase-3 are a known and
useful marker of neuronal apoptosis (29). Caspase-3 is a key cell death marker
and apoptosis effector enzyme (29,30). The
current study revealed that isoflurane significantly induced
caspase-3 activation (P<0.05; Fig. 2A
and B). However, rutin significantly downregulated (P<0.05)
caspase-3 expression levels, with the 40 mg dose presenting a more
marked reduction compared with the isoflurane group. The lower dose
of rutin (10 mg) reduced caspase-3 expression levels; however the
reduction was not statistically significant.
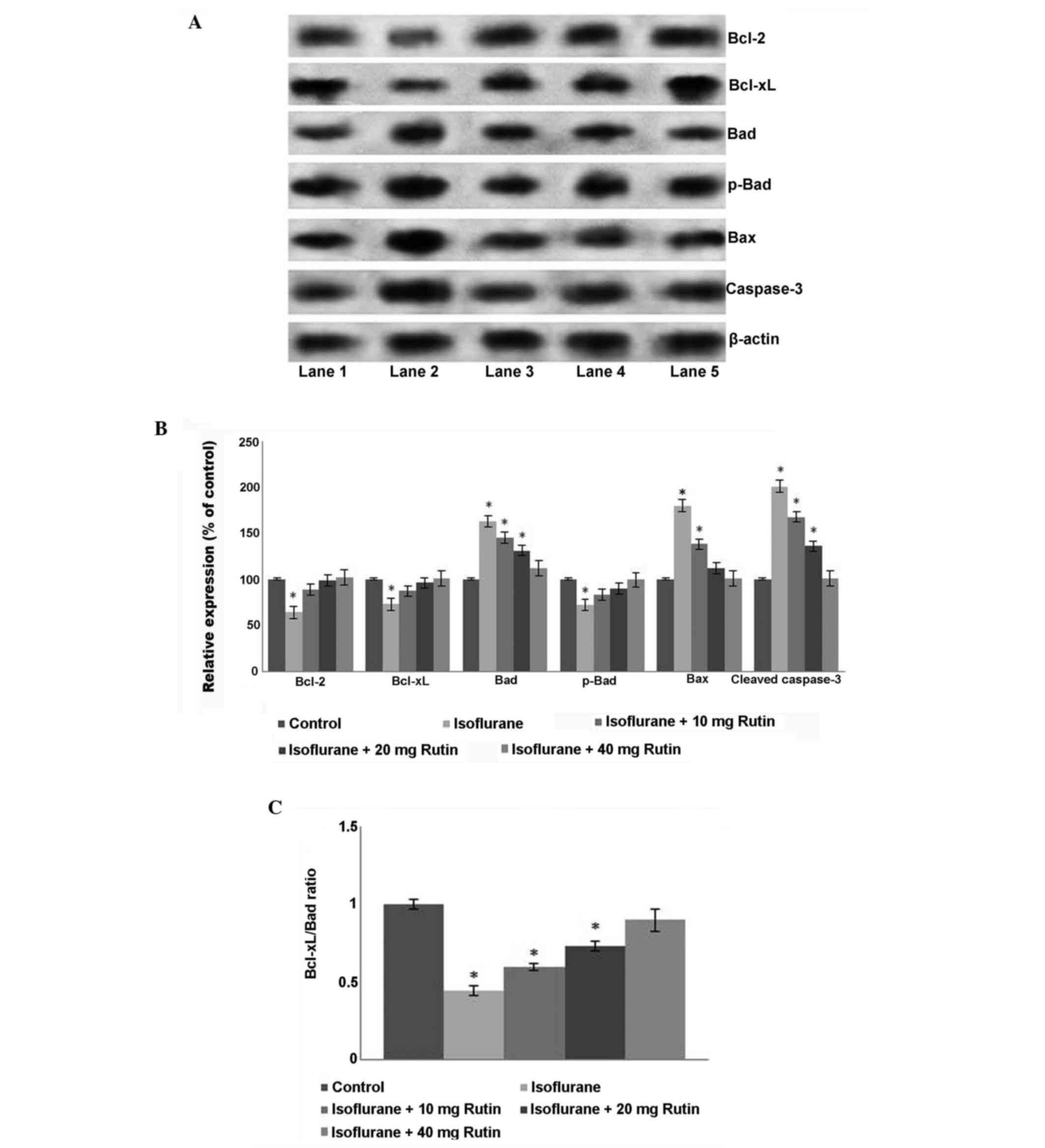 | Figure 2.Effects of rutin on the expression
levels of proteins in the apoptotic pathway. (A) Western blot
analysis to determine the expression levels of apoptotic pathway
proteins. Lane 1: Control, Lane 2: Isoflurane, Lane 3:
Isoflurane+10 mg rutin, Lane 4: Isoflurane+20 mg rutin, Lane 5:
Isoflurane+40 mg rutin. (B) Relative expression of apoptotic
cascade proteins. Quantification of the western blot analysis
indicated that isoflurane increased the expression levels of
caspase-3, Bax and total Bad, and decreased Bcl-2 and Bcl-xL
expression levels. (C) Rutin significantly rescued the altered
expression levels of the proteins, increasing the Bcl-xL/Bad ratio
(C). Values are presented as the mean ± standard deviation, n=3.
*P<0.05 vs. control, #P<0.05 vs. isoflurane only
and ##P<0.05, as determined by one-way analysis of
variance. Bcl, B-cell lymphoma, B-cell lymphoma; Bad,
Bcl2-associated agonist of cell death. |
Furthermore, isoflurane treatment alone resulted in
the decreased expression of Bcl-xL and Bcl-2, the anti-apoptotic
proteins in the hippocampi of P7 rats, but upregulated the
expression of Bax, a protein that promotes apoptosis by binding to
and antagonizing the Bcl-2 protein (20). In the present study, the increase in
Bax expression correlated with the downregulation of Bcl-2,
supporting this conclusion. Isoflurane however, decreased
phospho-Bad expression levels, yet the total expression levels of
Bad increased compared with the control group. A significant
decrease in the Bcl-xL/Bad ratio was observed following isoflurane
exposure compared with the control group (P<0.05; Fig. 2C). Rutin at all the doses
significantly ameliorated (P<0.05) the alteration in expression
levels of the studied proteins (Fig. 2B
and C). Rutin significantly upregulated (P<0.05) Bcl-xL and
Bcl-2 expression levels and downregulated Bax expression levels
compared with the isoflurane group. The phosphorylation of Bad has
an anti-apoptotic effect, whereas its de-phosphorylation is
pro-apoptotic (20). The increased
levels of total Bad observed following isoflurane exposure were
significantly reduced by treatment with rutin (P<0.05),
suggesting the effectiveness of rutin for inhibiting
isoflurane-induced neuroapoptosis.
Neuroprotection by rutin involves MAPK
signalling cascades
To further evaluate the molecular effects of rutin
on MAPK pathway proteins, the expression levels of major MAPK
proteins, including JNK, ERK and p38, were analyzed by western
blotting (Fig. 3A). Exposure to
isoflurane for 6 h caused a significant increase in the
phosphorylated JNK, ERK1/2 and p38 levels (P<0.05; Fig. 3B). The expression levels of total
JNK, ERK1/2 and p38 were also significantly increased (P<0.05).
However, rutin exposure resulted in significant reductions
(P<0.05) in the elevated levels of phospho-JNK and phospho-c-Jun
compared with the isoflurane-treated group. The administration of
40 mg/kg b.wt rutin caused a significant suppression (P<0.05) in
the phosphorylated forms of ERK1/2 and p38 in a dose-dependent
manner. The administration of 40 mg rutin resulted in similar
protein expression levels to those of pups in the control group
that were not exposed to anesthesia, which is suggestive of its
protective capacity against isoflurane-induced alterations of
protein expression (Fig. 3).
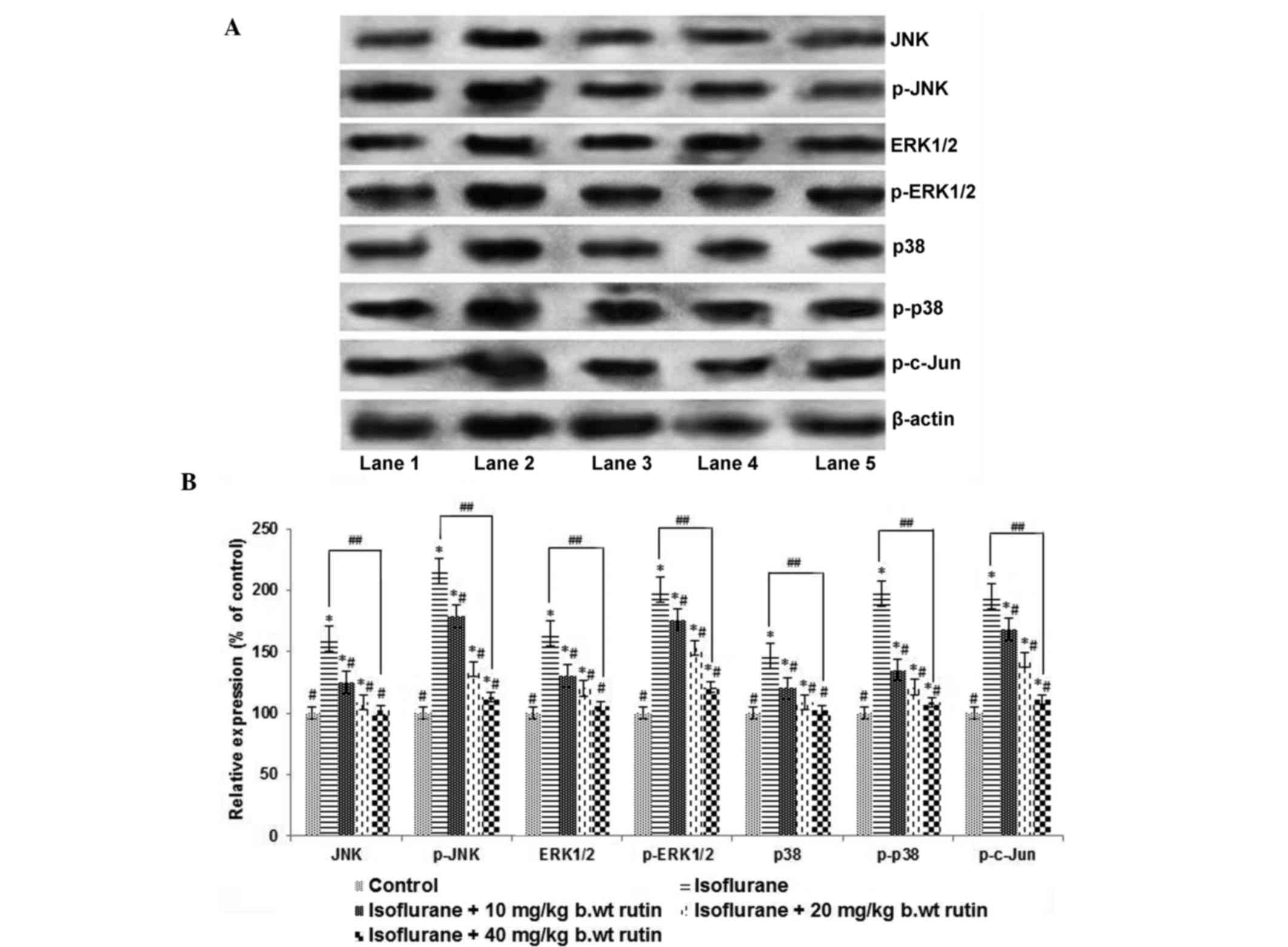 | Figure 3.Rutin modulates the MAPK signalling
cascade. (A) Western blot analysis to determine the expression
levels of proteins in the MAPK signalling cascade. Lane 1: Control,
Lane 2: Isoflurane, Lane 3: Isoflurane+10 mg rutin, Lane 4:
Isoflurane+20 mg rutin, Lane 5: Isoflurane+40 mg rutin) (B)
Relative expression levels of signal cascade proteins. The
significant upregulation in the expression levels of MAPK proteins
following 6 h of isoflurane exposure was effectively modulated by
rutin (10, 20 or 40 mg). Values are presented as the mean ±
standard deviation, n=3. *P<0.05 vs. control,
#P<0.05 vs. isoflurane only and
##P<0.05, as determined by one-way analysis of
variance. JNK, c-Jun N-terminal kinase; ERK,
extracellular-signal-regulated kinase. |
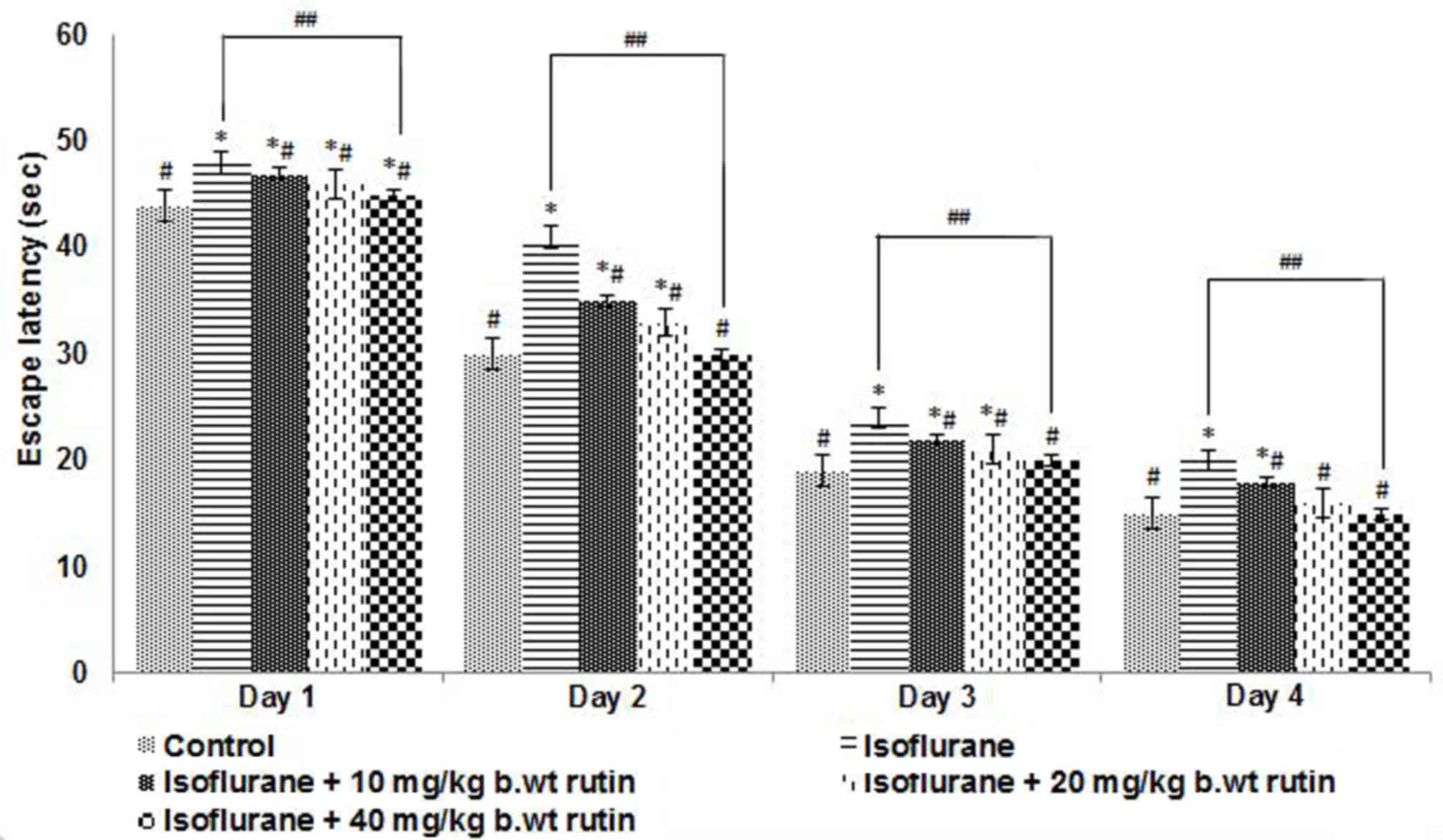
Effects of rutin on the behavior and
memory of rats exposed to inhalation anesthesia
To evaluate the effect of neonatal exposure to
isoflurane on potential learning and memory deficits, animals were
subjected to the Morris water maze test, the main test used to
assess spatial learning and memory (31). The rat pups exposed to isoflurane
anesthesia were trained to explore the swimming pool and to reach
the platform. The escape latency, which is the duration each rat
takes to reach the submerged platform, was recorded. The latency
was observed to decrease with each training session for all rats
irrespective of whether they had been administered isoflurane and
rutin or isoflurane alone (Fig. 4).
The escape latencies of rutin administered groups were found to be
similar to control values.
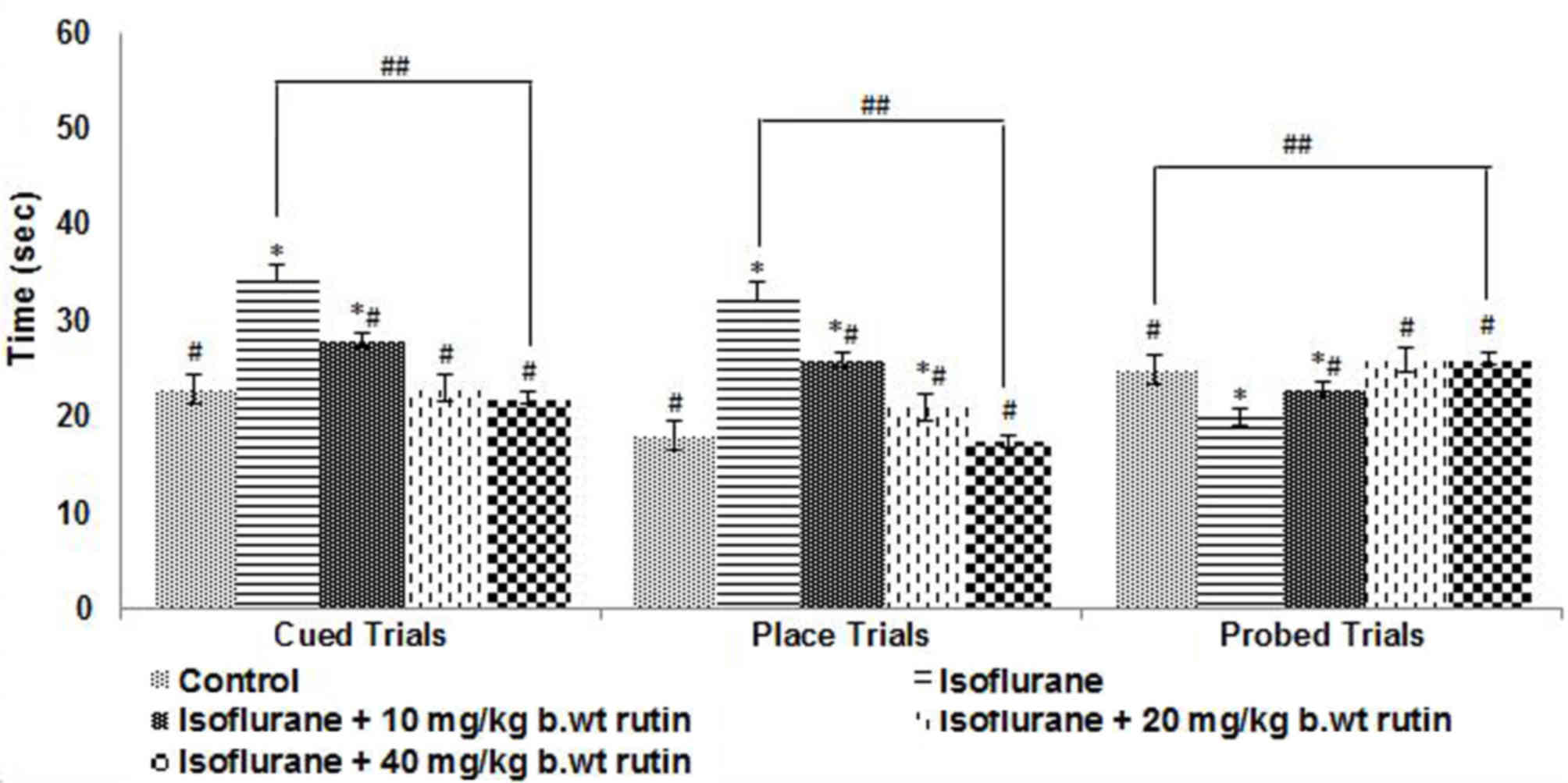
Cued trials were conducted on P31 to evaluate the
swimming and visual abilities of the rat pups. The rats exposed to
isoflurane anesthesia took significantly longer to reach the
submerged platform compared with pups that received no anesthesia
(P<0.05; Fig. 5). Rutin
supplementation was observed to significantly improve the
performance of the rats (P<0.05). The animals that received
rutin were able to reach the platform quicker compared with the
rats that received isoflurane alone. Furthermore, the rats that
received the higher dose of rutin (40 mg/kg b.wt) reached the
platform in a shorter time compared with those that received lower
doses in the cued and place trials (Fig.
5). Place and probe trials were performed to assess the ability
of the rat pups to learn and remember the location of a new
platform (Fig. 5). In place trials,
the rats that received 20 and 40 mg/kg b.wt rutin exhibited a
significant improvement in performance (P<0.05), as compared
with the isoflurane-only group. The rutin (20 and 40 mg)-treated
rats reached the platform quicker. The 10 mg/kg b.wt dose did not
elicit an enhancement in the performance of the rats.
The memory retention following exposure to
isoflurane on P7 was assessed by removing the platform from the
pool. Isoflurane exposure had a significantly detrimental impact on
the memory of the rats compared with the control group (P<0.05;
Fig. 5), as determined by the time
the animals spent in the target quadrant. The rats exposed to
isoflurane spent less time in the target quadrant compared with the
control group, which was indicative of the memory and learning
deficits caused by isoflurane. The rats that were administered
rutin spent more time on the target quadrant searching for the
platform compared with the isoflurane group (P<0.05), indicating
that there was an improvement in the memory of rat pups with 40
mg/kg b.wt dose of rutin compared wuth rat pups who received
isoflurane alone.
Discussion
Previous studies have demonstrated the potential
detrimental effects of anesthetic exposure to neonatal animals in
terms of causing neurohistopathological changes and long-term
behavior deficits and cognitive dysfunctions (6,32,33).
Apoptotic cell death is an essential part of normal brain
development and maturation, leading to the elimination of ~50–70%
of neurons and progenitor cells during the development of the
central nervous system (34,35). However, during pathological processes
such as hypoxia-ischemia, an absence of neurotrophic factors, or
prolonged exposure to anesthetic, the rate of apoptosis surpasses
the normal apoptotic rate (36,37).
Isoflurane, a commonly used volatile anesthetic, has
been reported to induce neurodegeneration in the developing brain
of animal models (2,4,5,7). The present study focused on an analysis
of the effects of flavonoid rutin on isoflurane-induced
neurotoxicity.
The present study demonstrated that there was an
increased level of apoptosis in the brain tissues of neonatal rats
exposed to 0.75% isoflurane for 6 h. Although anesthetic-induced
neurotoxicity is widespread throughout brain tissue, the current
study observed the effects on the hippocampal region, as previous
reports have suggested that hippocampal lesions following
isoflurane exposure result in abnormal behavioral responses in
neonatal rats (2,3,5,38). Increased apoptotic cell counts that
were observed in the CA1, CA3 and DG regions of the hippocampus
following isoflurane exposure were significantly reduced with rutin
treatment, demonstrating the neuroprotective effects of rutin.
Previous studies have suggested that neurogenesis in the DG region
is important to hippocampus-dependent episodic learning and memory,
and any interference in the process may result in impaired
hippocampal learning (39,40).
Furthermore, the raised cleaved caspase-3 expression
was found to be consistent with TUNEL-positive cell counts as a
result of anesthetic exposure. Caspase-3 serves a vital role in the
apoptotic pathway (41), and in
previous studies, cleaved caspase-3 expression levels were used as
a marker of apoptotic cell death (2,4,5,33). Rutin
effectively downregulated the expression levels of activated
caspase-3, in addition to the apoptotic cell count. Furthermore,
the expression levels of Bax protein were upregulated following
exposure to isoflurane. The anti-apoptotic protein Bcl-xL is widely
expressed in the central nervous system, which enhances cell
survival by maintaining mitochondrial membrane integrity and
inhibits the release of cytochrome c (42).
The Bcl-2 gene family encodes proteins that regulate
the apoptosis program. The Bcl-2 family includes the pro-apoptotic
(Bax and Bak) and anti-apoptotic (Bcl-2 and Bcl-xL) members
(20). The delicate balance of the
Bcl-2 family determines the release of cytochrome c, in
addition to the subsequent activation of the caspase cascade,
leading to apoptosis (19,20). In the present study, isoflurane
decreased the expression levels of Bcl-2, Bcl-xL and phospho-Bad.
However, the elevated levels of total Bad observed following
isoflurane exposure may have caused the decrease in the Bcl-xL/Bad
ratio. The current results demonstrated that rutin effectively
reversed the isoflurane-induced downregulation of Bcl-2, Bcl-xL and
phospho-Bad, and improved the Bcl-xL/Bad ratio, which may
contribute to the stabilization of the inner mitochondrial
membrane, and thus inhibit isoflurane-induced neuroapoptosis.
The MAPK pathway has been reported to be involved in
anesthetic-induced neurodegeneration (7,21). The
JNK signalling pathway is involved in neuronal apoptosis triggered
by several brain injury stimuli, such as ischemic reperfusion
injury (43–45). Previous studies have demonstrated
that the activation of JNK signalling is involved in
isoflurane-induced neuronal apoptosis (7). Activated JNK phosphorylates a nuclear
substrate, the transcription factor c-Jun, which results in an
increase of activator protein-1 transcription activity to modulate
the transcription of genes associated with apoptosis. By contrast,
activated JNK regulates the activation of non-nuclear substrates,
including Bcl-2 family members (43,45).
The present results indicated that rutin
pretreatment inactivated the JNK nuclear pathway by reducing the
isoflurane-induced increase in the phosphorylation of JNK and
transcription factor c-Jun. Rutin also inhibited the JNK
non-nuclear pathway by preventing the isoflurane-induced
downregulation of Bcl-2, and increasing the expression levels of
Bax. Furthermore, rutin caused a marked decrease in the expression
levels of phospho-ERK1/2 that had been upregulated following
exposure to isoflurane, which was similar to the protective effect
observed with dexmedetomidine (22).
Zheng and Zuo (46) reported that
isoflurane activated p38 MAPK in the brains of rats. The current
study reported an increase in the activation of p38 following the
exposure of neonatal rats pups to isoflurane on P7. However, rutin
decreased phospho-p38 expression levels in a dose-dependent manner.
Rutin (40 mg/kg b.wt) resulted in the regulation of the expression
patterns of the MAPK pathway proteins compared with lower doses.
These results suggest the potential involvement of the MAPK
signalling pathway in both isoflurane-induced neuronal apoptosis,
and in the neuroprotection caused by rutin.
Several studies have reported deficits in learning
and memory in rodents after neonatal exposure to volatile
anesthetics (2,3). Working memory refers to cognitive
functions that provide synchronized temporary storage and
manipulation of the information required to perform intricate
cognitive tasks. Working memory is involved in higher cognitive
functioning such as planning and sequential behavior. Any deficits
in the working memory are directly associated with deficits in
behavioral flexibility, and learning deficits have been shown to be
associated with hippocampal damage (47).
The Morris water maze test was selected to evaluate
the cognitive behavior of rat pups as it is a reliable measure of
hippocampus-dependent spatial navigation and reference memory
(48). The escape latencies
demonstrated by the rats that received isoflurane was significantly
longer compared with the controls when assessed ~4 weeks after the
administration of isoflurane. The observations of the present study
are suggestive of the possibility of impairments with respect to
long-term memory caused by exposure to isoflurane. This could be
attributed to the observed isoflurane-induced neurodegeneration.
Jevtovic-Todorovic et al (2)
reported that, on P7, rats are extremely sensitive to neurotoxic
challenge. Thus, any decrease in neural cell proliferation in
neonates may eventually lead to cognitive dysfunction by disrupting
the neural architecture of the hippocampus during a critical period
of development, or by depleting the pool of precursor cells present
throughout the duration of the animal's life (49).
The marked reduction in neuronal apoptosis as a
result of treatment with rutin may have contributed to the
significant improvements observed in the performance of the rats in
the Morris water maze tests when comparing the isoflurane-only and
rutin-treated groups. This may also improve the working memory of
the rats.
In conclusion, rutin effectively reduced neuronal
apoptosis by regulating JNK/ERK/p38MAPK signalling pathways and
modulating the expression of apoptotic proteins. Thus, additional
exploration of the effects of rutin is warranted to identify its
molecular targets, which may elucidate anesthetic-induced neuronal
toxicities.
References
|
1
|
Istaphanous GK and Loepke AW: General
anesthetics and the developing brain. Curr Opin Anesthesiol.
22:368–373. 2009. View Article : Google Scholar
|
|
2
|
Jevtovic-Todorovic V, Hartman RE, Izumi Y,
Benshoff ND, Dikranian K, Zorumski CF, Olney JW and Wozniak DF:
Early exposure to common anesthetic agents causes widespread
neurodegeneration in the developing rat brain and persistent
learning deficits. J Neurosci. 23:876–882. 2003.PubMed/NCBI
|
|
3
|
Satomoto M, Satoh Y, Terui K, Miyao H,
Takishima K, Ito M and Imaki J: Neonatal exposure to sevoflurane
induces abnormal social behaviors and deficitsin fear conditioning
in mice. Anesthesiology. 110:628–637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brambrink AM, Evers AS, Avidan MS, Farber
NB, Smith DJ, Zhang X, Dissen GA, Creeley CE and Olney JW:
Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque
brain. Anesthesiology. 112:834–841. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kong F, Xu L, He D, Zhang X and Lu H:
Effects of gestational isoflurane exposure on postnatal memory and
learning in rats. Eur J Pharmacol. 670:168–174. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Liu C, Zhao Y, Hu K, Zhang J, Zeng
M, Luo T, Jiang W and Wang H: Sevoflurane induces short-term
changes in proteins in the cerebral cortices of developing rats.
Acta Anaesthesiol Scand. 57:380–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wang F, Liu C, Zeng M, Han X, Luo T,
Jiang W, Xu J and Wang H: JNK pathway may be involved in
isoflurane-induced apoptosis in the hippocampi of neonatal rats.
Neurosci Lett. 545:17–22. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
DiMaggio C, Sun LS and Li G: Early
childhood exposure to anesthesia and risk of developmental and
behavioral disorders in a sibling birth cohort. Anesth Analg.
113:1143–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ing C, DiMaggio C, Whitehouse A, Hegarty
MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G and
Sun LS: Long-term differences in language and cognitive function
after childhood exposure to anesthesia. Pediatrics. 130:e476–e485.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Liang G, Chen Q, Joseph DJ, Meng
Q, Eckenhoff RG, Eckenhoff MF and Wei H: Anesthetic-induced
neurodegeneration mediated via inositol 1,4,5-trisphosphate
receptors. J Pharmacol Exp Ther. 333:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao YL, Xiang Q, Shi QY, Li SY, Tan L,
Wang JT, Jin XG and Luo AL: GABA ergic excitotoxicity injury of the
immature hippocampal pyramidal neurons' exposure to isoflurane.
Anesth Analg. 113:1152–1160. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei H, Kang B, Wei W, Liang G, Meng QC, Li
Y and Eckenhoff RG: Isoflurane and sevoflurane affect cell survival
and BCL-2/BAX ratio differently. Brain Res. 1037:139–147. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yon JH, Daniel-Johnson J, Carter LB and
Jevtovic-Todorovic V: Anesthesia induces neuronal cell death in the
developing rat brain via the intrinsic and extrinsic apoptotic
pathways. Neuroscience. 135:815–827. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harper SJ and Wilkie N: MAPKs: New targets
for neurodegeneration. Expert Opin Ther Targets. 7:187–200. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaminska B, Gozdz A, Zawadzka M,
Ellert-Miklaszewska A and Lipko M: MAPK signal transduction
underlying brain inflammation and gliosis as therapeutic target.
Anat Rec (Hoboken). 292:1902–1913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ji RR, Gereau RW IV, Malcangio M and
Strichartz GR: MAP kinase and pain. Brain Res Rev. 60:135–148.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mousa A and Bakhiet M: Role of cytokine
signaling during nervous system development. Int J Mol Sci.
14:13931–13957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Behrens A, Sibilia M and Wagner EF:
Amino-terminal phosphorylation of c-Jun regulates stress-induced
apoptosis and cellular proliferation. Nat Genet. 21:326–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jeong HS, Choi HY, Choi TW, Kim BW, Kim
JH, Lee ER and Cho SG: Differential regulation of the antiapoptotic
action of B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma extra-long
(Bcl-xL) by c-Jun N-terminal protein kinase (JNK) 1-involved
pathway in neuroglioma cells. Biol Pharm Bull. 31:1686–1690. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chu R, Upreti M, Ding WX, Yin XM and
Chambers TC: Regulation of Bax by c-Jun NH2-terminal kinase and
Bcl-xL in vinblastine-induced apoptosis. Biochem Pharmacol.
78:241–248. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang WY, Yang R, Hu SF, Wang H, Ma ZW and
Lu Y: N-stearoyl-l-tyrosine ameliorates sevoflurane induced
neuroapoptosis via MEK/ERK1/2MAPK signaling pathway in the
developing brain. Neurosci Lett. 541:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sanders RD, Sun P, Patel S, Li M, Maze M
and Ma D: Dexmedetomidine provides cortical neuroprotection: Impact
on anaesthetic-induced neuroapoptosisin the rat developing brain.
Acta Anaesthesiol Scand. 54:710–716. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Metodiewa D, Kochman A and Karolczak S:
Evidence for antiradical and antioxidant properties of four
biologically active N, N-diethylaminoethyl ethers of flavaone
oximes: A comparison with natural polyphenolic flavonoid (rutin)
action. Biochem Mol Biol Int. 41:1067–1075. 1997.PubMed/NCBI
|
|
24
|
Jung CH, Lee JY, Cho CH and Kim CJ:
Anti-asthmatic action of quercetin and rutin in conscious
guinea-pigs challenged with aerosolized ovalbumin. Arch Pharm Res.
30:1599–1607. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santos KF, Oliveira TT, Nagem TJ, Pinto AS
and Oliveira MG: Hypolipidaemic effects of naringenin, rutin,
nicotinic acid and their associations. Pharmacol Res. 40:493–496.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garber JC: Committee for the Update of the
Guide for the Care and Use of Laboratory AnimalsGuide for the Care
and Use of Laboratory Animals. 8th. National Academy of Sciences;
2011, PubMed/NCBI
|
|
27
|
Orliaguet G, Vivien B, Langeron O,
Bouhemad B, Coriat P and Riou B: Minimum alveolar concentration of
volatile anesthetics in rats during postnatal maturation.
Anesthesiology. 95:734–739. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Liang G, Wang S, Meng Q, Wang Q and
Wei H: Effect of fetal exposure to isoflurane on postnatal memory
and learning in rats. Neuropharmacology. 53:942–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thornberry NA and Lazebnik Y: Caspases:
Enemies within. Science. 281:1312–1316. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zimmermann KC and Green DR: How cells die:
Apoptosis pathways. J Allergy Clin Immnol. 108:(4 Suppl). S99–S103.
2001. View Article : Google Scholar
|
|
31
|
Vorhees CV and Williams MT: Morris water
maze: Procedures for assessing spatial and related forms of
learning and memory. Nat Protoc. 1:848–858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang G, Ward C, Peng J, Zhao Y, Huang B
and Wei H: Isoflurane causes greater neurodegeneration than an
equivalent exposure of sevoflurane in the developing brain of
neonatal mice. Anesthesiology. 112:1325–1334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Istaphanous GK, Howard J, Nan X, Hughes
EA, McCann JC, McAuliffe JJ, Danzer SC and Loepke AW: Comparison of
the neuroapoptotic properties of equipotent anesthetic
concentrations of desflurane, isoflurane, or sevoflurane in
neonatal mice. Anesthesiology. 114:578–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oppenheim RW: Cell death during
development of the nervous system. Annu Rev Neurosci. 14:453–501.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rakic S and Zecevic N: Programmed cell
death in the developing human telencephalon. Eur J Neurosci.
12:2721–2734. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Blomgren K, Leist M and Groc L:
Pathological apoptosis in the developing brain. Apoptosis.
12:993–1010. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Loepke AW and Soriano SG: An assessment of
the effects of general anesthetics on developing brain structure
and neurocognitive function. Anesth Analg. 106:1681–1707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sanders RD, Xu J, Shu Y, Januszewski A,
Halder S, Fidalgo A, Sun P, Hossain M, Ma D and Maze M:
Dexmedetomidine attenuates isoflurane-induced neurocognitive
impairment in neonatal rats. Anesthesiology. 110:1077–1085. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Madsen TM, Kristjansen PE, Bolwig TG and
Wörtwein G: Arrested neuronal proliferation and impaired
hippocampal function following fractionated brain irradiation in
the adult rat. Neuroscience. 119:635–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rola R, Raber J, Rizk A, Otsuka S,
VandenBerg SR, Morhardt DR and Fike JR: Radiation-induced
impairment of hippocampal neurogenesis is associated with cognitive
deficits in young mice. Exp Neurol. 188:316–330. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gown AM and Willingham MC: Improved
detection of apoptotic cells in archival paraffin sections:
Immunohistochemistry using antibodies to cleaved caspase 3. J
Histochem Cytochem. 50:449–454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao H, Yenari MA, Cheng D, Sapolsky RM
and Steinberg GK: Bcl-2 overexpression protects against neuron loss
within the ischemic margin following experimental stroke and
inhibits cytochrome c translocation and caspase-3 activity.
J Neurochem. 85:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guan QH, Pei DS, Zhang QG, Hao ZB, Xu TL
and Zhang GY: The neuroprotective action of SP600125, a new
inhibitor of JNK, on transient brain ischemia/reperfusion-induced
neuronal death in rat hippocampal CA1 via nuclear and non-nuclear
pathways. Brain Res. 1035:51–59. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guan QH, Pei DS, Zong YY, Xu TL and Zhang
GY: Neuroprotection against ischemic brain injury by a small
peptide inhibitor of c-Jun N-terminal kinase (JNK) via nuclear and
non-nuclear pathways. Neuroscience. 139:609–627. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Han JY, Jeong EY, Kim YS, Roh GS, Kim HJ,
Kang SS, Cho GJ and Choi WS: C-jun N-terminal kinase regulates the
interaction between 14-3-3 and Bad in ethanol-induced cell death. J
Neurosci Res. 86:3221–3229. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zheng S and Zuo Z: Isoflurane
preconditioning induces neuroprotection against ischemia via
activation of P38 mitogen-activated protein kinases. Mol Pharmacol.
65:1172–1180. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Morris RG, Garrud P, Rawlins JN and
O'Keefe J: Place navigation impaired in rats with hippocampal
lesions. Nature. 297:681–683. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
D'Hooge R and De Deyn PP: Applications of
the Morris water maze in the study of learning and memory. Brain
Res Brain Res Rev. 36:60–90. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sall JW, Stratmann G, Leong J, McKleroy W,
Mason D, Shenoy S, Pleasure SJ and Bickler PE: Isoflurane inhibits
growth but does not cause cell death in hippocampal neural
precursor cells grown in culture. Anesthesiology. 110:826–833.
2009. View Article : Google Scholar : PubMed/NCBI
|