Introduction
Chronic renal allograft dysfunction (CRAD), defined
as an irreversible decline of renal allograft function, is one of
the main factors reducing long-term survival of renal allograft
tissue (1–3). At present, chronic active
antibody-mediated rejection (ABMR) is generally considered as an
inducing factor contributing to CRAD. The pathological mechanism of
CRAD can be expressed as interstitial fibrosis and tubular atrophy
(IF/TA), which is closely related to the decline of renal allograft
function. In addition, epithelial-mesenchymal transdifferentiation
(EMT) has an important role in the process of interstitial fibrosis
(4). Studies on animal models and
in vitro experiments have shown that the EMT participates in
the progression of interstitial fibrosis in renal allografts with
CRAD. It has been indicated that integrin-linked kinase (ILK) and
transforming growth factor (TGF)-β1 are the key factors inducing
EMT (5). Furthermore, a previous
study by our group identified a positive correlation between the
expression of ILK and the development of CRAD (6). However, to date, no study has assessed
the roles of Akt (also known as protein kinase B) and glycogen
synthase kinase (GSK)-3β in the development of EMT in renal
allografts with ABMR. Therefore, immunohistochemical staining and
semi-quantitative methods were applied in the present study to
assess the levels of phosphorylated (p)-Akt, GSK-3β, TGF-β1, ILK,
E-cadherin and α-smooth muscle actin (SMA). The results suggested
that the Akt/GSK-3β signaling pathway is involved in the
development of EMT induced by TGF-β1 and ILK in human renal
allografts with ABMR, and therefore participates in the
pathogenesis of IF/TA inducing ABMR.
Materials and methods
Patients and samples
Samples were collected from 38 renal transplant
recipients who were pathologically diagnosed with chronic active
ABMR. Renal allograft biopsy was performed in all of the recipients
due to upward-creeping serum creatinine or resistant proteinuria
from June 2010 to January 2012. The diagnosis of chronic ABMR was
made according to the Banff 2009 classification (7,8). Among
the 38 cases of ABMR, 22 were male (age, 44±9 years) 16 were female
(age, 40±9 years). The duration after kidney transplantation was
1–9 years (mean, 4 years). For the immunosuppressant protocols, 20
recipients received cyclosporine + mycophenolate mofetil +
prednisone therapy, 17 received tacrolimus + mycophenolate mofetil
+ prednisone therapy and one recipient received sirolimus +
mycophenolate mofetil + prednisone therapy. Prior to renal biopsy,
all of the renal allografts were detected by color ultrasound and
the blood drug concentration was determined to further exclude
acute rejection, calcineurin inhibitor renal toxicity, ureteral
obstruction/regurgitation and other renal diseases. All of the
recipients had matching blood groups to donors and two or more loci
matching with regard to human leukocyte antigen (HLA)-A, HLA-B and
HLA-DR antigens. The results of the lymphocytotoxicity test were
<10% crossmatch (negative) and panel reactive antibody scores
were <10%. Nine specimens of renal tissue used in the present
study came from nine normal donor kidneys from healthy donors,
verified by pre-transplant biopsy and clinical follow-up of the
recipients.
Histological examination
The paraffin-embedded kidney specimens were cut into
3-µm tissue sections that were de-paraffinized with xylene and
hydrated with a graded series of ethanols (100, 96, 90 and 70%) and
distilled water. Staining was performed according to standard
histology procedures, including hematoxylin/eosin stain,
periodic-acid schiff stain, masson trichrome and periodic
schiff-methenamine stain. The slides were observed under a
microscope in a blinded manner for inflammatory-cell infiltration
in renal tissue, increased mesangial and extracellular matrix,
proliferation of mesangial cells, epithelium and endothelium,
adhesions and sclerosis, thickening of glomerular basement
membranes and double track sign, thickening of peritubular
capillary basement membrane, interstitial fibrosis, inflammatory
cell infiltration, tubular atrophy and intimal thickening of
arteries.
Immunohistochemical staining
The EnVision method was used for immunohistochemical
staining. Paraffin sections (3-µm thickness) received routine
baking and were dewaxed with xylene and rehydrated with a series of
graded ethanols. 3% hydrogen peroxide was adopted to clear
endogenous hydrogen peroxidase. Prior to immunohistochemial
staining, antigen retrieval was performed in sodium citrate buffer
in a microwave (550 watts) for 15 min for the analysis p-Akt and
GSK-3β and for 3×3 min for all other proteins apart from α-SMA, for
which no antigen retrieval was required. Samples were then
incubated with rabbit anti-human polyclonal p-Akt antibody (cat.
no. 9611S; 1:300 dilution; Cell Signaling Technology, Inc.,
Danvers, MA, USA), rabbit anti-human polyclonal GSK-3β antibody
(cat. no. BA-0906; 1:100 dilution; Wuhan Boshide Co., Wuhan,
China), mouse anti-human monoclonal ILK antibody (cat. no.
SC-20019; 1:200 dilution; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA), rabbit anti-human TGF-β1 antibody (cat. no. RAB-0238;
working solution; Fuzhou Maixin Co.), mouse anti-human E-cadherin
antibody (cat. no. MAB-0589; working solution; Fuzhou Maixin Co.)
or mouse anti-human α-SMA antibody (cat. no. MAB-0003; working
solution; Fuzhou Maixin Co.) overnight at 4°C. After washing with
PBS, anti-rabbit (cat. no. KIT-9902; Fuzhou Maixin Co.) was added
and the sections were incubated for 30 min at 37°C. Subsequent to
washing with PBS, antibodies were visualized with diaminobenzidine
and counterstained with hematoxylin prior to microscopic
observation.
Staining was considered positive when pale yellow,
brownish yellow and yellowish-brown particles occurred in tissue.
Immunohistochemical staining in each group was compared with that
in a control group with PBS as a substitute for the primary
antibody. At the same time, the deposition of immunoglobulin (Ig)A,
IgG, IgM, C3, C4, C1q and C4d were routinely tested for renal
biopsy.
Pathological classification
According to the Banff 2009 classification (7) the pathological manifestation of renal
allografts with ABMR can be classified as: i) C4d positive,
coexistence of circulating anti-donor antibodies; ii) IF/TA and
thickening of the glomerular basement membrane. The severity grade
of IF/TA was divided into three groups: IF/TA-I, mild IF/TA
(<25% cortex involved); IF/TA-II, moderate IF/TA (26–50% cortex
involved) and IF/TA-III, severe IF/TA (>50% cortex involved),
which may include nonspecific sclerosis of blood vessels and
glomeruli, but is certainly accompanied by tubular interstitial
lesion.
Semiquantitative analysis
Semiquantitative analysis was performed with a
DMR+Q550 renal color patho-image analysis system (Leica, Wetzlar,
Germany). 10 discontinuous tubulointerstitial fields
(magnification, ×400; excluding glomeruli, veins and arteries) were
randomly selected in different regions including renal cortex,
cortex-medulla juncture and medullary interstitium. More than 60
tubules were involved in each part. Positive results were indicated
by yellow-stained cells. ImagePro Plus software version 6.0.0.260
(Media Cybernetics, Rockville, MD, USA) was utilized to determine
the ratio of the positive to total area (area of tubular lumens
excluded). The relative amount of each type of protein in the renal
tubulointerstitium was represented as the mean value.
Statistical analysis
All experimental data were expressed as the mean ±
standard deviation. SPSS 13.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was adopted for data analysis. Differences in the
levels of p-AKT, GSK-3β, ILK, TGF-β1, E-cadherin and α-SMA were
analyzed by single-factor analysis of variance. Pearson's linear
correlation analysis was used for two-variable correlation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Histological examination
By observation under the light microscope, the
pathological manifestations of ABMR in renal allografts of the
recipients were identified to include focal interstitial diffuse
fibrosis, different degrees of tubular atrophy and disordered
arrangements, accompanied by plasma cell and lymphocyte invasion
(Fig. 1A).
Grouping based on IF/TA grades
According to the Banff 2009 classification,
recipients diagnosed with ABMR were divided into three groups:
Group IF/TA-I (12 cases), IF/TA-II (14 cases) and IF/TA-III (12
cases), based on the grade of IF/TA (I, II or III).
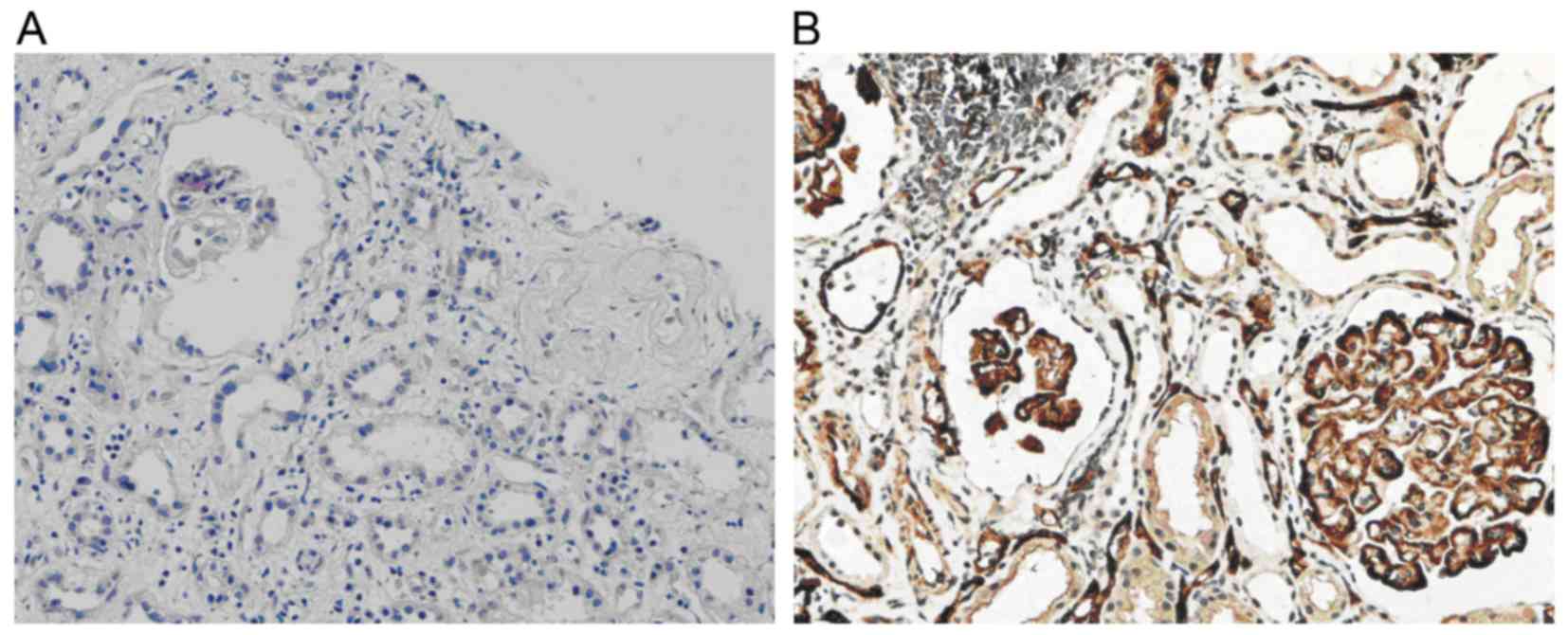
C4d deposition
In normal renal tissue, C4d deposition is present in
the glomerular mesangium and segmental endarterium, while it is
rarely shown in glomerular and peritubular capillaries. However, in
the renal allograft tissue with chronic active ABMR, diffuse and
linear deposition of C4d was obvious in endothelial cells of
peritubular capillaries (Fig.
1B).
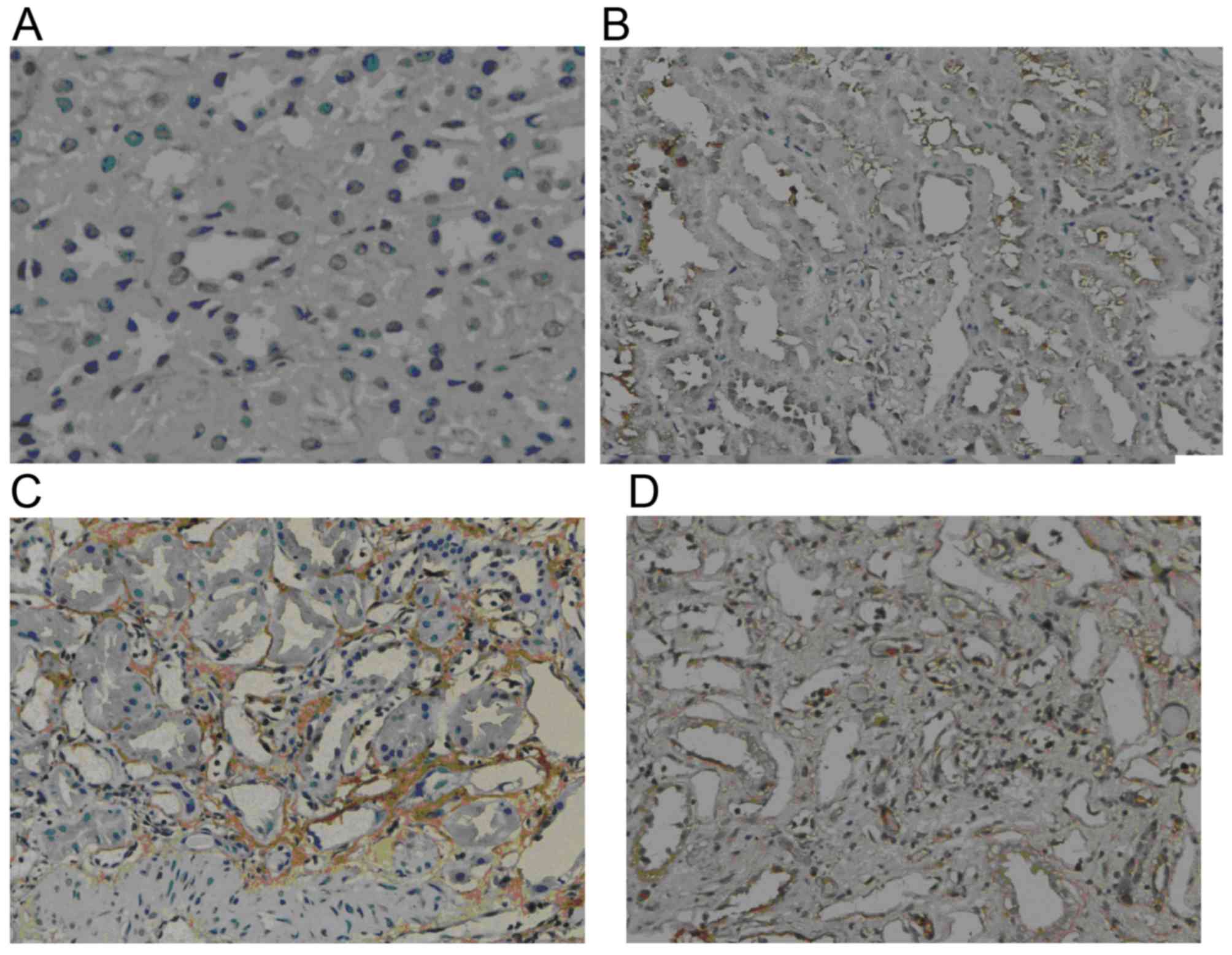
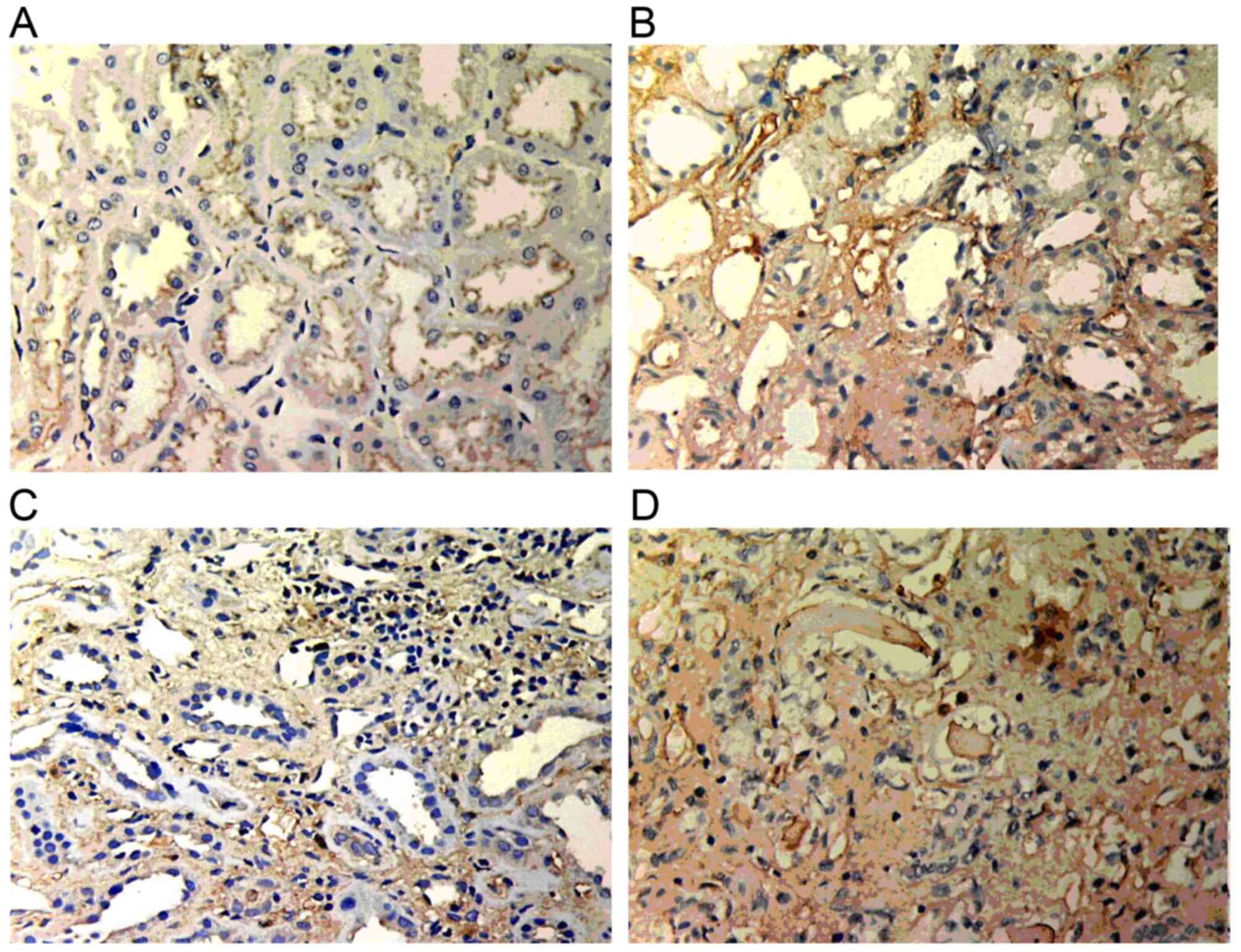
GSK-3β expression
Weak GSK-3β expression was present in normal renal
tissue. However, in the renal allograft tissue with chronic active
ABMR, GSK-3β expression was markedly increased. The expression was
mainly located in the endochylema of tubular cells and was enhanced
with increasing IF/TA pathological grade (Fig. 2).
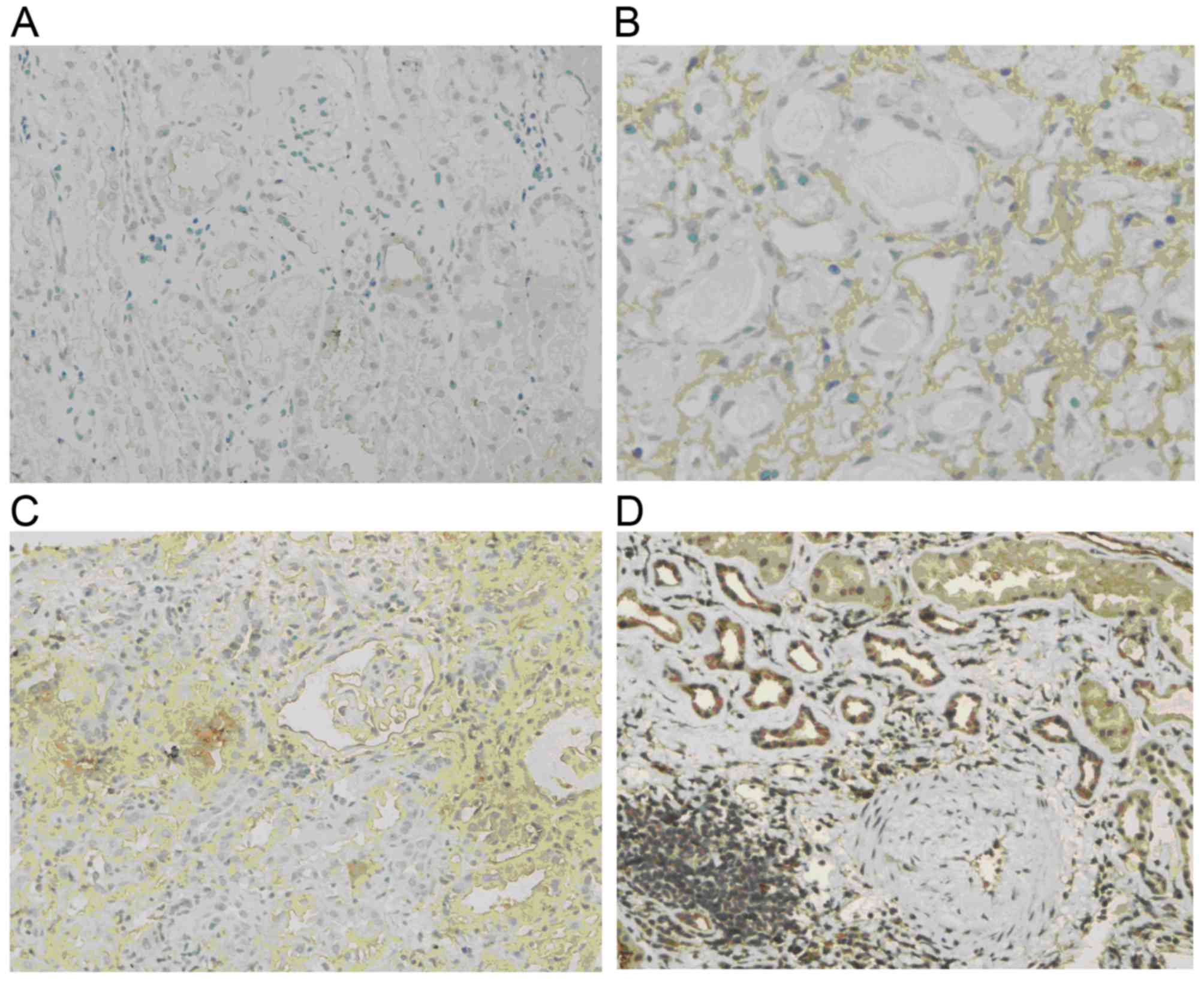
p-Akt levels
Normal renal tissue was almost negative for p-Akt.
In comparison, p-Akt was obviously increased in renal allograft
tissue with chronic active ABMR. The expression was mostly located
in the endochylema of tubular epithelial cells and interstitial
cells and tended to be enhanced with increases in the IF/TA grade
(Fig. 3).
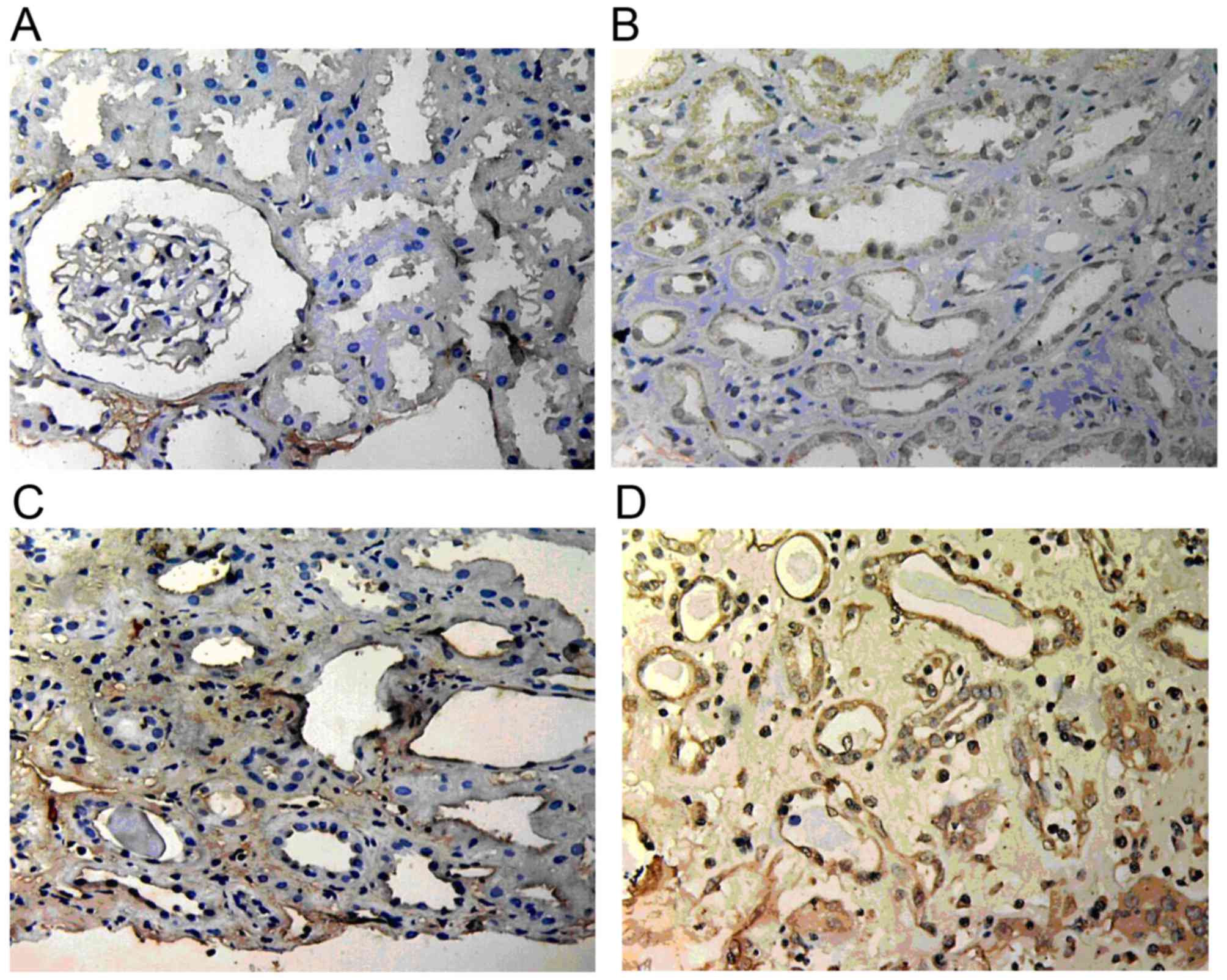
ILK expression
ILK expression in normal renal tissue was low or
absent; however, it was markedly increased in renal allograft
tissue with ABMR and was mainly located in the endochylema of
tubular epithelial cells and interstitial cells. Atrophic renal
tubules showed the highest ILK staining. With the increase of the
pathological grade of IF/TA, ILK expression became stronger and its
scope became wider (Fig. 4).
TGF-β1 expression
TGF-β1 expression in normal renal tissue was mainly
located in the endochylema of tubular epithelial cells and was
weakly positive. In renal allograft tissue, TGF-β1 expression in
tubular epithelial cells, interstitial cells and the interstitial
matrix area was in positive. Moreover, the expression was increased
in the IF/TA-I group and showed further increases in the IF/TA-II
and IF/TA-III groups (Fig. 5).
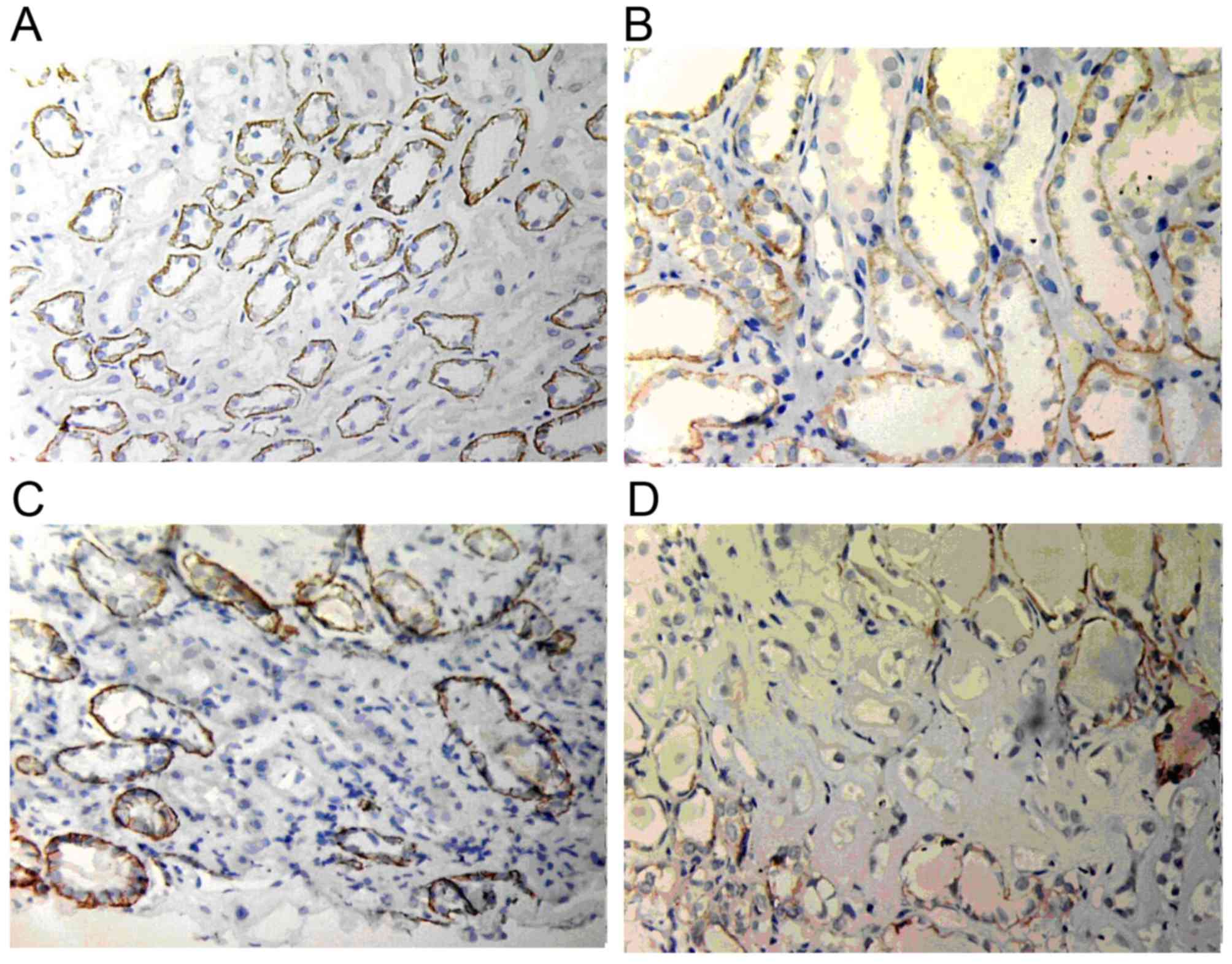
E-cadherin expression
In normal renal tissues, E-cadherin expression was
mainly located in the basement membrane of glomeruli and tubular
epithelial cells but not in the endochylema of tubular cells.
However, in renal allograft tissue with ABMR and IF/TA grade I,
E-cadherin expression started to reduce. In addition, in the IF/TA
grade II group, E-cadherin expression was markedly reduced and only
few cells expressed E-cadherin in the IF/TA grade III group
(Fig. 6).
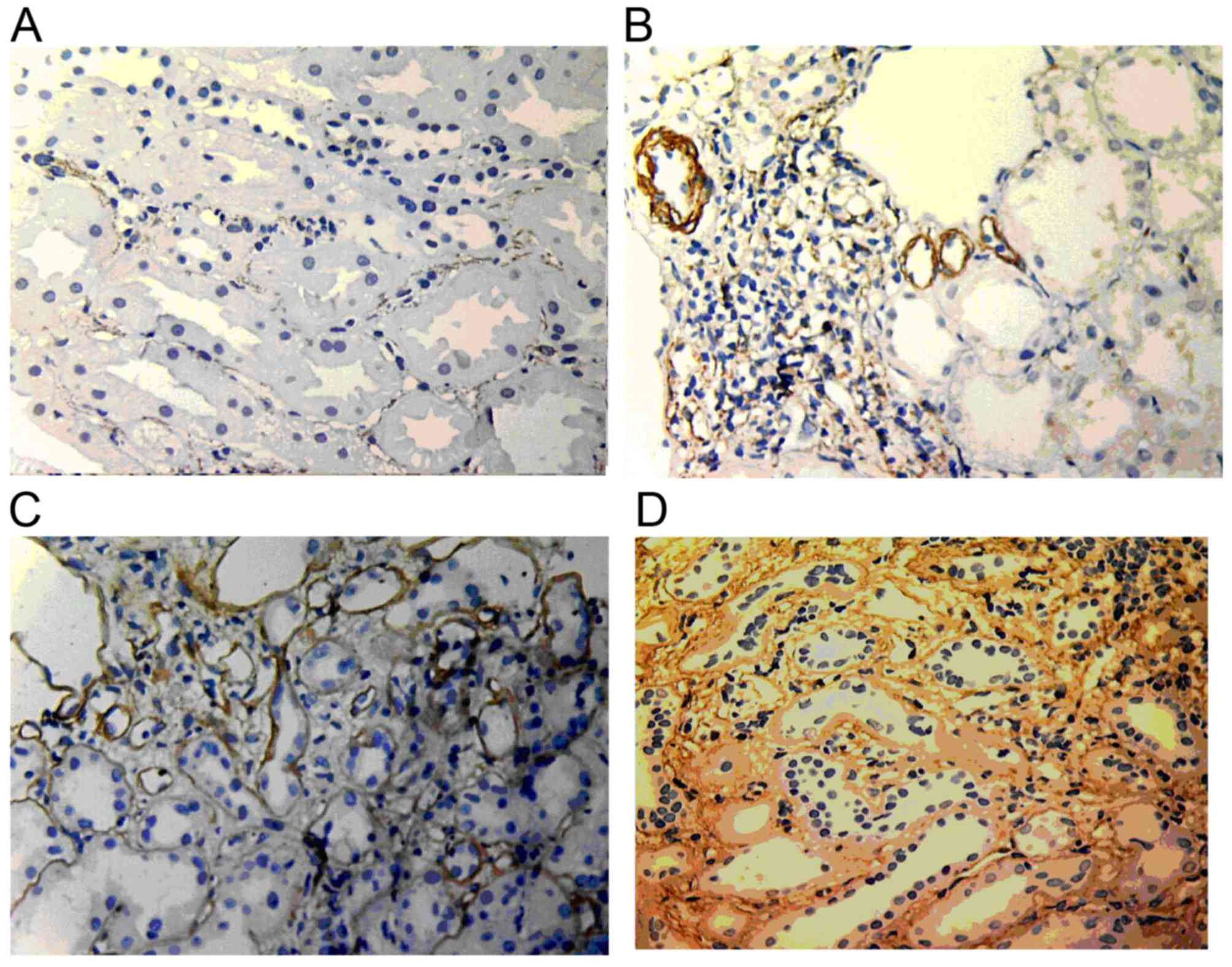
α-SMA expression
In normal renal tissue, α-SMA was only expressed in
the muscle layer of vascular smooth muscle and randomly expressed
in the renal interstitium. In renal allografts with ABMR, the
expression increased along with the increase of the IF/TA grade.
α-SMA expression was widely distributed in the renal interstitium.
Furthermore, α-SMA expression was also found in renal tubules with
lumen damage and tubular atrophy (Fig.
7).
Association between the levels of
p-Akt, GSK-3β, ILK, TGF-β, α-SMA and the IF/TA grade
Along with the increase of the IF/TA grade, the
levels of p-Akt, GSK-3β, ILK, TGF-β1 and α-SMA in renal allografts
with ABMR were obviously increased compared to those in normal
renal tissue (P<0.001). By contrast, E-cadherin was
significantly reduced (P<0.001) (Table I).
 | Table I.Positive area ratio of p-Akt, GSK-3β,
ILK, TGF-β1, E-cadherin and α-SMA in the renal tubules and
tubulointerstitium of normal controls and renal transplant
recipients with antibody-mediated rejection. |
Table I.
Positive area ratio of p-Akt, GSK-3β,
ILK, TGF-β1, E-cadherin and α-SMA in the renal tubules and
tubulointerstitium of normal controls and renal transplant
recipients with antibody-mediated rejection.
| Group | N | p-Akt | GSK-3β | ILK | TGF-β1 | E-cadherin | α-SMA |
|---|
| Normal control | 9 | 2.99±1.18 | 11.12±6.56 | 1.67±0.80 | 4.01±1.16 | 30.71±7.36 | 0.34±0.33 |
| IF/TA-I | 12 |
27.21±7.80a |
31.60±6.85a |
14.20±5.93a |
23.44±4.18a |
12.04±3.47a |
12.09±4.39a |
| IF/TA-II | 14 |
45.72±7.18a,b |
42.08±10.63a,b |
23.94±5.90a,b |
41.14±6.21a,b |
7.49±1.83a,b |
26.25±5.39a,b |
| IF/TA-III | 12 |
61.97±9.28a–c |
57.42±11.43a–c |
37.92±8.12a–c |
64.82±8.56a–c |
3.27±1.36a–c |
32.16±5.06a,b,d |
Correlation among the expression of
p-Akt, GSK-3β, ILK, TGF-β and α-SMA
Linear correlation analysis showed that the
expression of p-Akt was positively correlated with ILK, TGF-β and
α-SMA (r=0.871, 0.912 and 0.878, respectively; P<0.001). The
expression of GSK-3β was also positively correlated with p-Akt,
ILK, TGF-β1 and α-SMA (r=0.828, 0.793, 0.874 and 0.781
respectively; P<0.001). However, the expression of p-Akt and
GSK-3β was negatively correlated with E-cadherin expression
(r=−0.849 and −0.781; P<0.001).
Discussion
CRAD is the main factor affecting the long-term
survival of renal allografts and its pathological manifestation is
interstitial fibrosis and tubular atrophy. Factors leading to
chronic renal allograft dysfunction can be divided into immune
factors and non-immune factors, and chronic active ABMR is the main
immune factor contributing to CRAD (8). However, the pathogenesis of tubular EMT
caused by ABMR has remained to be fully elucidated. Consequently,
it is of great significance to investigate the pathogenesis of EMT
in renal allograft recipients undergoing ABMR.
EMT is a key factor leading to interstitial
fibrosis. It refers to a process by which epithelial cells lose
their phenotype under pathogenesis and transdifferentiate into the
phenotype of mesenchymal cells. E-cadherin has an important role in
maintaining epithelial cell polarity and function. The loss of its
expression and the damage of epithelial integrity are the early
events in the EMT process of tubular epithelial cells. α-SMA is a
marker protein of myofibroblasts. It is not expressed in normal
renal tissue but in renal allografts with CRAD. The EMT can be
stimulated by various inflammatory factors and cell factors,
leading to the transformation of epithelial cells into
myofibroblasts and expression of α-SMA. Therefore, α-SMA is a
marker of the EMT, which occurs once α-SMA is expressed (9). The EMT is involved in the pathogenesis
ABMR and associated mechanisms may offer approaches for preventing
CRAD.
In the present study, p-Akt and GSK-3β were present
at low levels in normal renal tissue and were markedly increased
along with the aggravation of IF/TA, the major pathological
manifestation of ABMR. The present study also showed that p-Akt,
GSK-3β, TGF and ILK were positively correlated with α-SMA
expression but negatively correlated with E-cadherin expression,
suggesting that all of these factors participate in the EMT process
associated with ABMR leading to the development of CRAD.
TGF-β1, as the most important factor causing renal
interstitial fibrosis, is the only cell factor that induces and
maintains the EMT process in renal tubules. It has been suggested
that the effect of TGF-β1 to induce renal interstitial fibrosis may
be accomplished by inducing EMT (10). Furthermore, ILK has been suggested to
be an important downstream factor of TGF-β1. Through interacting
with integrin b1 and b3, ILK can participate in various
transduction pathways of cell signals, including Akt and GSK-3β,
and has a significant role in adjusting cellular adhesion,
apoptosis, growth and removal as well as extracellular matrix
accumulation, and is closely associated with renal disease
progression. Furthermore, TGF-β1 expression in tubular epithelial
cells is induced by ILK and the expression of these two factors is
inter-dependent with regard to time and dosage (11). A study using a rat kidney
transplantation model showed that ILK is correlated with tubular
interstitial fibrosis and mononuclear cell invasion (12). Li et al (5) suggested the existence of the
TGF-β1/Smad/ILK/EMT axis, in which TGF-β1 induces ILK to be
expressed in tubular epithelial cells by means of Smad signaling,
and if transduction pathways were restrained, ILK expression and
the EMT process induced by TGF-β1 were effectively prevented.
However, by restraining other transduction pathways downstream of
TGF-β1, ILK expression and the EMT process were not completely
inhibited. Therefore, ILK markedly influences the EMT process
(13).
The results of a previous study by our group
suggested the trend that the expression of TGF-β1 and ILK in
allograft recipients with ABMR was markedly increased along with
the aggravation of the pathological grade of IF/TA (6). In the present study, the expression of
ILK in the ABMR group was found to be positively correlated with
that of TGF-β1 and α-SMA, while being negatively correlated with
that of E-cadherin, which indicates that ILK affects the EMT
process of renal allograft tissue and furthermore triggers tubular
interstitial fibrosis.
Akt is a serine/threonine kinase with a relative
molecular weight of 57 kDa and participates in important cellular
signal transduction pathways. Numerous growth factors and mouse
eotaxins have been found to take effect through the Akt signaling
pathway. Akt can be activated by the phosphorylation of its ser473
residue and take part in cellular growth, differentiation and
apoptotic processes. Subsequent to Akt activation, TGF-β1 and ILK
are activated and have a role in stimulating cellular growth and
reducing apoptosis (14,15). p-Akt is also a key factor in the EMT
process (16). A study on EMT in
type II diabetes-associated renal disease in rats showed that
TGF-β1 was able to activate Akt and that the activation level of
Akt was positively correlated with TGF-β1 expression (14). In the kidney under chronic hypoxia
caused by biliverdin, the EMT process can also be induced though
the Akt signaling pathway (17). A
study on tumor progression found that ILK stimulates the EMT
process of tumor cells via Akt (18). Subsequent to activation, Akt
stimulates nuclear factor Snail and inhibits the expression of cell
adhesion molecule E-cadherin. Thus, the activation of Akt lays a
solid foundation for the EMT process (19).
The present study showed that in renal allograft
tissues, the expression of Akt increased along with the aggravation
of the pathological grade of IF/TA and that there was a positive
correlation between the levels of p-Akt and the individual
expression of TGF-β1, ILK and α-SMA, while there was a negative
correlation between p-Akt and E-cadherin, indicating that Akt takes
part in the EMT process associated with ABMR resulting in CRAD. The
results of the present study suggested that Akt signal transduction
is necessary in the EMT process induced by TGF-β1 and ILK in human
renal allografts with ABMR, and that the induction of the EMT
process by Akt may proceed via the TGF-β1/ILK signaling
pathway.
In studies on diseases rather than kidney
transplants, p-Akt was shown to influence the downstream signaling
molecule GSK-3β. GSK-3β is a serine/threonine kinase, which is
commonly expressed in eukaryotic organisms. GSK-3β is mainly
expressed in the nucleus and affects the activation status of
downstream molecules via phosphorylation. Besides, GSK-3β is an
important substrate of p-Akt. Phosphorylation of Akt deactivates
GSK-3β and studies have shown that GSK-3β, as a major component of
the Akt pathway, has a role in the EMT process (14).
Gong et al (20,21) have
verified that GSK-3β controls the inflammatory reaction in
allografts undergoing CRAD and showed that GSK-3β was highly
expressed in damaged renal tubules. Increased levels of deactivated
p-GSK-3β were negatively correlated with interstitial fibrosis and
tubular atrophy. Akt and ILK can trigger the activation of nuclear
transcription factor β-catenin and activator protein (AP)-1 by
GSK-3β, which makes them enter the nucleus, activate nuclear
transcription processes, causes the reduction of epithelial cell
adhesion, increases the synthesis of matrix metalloproteinase
(MMP)-9 and α-SMA and finally affects the induction and progression
of the EMT process (22,23). In a rat model of diabetes-associated
renal disease, it was found that the inhibition of the GSK-3β
signaling pathway promotes the EMT process induced by high glucose
and delays the pathological change of interstitial fibrosis
(24). In addition, ILK can also
lead to the enhancement of AP-1 and β-catenin activation and the
multiplication of cells by phosphorylating and thereby deactivating
GSK-3β (25,26). Enhancement of AP-1 leads to increased
expression and secretion of MMP-2, breaks the integrity of the
glomerular basement membrane, increases the invasion of cells and
stimulates the transformation of epithelial cells in the basement
membrane to cause interstitial fibrosis (27). Through the nuclear factor (NF)-κB
signaling pathway, GSK-3β can also influence the emergence of
inflammatory factors and take part in the inflammation reaction, as
the EMT process is triggered in renal tubules after NF-κB activates
mononuclear cells (28). Studies on
tubular epithelial cells have found found that inhibition of GSK-3β
phosphorylation may restrain the activation of NF-κB p65 and
consequently prevent the expression of inflammatory factors and the
emergence of the inflammatory reaction (20,21).
In a previous study by our group, it was
demonstrated that in renal allograft tissue, the expression of
GSK-3β increases with the aggravation of the pathological grade of
IF/TA (29). Furthermore, in the
present study, the expression of GSK-3β was found to be positively
correlated with the individual levels of ILK, p-Akt and α-SMA in
renal allografts with ABMR, which indicated that GSK-3β
participates in the EMT process induced by ILK. However, the
necessity of Akt to induce ABMR also indicates the possible
connection point between ILK and GSK-3β. Based on the results of
the present study, it is suggested that tubular fibrosis may be
blocked by interference with Akt/GSK-3β signaling.
TGF-β1 stimulates the phosphorylation of Akt and
helps LY294002, an inhibitor of the phosphoinositide-3 kinase/Akt
pathway, to effectively reduce the expression of collagen I induced
by TGF-β1 (30). The GSK-3β
inhibitor lithium oxide improved the restoration of renal tissue
and damaged epithelial cells through Wnt signaling (31). Troglitazone may improve tubular EMT
caused by high glucose in experimental models (22).
Based on the results of previous studies by our and
other groups as well as the present study, it is summarized that in
renal allograft tissue undergoing ABMR resulting in CRAD, ILK,
together with the downstream signaling molecules Akt and GSK-3β,
may take part in EMT and interstitial fibrotic processes induced by
TGF-β1. As a key mediator, ILK connects the upstream TGF-β1 with
the downstream factors Akt and GSK-3β. It is suggested that early
detection of ILK, Akt and GSK-3β expression in renal allograft
tissues provides a basis for clinical intervention by means of
inhibiting the expression or activity of the above factors, which
may represent a novel strategy to extend the survival of renal
allograft tissues.
Acknowledgements
The project was supported by the Natural Science
Foundation of Guangxi (no. 2014GXNSFAA118179), self-funded research
projects of Guangxi Health Department (no. Z2014533) and the
Science and Technology Planning Project of Guilin (no.
20130121-6).
References
|
1
|
Maluf DG, Mas VR, Archer KJ, Yanek K,
Gibney EM, King AL, Cotterell A, Fisher RA and Posner MP: Molecular
pathways involved in loss of kidney graft function with tubular
atrophy and interstitial fibrosis. Mol Med. 14:276–285. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chapman J: Addressing the challenges for
improving long-term outcomes in renal transplantation. Transplant
Proc. 40:(10 Suppl). S2–S4. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robertson H, Ali S, McDonnell BJ, Burt AD
and Kirby JA: Chronic renal allograft dysfunction: The role of T
cell-mediated tubular epithelial to mesenchymal cell transition. J
Am Soc Nephrol. 15:390–397. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mannon RB: Therapeutic targets in the
treatment of allograft fibrosis. Am J Transplant. 6:867–875. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li Y, Yang J, Dai C, Wu C and Liu Y: Role
for integrin-linked kinase in mediating tubular epithelial to
mesenchymal transition and renal interstitial fibrogenesis. J Clin
Invest. 112:503–516. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yan Q, Sui W, Xie S, Chen H, Xie S, Zou G,
Guo J and Zou H: Expression and role of integrin-linked kinase and
collagen IV in human renal allografts with interstitial fibrosis
and tubular atrophy. Transpl Immunol. 23:1–5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sis B, Mengel M, Haas M, Colvin RB,
Halloran PF, Racusen LC, Solez K, Baldwin WM 3rd, Bracamonte ER,
Broecker V, et al: Banff '09 meeting report: Antibody mediated
graft deterioration and implementation of Banff working groups. Am
J Transplant. 10:464–471. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loupy A, Hill GS, Nochy D and Legendre C:
Antibody-mediated microcirculation injury is the major cause of
late kidney transplant failure. Am J Transplant. 10:9532010.
View Article : Google Scholar
|
|
9
|
Liu Y: Epithelial to mesenchymal
transition in renal fibrogenesis: Pathologic significance,
molecular mechanism, and therapeutic intervention. J Am Soc
Nephrol. 15:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lan HY: Tubular epithelial-myofibroblast
transdifferentiation mechanisms in proximal tubule cells. Curr Opin
Nephrol Hypertens. 12:25–29. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Guo L, Blattner SM, Mundel P,
Kretzler M and Wu C: Formation and phosphorylation of the
PINCH-1-integrin linked kinase-alpha-parvin complex are important
for regulation of renal glomerular podocyte adhesion, architecture,
and survival. J Am Soc Nephrol. 16:1966–1976. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han C, Zou H, Li Q, Wang Y, Shi Y, Lv T,
Chen L and Zhou W: Expression of the integrin-linked kinase in a
rat kidney model of chronic allograft nephropathy. Cell Biochem
Biophys. 61:73–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee YI, Kwon YJ and Joo CK:
Integrin-linked kinase function is required for transforming growth
factor beta-mediated epithelial to mesenchymal transition. Biochem
Biophys Res Commun. 316:997–1001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kattla JJ, Carew RM, Heljic M, Godson C
and Brazil DP: Protein kinase B/Akt activity is involved in renal
TGF-beta1-driven epithelial-mesenchymal transition in vitro and in
vivo. Am J Physiol Renal Physiol. 295:F215–F225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Delcommenne M, Tan C, Gray V, Rue L,
Woodgett J and Dedhar S: Phosphoinositide-3-OH kinase-dependent
regulation of glycogen synthase kinase 3 and protein kinase B/AKT
by the integrin-linked kinase. Proc Natl Acad Sci USA.
95:11211–11216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai C, Yang J and Liu Y: Transforming
growth factor-beta1 potentiates renal tubular epithelial cell death
by a mechanism independent of Smad signaling. J Biol Chem.
278:12537–12545. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng R, Yao Y, Han M, Zhao X, Liu XC, Wei
J, Luo Y, Zhang J, Zhou J, Wang S, et al: Biliverdin reductase
mediates hypoxia-induced EMT via PI3-kinase and Akt. J Am Soc
Nephrol. 19:380–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Persad S and Dedhar S: The role of
integrin-linked kinase (ILK) in cancer progression. Cancer
Metastasis Rev. 22:375–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Larue L and Bellacosa A:
Epithelial-mesenchymal transition in development and cancer: Role
of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene.
24:7443–7454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong R, Rifai A, Ge Y, Chen S and Dworkin
LD: Hepatocyte growth factor suppresses proinflammatory NFkappaB
activation through GSK-3Β3beta inactivation in renal tubular
epithelial cells. J Biol Chem. 283:7401–7410. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gong R, Ge Y, Chen S, Liang E, Esparza A,
Sabo E, Yango A, Gohh R, Rifai A and Dworkin LD: Glycogen synthase
kinase 3beta: A novel marker and modulator of inflammatory injury
in chronic renal allograft disease. Am J Transplant. 8:1852–1863.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim K, Lu Z and Hay ED: Direct evidence
for a role of beta-catenin/LEF-1 signaling pathway in induction of
EMT. Cell Biol Int. 26:463–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bijur GN and Jope RS: Glycogen synthase
kinase-3 beta is highly activated in nuclei and mitochondria.
Neuroreport. 14:2415–2419. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YJ and Han HJ: Troglitazone
ameliorates high glucose-induced EMT and dysfunction of SGLTs
through PI3K/Akt, GSK-3Β-3Β3beta, Snail1 and beta-catenin in renal
proximal tubule cells. Am J Physiol Renal Physiol. 298:F1263–F1275.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Novak A, Hsu SC, Leung-Hagesteijn C,
Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R and
Dedhar S: Cell adhesion and the integrin-linked kinase regulate the
LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci USA.
95:4374–4379. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Troussard AA, Tan C, Yoganathan TN and
Dedhar S: Cell-extracellular matrix interactions stimulate the AP-1
transcription factor in an integrin-linked kinase- and glycogen
synthase kinase 3-dependent manner. Mol Cell Biol. 19:7420–7427.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Troussard AA, Costello P, Yoganathan TN,
Kumagai S, Roskelley CD and Dedhar S: The integrin linked kinase
(ILK) induces an invasive phenotype via AP-1 transcription
factor-dependent upregulation of matrix metalloproteinase 9
(MMP-9). Oncogene. 19:5444–5452. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Q, Liu BC, Lv LL, Ma KL, Zhang XL and
Philiip AO: Monocytes induce proximal tubular
epithelial-mesenchymal transition through NF-kappa B dependent
upregulation of ICAM-1. J Cell Biochem. 112:1585–1592. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan Q, Wang B, Sui W, Zou G, Chen H, Xie S
and Zou H: Expression of GSK-3Β-3Β3b in renal allograft tissue and
its significance in pathogenesis of chronic allograft dysfunction.
Diagn Pathol. 7:52012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Runyan CE, Schnaper HW and Poncelet AC:
The phosphatidylinositol 3-kinase/Akt pathway enhances
Smad3-stimulated mesangial cell collagen I expression in response
to transforming growth factor-beta1. J Biol Chem. 279:2632–2639.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sinha D, Wang Z, Ruchalski KL, Levine JS,
Krishnan S, Lieberthal W, Schwartz JH and Borkan SC: Lithium
activates the Wnt and phosphatidylinositol 3-kinase Akt signaling
pathways to promote cell survival in the absence of soluble
survival factors. Am J Physiol Renal Physiol. 288:F703–F713. 2005.
View Article : Google Scholar : PubMed/NCBI
|