Introduction
Breast cancer leads to 15.4% of cancer-related
mortalities among females in developed countries, and is the
primary cause of cancer morbidity in poorly developed countries
(1). Breast cancer represented 1.7
million cases, 11.9%, of all cancer worldwide in 2015 (1,2), and is
the most prevalent cancer among women in Egypt, constituting 32% of
female cancer cases leading to death (3).
Obesity is characterized by the accumulation of
adipocytes in fat tissues and is considered as a serious health
problem due to its association with different disorders including
carcinogenesis (4). Obesity degree
is measured by body mass index (BMI) and it was estimated that
>1.3 billion individuals worldwide are obese according to World
Health Organization (WHO) (5,6). Obesity
is considered to be a breast cancer risk factor that may rise
steadily worldwide, and it is estimated that 21% of all cancer
morbidity worldwide is due to obesity (7,8). Among
risk factors related to obesity is the accumulation of adipose
tissue that secretes adipokines including leptin, resistin,
adiponectin and other cytokines (9).
Obese patients with breast cancer are characterized
by advanced pathological characteristics including high tumor
grade, advanced tumor stage and lymph node metastasis (10), in addition to cancer recurrence and
shorter disease free survival (11).
Furthermore, obesity may decrease the efficiency of chemotherapy
against breast cancer (12).
Leptin is a polypeptide (16 kDa) product of a gene
associated with obesity (13) that
mediates its physiological actions through the leptin receptor
(LEPR) (14). It is a cytokine
hormone that modulates energy balance and weight homeostasis
through stimulating the expression level of cytochrome P450 family
19 subfamily A member 1 (CYP19A1), and controlling serine/threonine
kinase 11 (STK11) and mitogen activated protein kinase (MAPK)
(13,15–17).
Furthermore, leptin possesses different biological and
physiological functions including immune responses, puberty,
lactation, cell proliferation and hematopoiesis (18,19).
Leptin and its receptor were previously identified to be associated
with aggressive breast tumor proliferation, cell migration and
stimulation of angiogenesis and invasion (20). It was demonstrated that leptin is
associated with breast cancer development by enhancing the janus
kinase/signal transducer and activator of transcription-3
(JAK/STAT3), extracellular signal-related kinases 1 and 2 (ERK1/2)
and phoshphoinositide 3-kinase pathways that lead to breast cancer
cell proliferation and cell survival in vitro studies
(21). A number of in vitro
studies have reported that leptin may stimulate estrogen expression
by increasing the expression of the intracellular aromatase enzyme,
which has also been implicated in breast cancer development
(22,23).
Leptin may induce breast cancer progression through
stimulating the adhesion process by enhancing the expression level
of E-cadherin in MCF-7 cell lines (24), migration and invasion processes by
activating the expression of matrix metalloproteinase 2 and 9 (MMP2
and MMP9) and epidermal growth factor receptor (EGFR) (25). Additionally, leptin may stimulate
angiogenesis and cell cycle processes via the activation of
vascular endothelial growth factor (VEGF) expression and cyclin D1,
respectively (26–28) and inhibiting apoptosis of breast
cancer cells (29). It has been
indicated that the small peptide leptin receptor antagonist (LPrA2)
decreases breast cancer growth in mice (27). The inhibition of leptin signalling
provides a target for breast cancer treatment that may be useful in
reducing the progression of breast cancer.
Studying the molecular mechanisms of leptin that
contribute to breast cancer development may guide the
identification of novel therapies to reduce breast cancer
progression and/or development. In the present study, leptin
expression in patients with breast cancer and the possible
proliferation pathway(s) responsible for breast cancer progression
were assessed and a significant positive association between leptin
expression, LEPR and activation of cell proliferation signalling
pathways (aromatase, MAPK and STAT3) in tissue samples of breast
cancer patients was observed. Furthermore, the concentration of
leptin in plasma of the breast tumor microenvironment and
peripheral blood of patients was assessed and the present study
demonstrates that the concentration of leptin in plasma from tumor
microenvironment blood was significantly higher compared with the
leptin in plasma from peripheral blood of obese patients with
estrogen receptor positive (ER+) breast cancer.
Materials and methods
Patient selection
The present study was approved by the Institutional
Review Board of the Ain Shams University Hospital Ethics Committee.
Each patient signed a consent form prior to participation.
Patients who visited the breast clinic of Ain Shams
University Hospital (Cairo, Egypt) and were subjected to medical
analysis by clinical examination, mammogram, ultrasound and biopsy
were enrolled in the present study.
A total of 44 female patients (age, 34–70 years;
weight, 70–120 kg) diagnosed with breast cancer and 24 healthy
donors (age, 30–65, weight, 70–100 kg) were enrolled between
February 2013 and August 2014. The clinical-pathological
characteristics: BMI, menopausal status and tumor invasion were
recorded based on pathological reports and medical records.
Prognostic factors including tumor grade, tumor size,
lymphovascular invasion, progesterone receptor (PR), estrogen
receptor (ER), human epidermal growth factor receptor-2 (HER2) and
Ki67 were documented by a professional pathologist, to be used as a
cell proliferating labelling index.
Subject groups
Patients were divided into groups; group i included
obese breast cancer patients (BMI ≥30; n=24), group ii included
overweight breast cancer patients (BMI between 25 and 30; n=20) and
group iii was control group of healthy donors (n=24). These groups
were subdivided according to menopausal status into the following
sub groups: Postmenopausal obese patients (group iA; n=18),
premenopausal obese patients (group iB; n=6), postmenopausal
overweight patients (group iiA; n=11) and premenopausal overweight
patients (group iiB; n=9). The control group was subdivided into
the following subgroups; (group iiiA; n=6), premenopausal obese
controls (group iiiB; n=6), postmenopausal overweight controls
(group iiiC; n=6) and premenopausal overweight controls (group
iiiD; n=6). Furthermore, patients were subdivided according to
estrogen receptor into subgroups; obese patients positive for
estrogen receptor (group iC; n=20), obese patients negative for
estrogen receptor (group iD; n=4), overweight patients positive for
estrogen receptor (group iiC; n=12) and overweight patients
negative for estrogen receptor (group iiD; n=8). Tissue samples
were collected from conservative breast surgery or modified radical
mastectomy and divided into 2 halves; one fixed for 24 h at room
temp in 10% neutral buffered formalin for immunohistochemistry and
second snap frozen in liquid nitrogen for molecular studies.
Plasma sample preparation
A total of 10 ml plasma was isolated from peripheral
blood and blood collected from tumor microenvironment prior to and
during surgical operation for each patient in EDTA tubes as
previously described (30). In
patients with breast cancer, venous withdrawal from the breast may
include cells of immunological importance, including tumor cells
and other biological factors obtained from the tumor
microenvironment. Therefore, biological characteristics of breast
tumor microenvironment may be defined by collecting axillary
tributaries during modified radical mastectomy prior to dilution in
circulation (30). A further 10 ml
peripheral blood was withdrawn from the antecubital vein from
healthy volunteers in anticoagulant tubes as a control (30). Blood was then centrifuged at 2,000 ×
g for 10 min at room temperature for plasma preparation. Plasma was
aliquoted and stored at −80°C until use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA was extracted from 29 tissue samples from
patients with breast cancer and 8 normal tissues using the GeneJET
RNA Purification kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's protocol. A total of 1 µg
total RNA was converted into cDNA using a Revert aid cDNA synthesis
kit (Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. PCR was performed using the Maxima
SYBR-Green Master Mix kit (Thermo Fisher Scientific, Inc.) to
amplify leptin, LEPR, aromatase, MAPK and STAT3 genes using
hypoxanthine-guanine phosphoribosyltransferase (HPRT) as a
housekeeping control gene. Primers used for qPCR were commercially
synthesized from Macrogen, Inc. (Seoul, Korea) and are listed in
Table I. qPCR was performed in
applied Biosystems Step One Plus (Thermo Fisher Scientific, Inc.)
and reactions were performed in duplicate. Each sample was
initially denatured at 95°C for 5 min, then subjected to 40 cycles
of the following: Denaturation at 95°C for 50 sec, and annealing
and extension at 60°C for 1 min. Each sample was exposed to a final
extension at 72°C for 10 min and finally held at 4°C followed by
amplification and melting curves. Following qPCR, Cq values were
measured, ∆∆Cq and fold expression were calculated to quantify the
results (31).
 | Table I.Primer sequences of target genes for
reverse transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences of target genes for
reverse transcription-quantitative polymerase chain reaction.
| Gene | Direction | Sequence |
|---|
| HPRT | Forward |
5′-CTCCTCCTGAGCAGTCAGC-3′ |
|
| Reverse |
5′-GTCATAACCTGGTTCATCATCACT-3′ |
| Leptin | Forward |
5′-AAAGATAGGGCCAAAGCCAC-3′ |
|
| Reverse |
5′-GTAGGAATCGCAGCGCC-3′ |
| Leptin
receptor | Forward |
5′-CCCAATGTAACAAAACCACACA-3′ |
|
| Reverse |
5′-CTTATGCTGGGATGTGCCTT-3′ |
| Aromatase | Forward |
5′-TCTCGATTCGGCAGCAAACT-3′ |
|
| Reverse |
5′-TGACCATACGAACAAGGCCG-3′ |
| MAPK | Forward |
5′-GGGGCTGATTTTCTTGATAGC-3′ |
|
| Reverse |
5′-ACCAACCTCTCGTACATCGG-3′ |
| STAT-3 | Forward |
5′-CTGCTCCAGGTACCGTGTGT-3′ |
|
| Reverse |
5′-CCTCTGCCGGAGAAACAG-3′ |
Immunohistochemistry (IHC) for
leptin
The expression of leptin in breast tissue was
evaluated in 23 female patients with breast cancer from the obese
(n=13) and overweight (n=10) groups and compared with obese and
overweight control samples (n=6) from healthy donors.
The paraffin embedded blocks were sliced using a
microtome into 4 µm-thick tissue sections. Tissue sections were
initially stained with hematoxylin and eosin, mounted using
positive charged slides and air-dried overnight. Following
de-waxing (by immersing in xylene for 5 min) and hydration (by
embedding slides in graded concentrations of alcohol; 100, 95, 80
and 50%; (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), positive
slides were incubated in citrate buffer (pH=6; 2.1 g citric acid
dissolved in 1 l distilled water) in a water bath for 1 h at 99°C.
Slides were subsequently kept at room temperature and dipped with
two changes of Tris-buffered saline with Tween-20 (TBST; 0.05 mol/l
tris-HCl, pH 7.6, 0.15 mol/l NaCl and 0.05% Tween-20) for 5 min of
washing. The slides were blocked using 3% hydrogen peroxide for 10
min (Dual Endogenous Enzyme block, K4065; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) and were washed with
TBST. Slides were then incubated at room temperature overnight with
rabbit polyclonal primary antibody against leptin (ab3583; 1:50;
Abcam, Cambridge, UK). The slides were rinsed in TBS two times for
5 min and incubated with 100 µl horseradish peroxidase-labelled
polymer rabbit (catalogue number not supplied; EnVision+ Dual link
system-HRP DAB+; 1:50; Dako; Agilent Technologies, Inc.) for 45 min
at room temperature and in TBST for 5 min. Diaminobenzidine with
substrate/chromogen was put on the slides and incubated at room
temperature for 5–10 min, depending on the appearance of a brown
color, then slides were washed in distilled water. Mayer's
hematoxylin was added to the slides for counterstaining. The slides
were washed in tap water, following dehydration and clearing steps,
and were covered using DPX mounting media (Thermo Fisher
Scientific, Inc.).
An immunohistochemical score of 0 was considered
negative, + represented faint staining, ++ represented moderate
staining and +++ was considered to be strong staining. Leptin
status was assessed as positive and negative for patients. The
staining was described as negative if no cancer cells were stained
and positive if cancer cells were stained and subsequently examined
using a light microscope (Optika S.r.l, Ponteranica, Italy) (<37
or 10 or >10%).
ELISA assay
Concentration of leptin in plasma from peripheral
blood and blood collected from the tumor microenvironment were
determined using the Leptin (Sandwich) ELISA kit (EIA 2395; Qiagen
AB, Sollentuna, Sweden) following the manufacturer's protocol.
Statistical analysis
The data were analysed using SPSS software version
18.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as mean ±
standard deviation and correlations between categorical variables
were assessed using Spearman correlations test and Student's
t-test. P≤0.01 represents statistically significant
differences.
Results
Clinical and pathological
characteristics of patients
Clinical and pathological characteristics are
presented in Table II and include
age, BMI, menopausal status, tumor grade, tumor size, lymph node
metastasis, lymph vascular invasion and expressions of ER, PR and
HER2 as explained below.
 | Table II.Patient and tumor
characteristics. |
Table II.
Patient and tumor
characteristics.
|
Characteristics | Data |
|---|
| Age (years) |
|
| Mean ±
standard deviation | 51.66±1.506 |
|
Range | 34–70 |
| Menopausal state, n
(%) |
|
|
Premenopausal | 15 (34.1) |
|
Postmenopausal | 29 (65.9) |
| BMI, kg/m2 (%) |
|
|
≥30 | 24 (54.54) |
|
<30 | 20 (45.46) |
| Tumor size
(cm) |
|
|
Mean | 28.6 |
|
Range | 0.17–110 |
| Tumor grade,
patient no. (%) |
|
| Grade
II | 43 (97.7) |
| Grade
III | 1 (2.3) |
| Metastatic lymph
nodes, n (%) |
|
| ≤4 | 35 (79.55) |
|
>4 | 9 (20.45) |
| Lymph vascular
invasion, n (%) |
|
|
Positive | 8 (18.2) |
|
Negative | 36 (81.8) |
| Estrogen receptor,
n (%) |
|
|
Positive | 32 (72.73) |
|
Negative | 12 (27.27) |
| Progesterone
receptor, n (%) |
|
|
Positive | 32 (72.73) |
|
Negative | 12 (27.27) |
| HER-2, n (%) |
|
|
Positive | 10 (22.7) |
|
Negative | 34 (77.3) |
The present study was applied in patients with
median age 51.66±1.506 years (range, 34–70). Among 44 female
patients, 29 (65.9%) were postmenopausal and 15 (34.1%) were
premenopausal. BMI between 18.5 and <25 is considered to be
normal, between 25 to <30 as overweight and ≥30 as obese
according to the WHO (5). A total of
24 female patients (54.54%) were obese and 20 patients (45.46%)
were overweight. The mean tumor sizes ranged from 0.17–110 cm (mean
size 28.6 cm). Among patients, 88.6% were negative for lymph
vascular invasion and 11.4% were positive for lymph vascular
invasion. Tumor grade staging was as follows: 97.7% of patients
were classified as grade 2, while 2.3% were classified as grade
3.
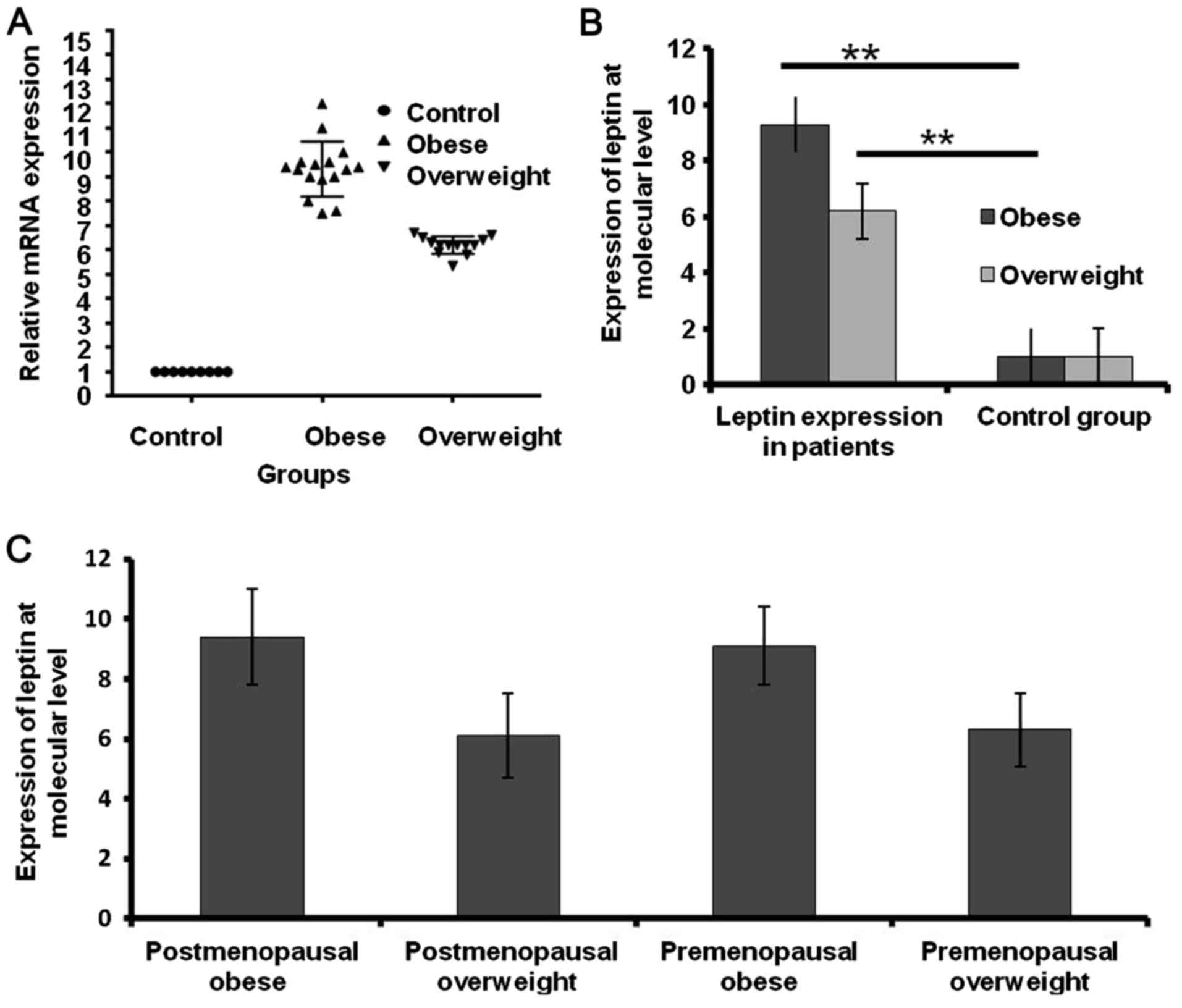
Expression of leptin in obese breast
cancer patient tissues
The mRNA expression level of leptin in the tissue of
patients with breast cancer was assessed. Leptin was significantly
overexpressed in obese patients compared with overweight patients
and healthy donors by 3.1-fold and 8.3-fold, respectively
(P<0.001; Fig. 1A and B). The
expression of leptin was higher in postmenopausal and premenopausal
obese patients than postmenopausal and premenopausal overweight
patients by 3.28-fold and 2.8-fold, respectively (Fig. 1C).
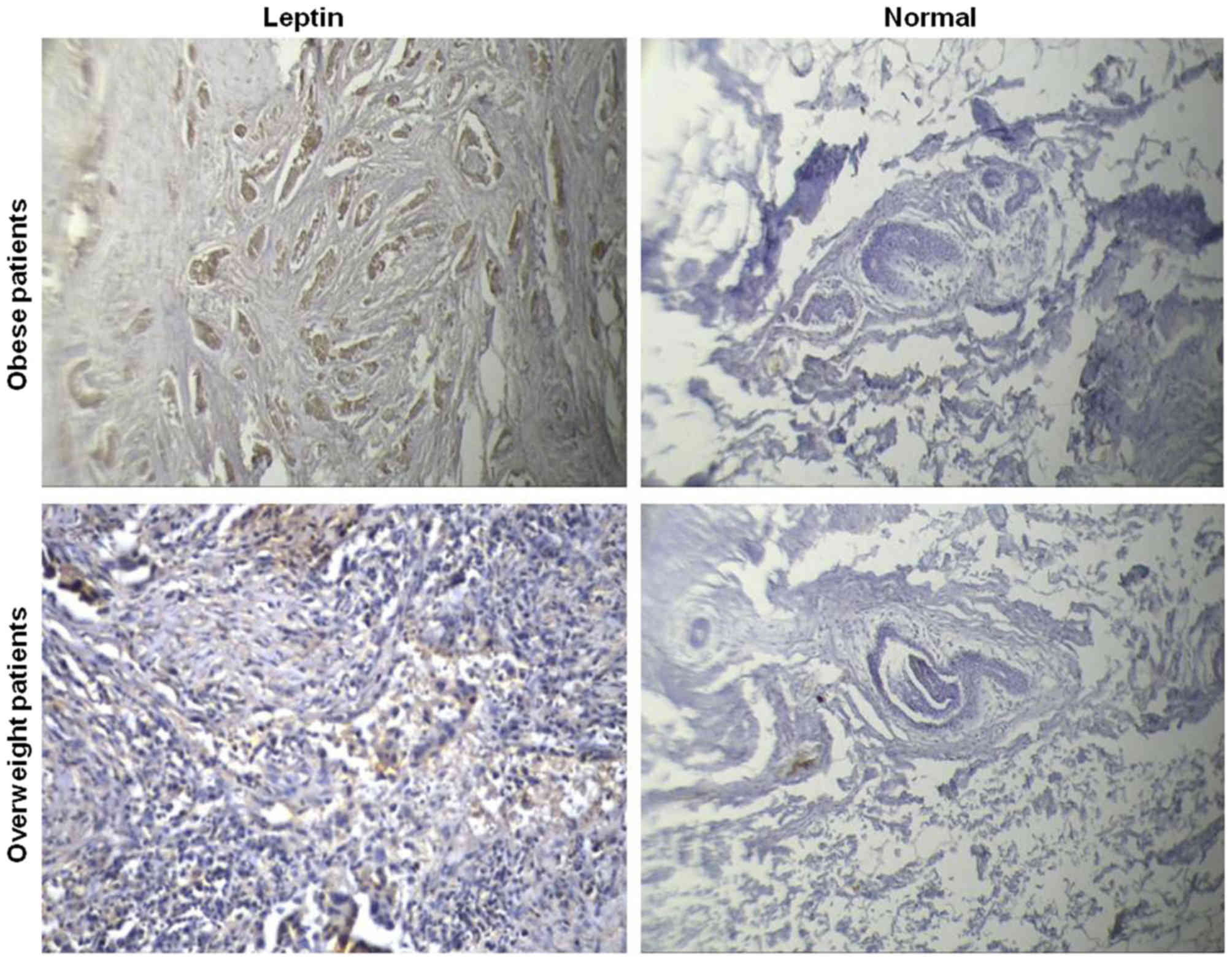
The same findings were obtained when the protein
expression of leptin in tissue was assessed by immunohistochemistry
(Table III and Fig. 2). The association between leptin
expression and clinical data of patients was assessed and indicated
that there is a positive correlation between expression of leptin
in breast cancer patient tissues and BMI using Student t-test
(r=0.916), whereas no significant association was identified
between leptin expression and menopausal status (r=0.373; Table III). A positive association was
identified between the expression of leptin and ER expression in
obese patients (Table III).
Conversely, a negative correlation was detected between the
expression of leptin and ER in overweight patients (r=0.9 and
r=−0.346 respectively; Table III).
No significant correlation was identified between the expression
level of leptin and PR or HER2 (r=0.182 and r=0.171 respectively;
Table III). Ki67 is a cell
proliferating label index that serves an important role in cell
proliferation. The correlation between the expression level of
leptin and expression of Ki67 in tissues from patients with breast
cancer was assessed. There was no significant correlation between
the expression level of leptin and the expression of Ki67 in
tissues from patients with breast cancer (r=0.283; Table III).
 | Table III.Association between leptin expression
and prognostic factors in tissues from patients with breast
cancer. |
Table III.
Association between leptin expression
and prognostic factors in tissues from patients with breast
cancer.
|
| Leptin (n=23) |
|---|
|
|
|
|---|
| Variable | High expression
(n=13) | Low expression
(n=10) | Correlation
coefficient | P-value |
|---|
| BMI, n (%) |
|
| 0.916 |
<0.001a |
|
Obese | 12 (92.3) | 0 (0) |
|
|
|
Overweight | 1 (7) | 10 (100) |
|
|
| Menopausal, n
(%) |
|
| 0.373 | 0.08 |
|
Post | 11 (84.6) | 5 (50) |
|
|
|
Pre | 2 (15.4) | 5 (50) |
|
|
| ER, n (%) |
|
| 0.182 | 0.405 |
|
Positive | 10 (76.92) | 6 (60) |
|
|
|
Negative | 3 (23.08) | 4 (40) |
|
|
| ER, n (%) |
|
| 0.9 | 0.01 |
|
Obese | 12 (92.3) | 0 (0) |
|
|
|
Overweight | 1 (7) | 10 (100) | −0.346 | 0.297 |
| PR, n (%) |
|
| 0.182 | 0.405 |
|
Positive | 10 (76.92) | 6 (60) |
|
|
|
Negative | 3 (23.08) | 4 (40) |
|
|
| HER-2, n (%) |
|
| 0.171 | 0.435 |
|
Positive | 3 (23.07) | 1 (10) |
|
|
|
Negative | 10 (76.93) | 9 (90) |
|
|
| Ki67, n (%) |
|
| 0.238 | 0.273 |
|
Positive | 7 (53.85) | 3 (30) |
|
|
|
Negative | 6 (46.15) | 7 (70) |
|
|
Assessment of leptin protein
expression in plasma of the tumor microenvironment and peripheral
plasma
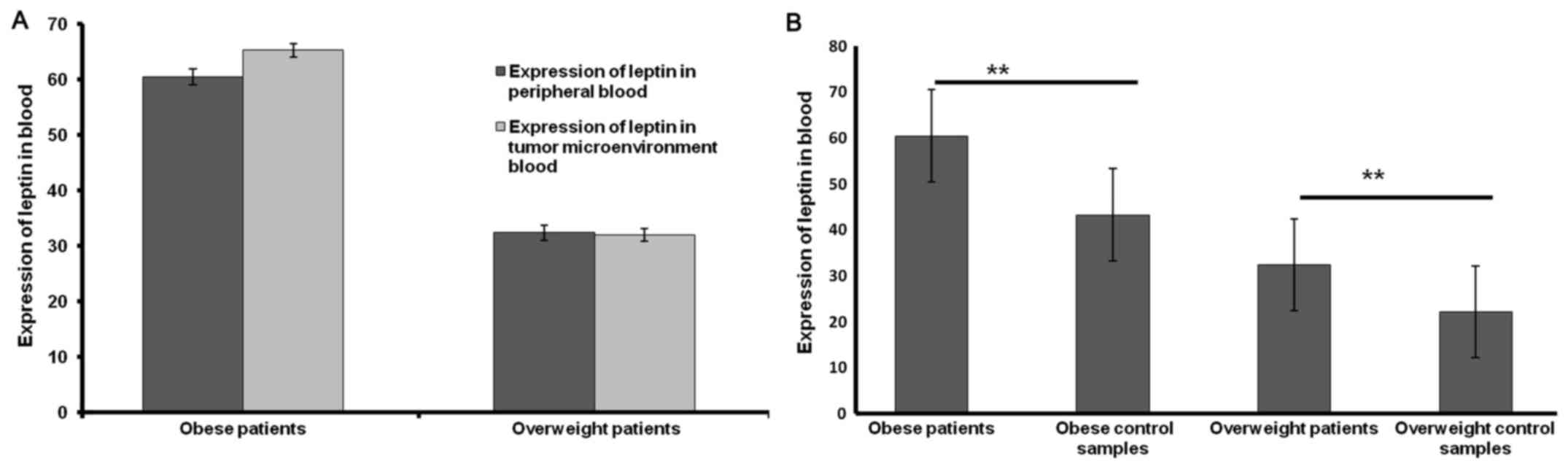
The results of the present study indicate that there
was a non-significant increase in the concentration of leptin in
plasma from the tumor microenvironment blood in obese patients
(Fig. 3A). Furthermore, a
significant difference was observed between the concentration of
leptin in peripheral plasma of obese and overweight patients with
breast cancer compared with that of obese and overweight
volunteers, respectively (both P<0.001; Fig. 3B).
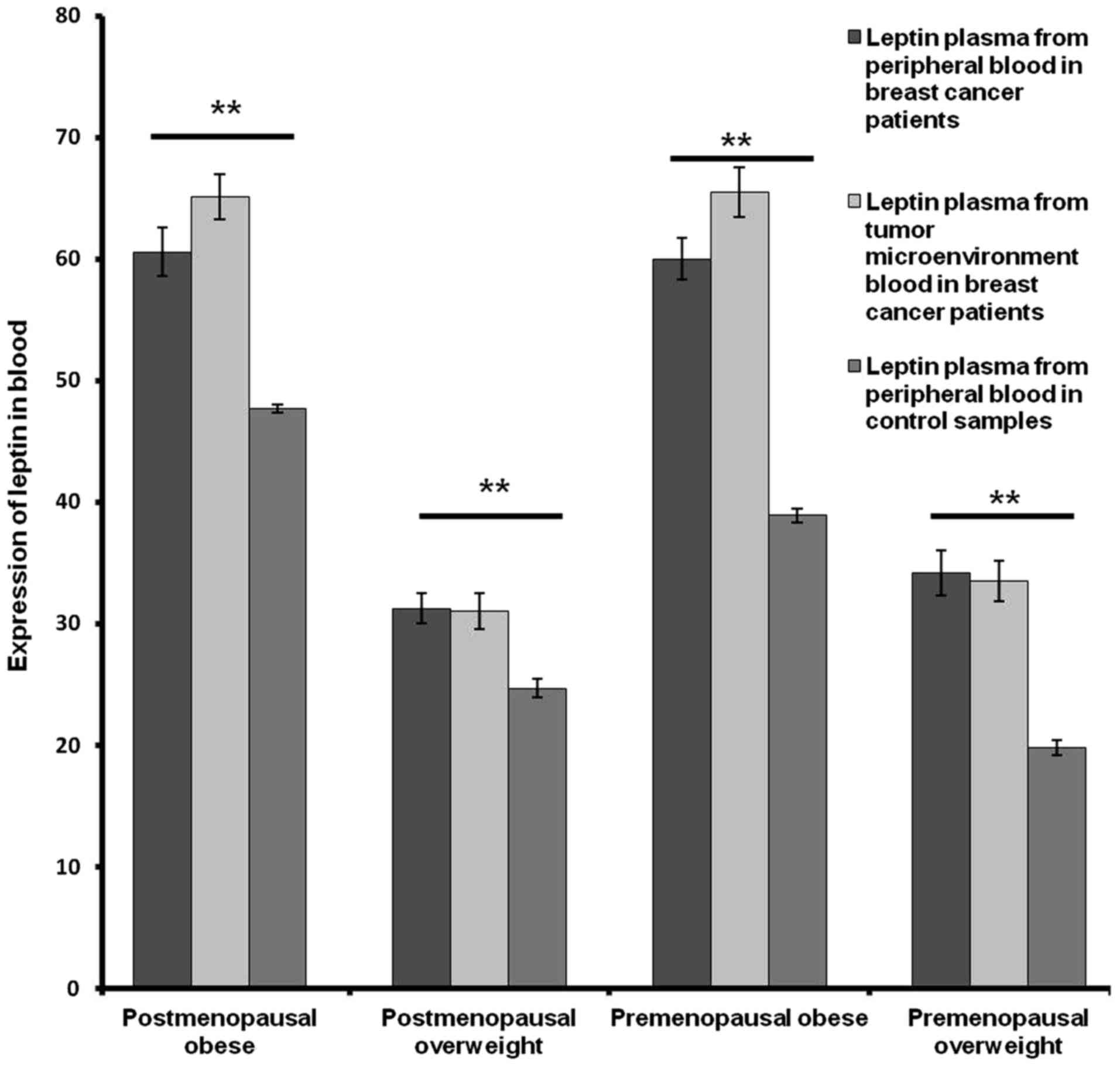
In addition, a significant difference was observed
between the concentration of leptin in the peripheral plasma
samples of post- and pre-menopausal obese patients and control
samples (P<0.001; Fig. 4). A
significant increase was also observed between the concentration of
leptin in plasma from peripheral blood between post- and
premenopausal overweight patients and control samples (P<0.001;
Fig. 4).
By contrast, no significant difference was observed
between the concentration in leptin in plasma from peripheral blood
among postmenopausal and premenopausal obese or overweight patients
(Fig. 4).
Expression of leptin in obese and
overweight patients with ER+ and ER- breast cancer
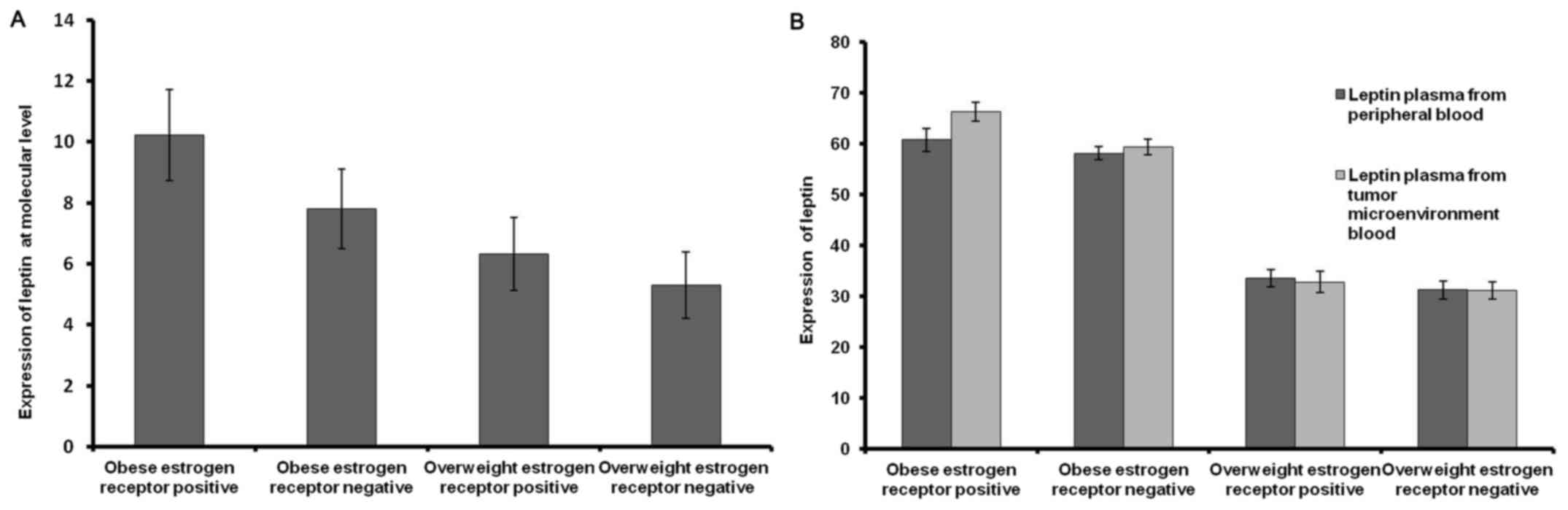
Patients were sub-grouped into ER+ and ER- in both
obese and overweight patients. Leptin expression increased by 2.42
fold more in obese ER+ compared with obese ER- breast cancer
patients, while it increased by only one fold in overweight ER+
compared with overweight ER- breast cancer patients (Fig. 5A). Furthermore, leptin expression was
higher in obese ER+ than overweight ER+ patients by 3.9-fold
(Fig. 5A).
A markedly higher concentration of leptin was
identified in plasma isolated from the tumor microenvironment blood
compared with plasma from peripheral blood in ER+ obese breast
cancer patients (Fig. 5B).
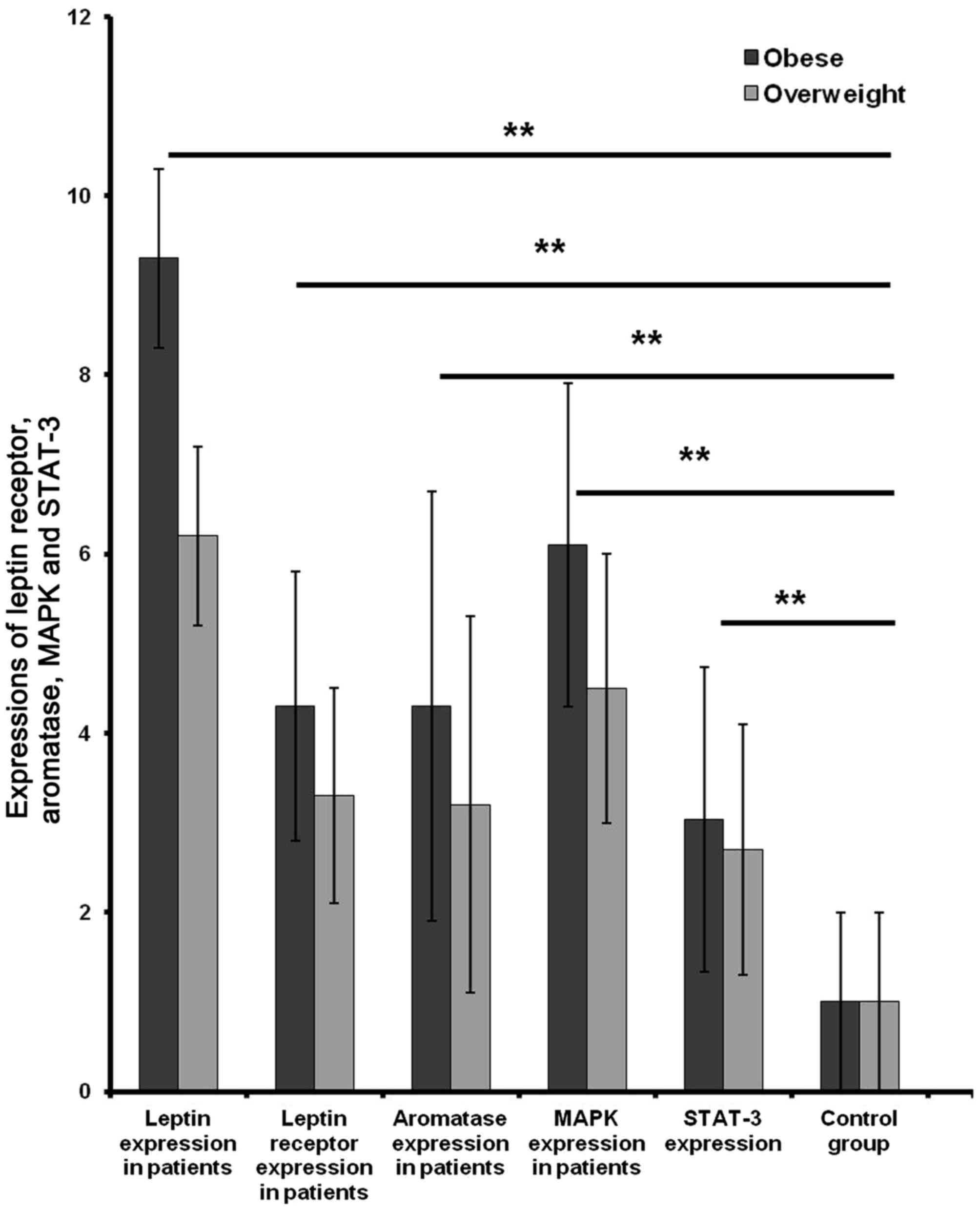
Leptin stimulated expression levels of LEPR,
aromatase, MAPK and STAT3 mRNA in tissues of obese patients with
breast cancer. The mRNA expression level of leptin and LEPR,
aromatase, MAPK and STAT3 in breast cancer patient tissues were
assessed. Leptin, LEPR, aromatase, MAPK and STAT-3 were
overexpressed in obese patients compared with overweight and normal
tissues (Fig. 6). The mRNA
expression level of leptin and BMI in tissues from patients was
assessed using the Spearman correlations test. Leptin in breast
cancer tissue samples was significantly associated with the obesity
status (r=0.663; Table IV).
Positive correlations between leptin expression and that of LEPR,
aromatase, MAPK and STAT3 were also identified (r=0.815, 0.772,
0.771 and 0.679 respectively; Table
IV).
 | Table IV.Association between mRNA leptin
expression and leptin receptor, aromatase, MAPK and STAT-3
expression in patients with breast cancer. |
Table IV.
Association between mRNA leptin
expression and leptin receptor, aromatase, MAPK and STAT-3
expression in patients with breast cancer.
|
| Expression of
leptin (n=29) |
|---|
|
|
|
|---|
| Variable | High expression
(n=14) | Low expression
(n=15) | Correlation
coefficient | P-value |
|---|
| BMI, n (%) |
|
|
|
<0.001a |
|
Obese | 14 (100) | 2 (13.33) | 0.663 |
|
|
Overweight | 0 (0) | 13 (86.67) |
|
|
| Leptin receptor, n
(%) |
|
|
|
<0.001a |
| High
expression | 13 (92.86) | 0 (0) | 0.815 |
|
| Low
expression | 1 (7.14) | 15 (100) |
|
|
| Aromatase, n
(%) |
|
|
|
<0.001a |
| High
expression | 14 (100) | 1 (60) | 0.772 |
|
| Low
expression | 0 (0) | 14 (40) |
|
|
| MAPK, n (%) |
|
|
|
<0.001a |
| High
expression | 14 (100) | 1 (6.67) | 0.771 |
|
| Low
expression | 0 (0) | 14 (93.33) |
|
|
| STAT-3, n (%) |
|
|
|
<0.001a |
| High
expression | 11 (78.57) | 0 (0) | 0.679 |
|
| Low
expression | 3 (21.43) | 15 (100) |
|
|
Discussion
The present study aimed to investigate the potential
role of leptin in breast cancer progression in obese patients.
Leptin is produced by adipose tissue, which constitutes the major
breast tissue structure (13,15,16).
Leptin serves a critical role in cell growth and differentiation in
normal cells (32). Since its
discovery in 1994, leptin has been identified to have an
association with obesity (13,15,16).
Reports of association between the expression of leptin and breast
cancer are inconsistent. A number of studies indicate that leptin
is associated with breast cancer development (33–35);
while other studies demonstrate that leptin is not associated with
breast cancer (36–38).
Obesity acts as a risk factor for a number of
serious medical conditions (7).
Obesity was identified to have an association with mortality in
patients with breast cancer, with a higher mortality rate observed
in obese patients compared with overweight patients (39). Obesity is associated with breast
cancer through the secretion of growth factors and leptin by
adipose stromal stem cells (ASCs) that, in turn, promote tumor
growth (40,41). Furthermore, brown adipose tissue has
been identified to activate breast cancer development through
activation of the angiogenesis process in mice (42).
In the present study, leptin expression in tissue
and plasma from both peripheral and tumor microenvironment blood of
patients with breast cancer was assessed to investigate the
association between leptin and breast cancer progression. To the
best of our knowledge, this is the first investigation to assess
the concentration of leptin in blood plasma collected from the
breast tumor microenvironment of patients. The expression of leptin
in the blood and tissues of patients with breast cancer was
assessed as previous studies indicated that expression of leptin in
blood was not associated with the expression of leptin in the
tissue (23,43). The present study indicated that
leptin was highly expressed in blood and tissue samples at
molecular and proteomic levels in patients with breast cancer.
Leptin expression was higher in obese ER+ patients compared with
obese ER-breast cancer patients by 2.42 fold. Additionally, leptin
was overexpressed in obese ER+ compared with overweight ER+ breast
cancer patients by 3.9 fold. The concentration of circulating
leptin in the blood was markedly associated with mRNA expression of
leptin in breast cancer patients, in agreement with a previous
study (44).
A positive correlation was identified between the
expression of leptin and estrogen receptor expression in obese
patients. By contrast, a negative correlation was detected between
the expression of leptin and estrogen receptor in overweight
patients. A non-significant difference between the expression of
leptin and progesterone receptor and human epidermal growth factor
has been demonstrated (35,45,46).
Previous results revealed a positive association between leptin
expression and cell proliferating marker (ki67 labelling index)
(35). Conversely, the present study
revealed no significant association between leptin expression and
ki67 labelling index, which is in accordance with a previous study
by Garofalo et al (33).
The present results revealed that the level of
leptin was higher in the plasma of obese and overweight breast
cancer patients than those of healthy individuals, agreeing with
previous studies (5,13,43–48).
Obesity may be associated with breast cancer through stimulation of
estrogen secretion, mediated by leptin in fat tissue during the
postmenopausal period. This suggestion disagrees with the results
of the present study as patients included both postmenopausal and
premenopausal patients while previous studies included
postmenopausal patients only. In addition, the enhancement of
insulin and insulin growth factors by leptin was associated with
metabolic disorders, and increased the production of adipokines
including leptin, which are secreted by adipose tissue. This may
lead to breast cancer progression (49). Also, leptin may stimulate tumor
development in breast cancer cells by stimulating the CYP19A1 gene
through activating MAPK and STAT3 pathways (17).
The plasma concentration of leptin higher in the
tumor microenvironment blood than in peripheral blood of obese
patients with ER+ breast cancer. The latter results are concurrent
with previous in vitro studies that identified higher levels
of leptin in ASCs of the breast tumor microenvironment in breast
cancer cells (50). ASCs produce
growth factors that protect breast cancer cells from immune
responses and stimulate breast cancer progression (41). The higher expression of leptin in
breast tumor microenvironment may be attributed to the potential
circulation of ASCs through blood to distant tumor regions where
they differentiate into vascular pericytes or produce growth
factors such as hepatocyte growth factor and insulin growth factor,
which elevate leptin levels and anchor the tumor microenvironment
(41,51). These growth factors are associated
with breast cancer development (52). Other studies suggest that ASCs
secrete proteases such as MMP2 and MMP9, and vascular
pro-angiogenic factors such as VEGF that elevate leptin levels at
the tumor site (53,54).
It has been demonstrated that obese adipose stromal
stem cells (obASCs) from obese patients with breast cancer express
higher leptin levels when compared with ASCs isolated from lean
patients (50). Previous studies
indicated that obesity may stimulate the production of ASCs within
white adipose tissue that activates proliferation of breast cancer
cells through an estrogen-induced response mediated by leptin
(55).
It was suggested that the higher concentration of
leptin in the breast tumor microenvironment in obese patients with
ER+ breast cancer may be due to their response to factors secreted
by obASCs and not secreted by ASCs in lean patients (50). Additionally, pathways in ER-patients
lack the estrogen receptor; and therefore, are unable to respond to
factors synthesized by obASCs (50).
In addition, higher levels of leptin in breast tumor
microenvironment of obese patients may attributed to the following:
Adipose tissue of obese patients is characterized by chronic
inflammation leading to stimulation of angiogenesis process
(56). It consists of higher numbers
of the macrophages and inflammatory cells that activate breast
cancer progression compared with lean patients (57). Obese patients overexpress adipose
triglyceride lipase, which is involved in breast tumor progression
(58), and they reduce pigment
epithelium-derived factor expression, which is associated with
aggressive metastatic risk for breast cancer (58). The present study hypothesized that
cells in the breast tumor microenvironment of obese patients
secrete higher levels of leptin due to activation by circulating
levels of insulin and insulin-like growth factors, inflammatory
cytokines and VEGF. Also, leptin stimulates the expression of MAPK
and STAT3 activating aromatase that increases the synthesis of
estrogen in obese ER+ patients with breast cancer. Estrogen
stimulates breast cancer progression through activation of numerous
processes including cell division, angiogenesis and proliferations
(59). The results of the present
study indicate that cells of obese patients with ER+ breast cancer
secrete higher levels of leptin, which produces estrogen and
activates breast cancer progression.
With respect to menopausal status, a positive
association was identified between the expression of leptin in
blood and obesity in breast cancer patients regardless of
menopausal status, which was in accordance with previous results
(35,43–47). By
contrast, previous studies have indicated that breast cancer risk
was associated with menopausal status (21,23,60) and
that obesity may increase breast cancer progression in
postmenopausal women by 30–50% (21).
Studies in vitro demonstrated that leptin is
associated with breast cancer progression as it stimulates the
JAK/STAT3, ERK1/2 and phoshphoinositide 3-kinase pathways leading
to breast cancer cell proliferation and cell survival (21). Few studies measured the expression of
LEPR and activation of cell proliferation signalling pathway
(aromatase) in patients with breast cancer. Leptin initiates its
actions through LEPR (14).
Aromatase is expressed in adipose stromal cells and epithelial
cancer cells (61). Leptin is able
to crosstalk with estrogen through increasing the expression of
aromatase enzyme and stimulating estrogen expression (61–64).
MAPK is a protein kinase involved in breast cancer progression
(65–67). STAT3 serves vital roles in cell
growth, survival, transformation and development (68). STAT3 controls multiple genes
including cyclinD1, B-cell lymphoma-2 (BCL2), BCL2-extra large and
c-Myc that participate in proliferation and cell growth (69). STAT3 is able to enhance the
proliferation of breast cancer (65–67).
The expression of potential genes regulated by
leptin in progression mechanism of patients with breast cancer were
assessed. The potential proliferation pathway(s) associated with
leptin expression may be responsible for breast cancer progression.
A positive association between the expression of leptin and
expression of leptin receptor, aromatase, MAPK and STAT3 genes was
identified in obese patients with breast cancer and these results
are concurrent with previous in vitro studies (33,61,64,65,67,70).
Accordingly, leptin may enhance breast cancer progression by
stimulating the estrogen pathway through increasing aromatase
expression, the ERK1/2 pathway via activating MAPK expression and
the JAK/STAT3 pathway through enhancing STAT3 expression.
Inhibition of the leptin proliferation signalling
pathway may be beneficial to identify novel therapeutic targets for
breast cancer. Identifying the molecular mechanism of leptin in
breast cancer progression may lead to novel targets for breast
cancer treatment. To the best of our knowledge, this is the first
investigation to determine the concentration of leptin in breast
tumor microenvironment in patients.
In conclusion, the concentration of leptin was
higher in plasma from tumor microenvironment blood than in plasma
from peripheral blood samples of obese patients with ER+ breast
cancer. Leptin may enhance breast cancer progression by inducing
the expression of JAK/STAT3, ERK1/2 and estrogen pathways in obese
patients with breast cancer.
Acknowledgements
The present study was supported by Avon-Foundation
(grant no. 02-2009-085 a and b; Robert J. Schneider and Mona
Mostafa Mohamed). The study was completed at the Cancer Biology
Research Laboratory (CBRL), Department of Zoology, Faculty of
Science, Cairo University, Giza, Egypt. The authors would like to
thank Dr. Eslam A. El-Ghonaimy (CBRL, Department of Zoology,
Faculty of Science, Cairo University) for his help in completing
the ELISA assay and special thanks to Dr. Heba Bassiony, Assistant
Professor of molecular biology (Department of Zoology, Faculty of
Science, Cairo University) for her assistance with statistical
analysis.
Glossary
Abbreviations
Abbreviations:
|
LEPR
|
leptin receptor
|
|
BMI
|
body mass index
|
|
CYP19A1
|
cytochrome P450 family 19 subfamily A
member 1
|
|
STK11
|
serine/threonine kinase 11
|
|
MAPK
|
mitogen activated protein kinase
|
|
ERK
|
extracellular signal-related
kinases
|
|
JAK/STAT3
|
janus kinase/signal transducer and
activator of transcription-3
|
|
MMP
|
matrix metalloproteinase
|
|
EGFR
|
epidermal growth factor receptor
|
|
VEGF
|
vascular endothelial growth factor
|
|
LPrA2
|
leptin receptor antagonist
|
|
PR
|
progesterone receptor
|
|
ER
|
estrogen receptor
|
|
HER2
|
human epidermal growth factor
receptor-2
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
IHC
|
immunohistochemistry
|
|
TBST
|
tris-buffered saline with Tween-20
|
|
ASCs
|
adipose stromal stem cells
|
|
obASC
|
obese adipose stromal stem cells
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ren JS, Masuyer E and Ferlay J:
Global estimates of cancer prevalence for 27 sites in the adult
population in 2008. Int J Cancer. 132:1133–1145. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ibrahim AS, Khaled HM, Mikhail NN, Baraka
H and Kamel H: Cancer incidence in egypt: Results of the national
population-based cancer registry program. J Cancer Epidemiol.
2014:4379712014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Newell B, Proust K, Dyball R and McManus
P: Seeing obesity as a systems problem. N S W Public Health Bull.
18:214–218. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kaur T and Zhang ZF: Obesity, breast
cancer and the role of adipocytokines. Asian Pac J Cancer Prev.
6:547–552. 2005.PubMed/NCBI
|
|
6
|
Popkin BM: Understanding global nutrition
dynamics as a step towards controlling cancer incidence. Nat Rev
Cancer. 7:61–67. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reeves GK, Pirie K, Beral V, Green J,
Spencer E and Bull D; Million Women Study Collaboration, : Cancer
incidence and mortality in relation to body mass index in the
Million Women Study: Cohort study. BMJ. 335:11342007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Danaei G, Hoorn S Vander, Lopez AD, Murray
CJ and Ezzati M; Comparative Risk Assessment collaborating group
(Cancers), : Causes of cancer in the world: Comparative risk
assessment of nine behavioural and environmental risk factors.
Lancet. 366:1784–1793. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Frühbeck G, Gómez-Ambrosi J, Muruzábal FJ
and Burrell MA: The adipocyte: A model for integration of endocrine
and metabolic signaling in energy metabolism regulation. Am J
Physiol Endocrinol Metab. 280:E827–E847. 2001.PubMed/NCBI
|
|
10
|
Porter GA, Inglis KM, Wood LA and
Veugelers PJ: Effect of obesity on presentation of breast cancer.
Ann Surg Oncol. 13:327–332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huber KE, Carey LA and Wazer DE: Breast
cancer molecular subtypes in patients with locally advanced
disease: Impact on prognosis, patterns of recurrence, and response
to therapy. Semin Radiat Oncol. 19:204–210. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diamond T, Vine J, Smart R and Butler P:
Thyrotoxic bone disease in women: A potentially reversible
disorder. Ann Intern Med. 120:8–11. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tartaglia LA: The leptin receptor. J Biol
Chem. 272:6093–6096. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Halaas JL, Gajiwala KS, Maffei M, Cohen
SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK and Friedman JM:
Weight-reducing effects of the plasma protein encoded by the obese
gene. Science. 269:543–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bray GA: The underlying basis for obesity:
Relationship to cancer. J Nutr. 132(11 Suppl): S3451–S3455.
2002.
|
|
17
|
Andò S and Catalano S: The multifactorial
role of leptin in driving the breast cancer microenvironment. Nat
Rev Endocrinol. 8:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahima RS and Flier JS: Adipose tissue as
an endocrine organ. Trends Endocrinol Metab. 11:327–332. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bonnet M, Delavaud C, Laud K, Gourdou I,
Leroux C, Djiane J and Chilliard Y: Mammary leptin synthesis, milk
leptin and their putative physiological roles. Reprod Nutr Dev.
42:399–413. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Snoussi K, Strosberg AD, Bouaouina N, Ben
Ahmed S, Helal AN and Chouchane L: Leptin and leptin receptor
polymorphisms are associated with increased risk and poor prognosis
of breast carcinoma. BMC Cancer. 6:382006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Garofalo C and Surmacz E: Leptin and
cancer. J Cell Physiol. 207:12–22. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Purohit A, Newman SP and Reed MJ: The role
of cytokines in regulating estrogen synthesis: Implications for the
etiology of breast cancer. Breast Cancer Res. 4:65–69. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vona-Davis L and Rose DP: Adipokines as
endocrine, paracrine, and autocrine factors in breast cancer risk
and progression. Endocr Relat Cancer. 14:189–206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mauro L, Catalano S, Bossi G, Pellegrino
M, Barone I, Morales S, Giordano C, Bartella V, Casaburi I and Andò
S: Evidences that leptin Up-regulates E-cadherin expression in
breast cancer: Effects on tumor growth and progression. Cancer Res.
67:3412–3421. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY,
Park BE, Jang Y, Cho SY and Kim HS: Potential role of leptin in
angiogenesis: Leptin induces endothelial cell proliferation and
expression of matrix metalloproteinases in vivo and in vitro. Exp
Mol Med. 33:95–102. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Frankenberry KA, Skinner H, Somasundar P,
McFadden DW and Vona-Davis LC: Leptin receptor expression and cell
signaling in breast cancer. Int J Oncol. 28:985–993.
2006.PubMed/NCBI
|
|
27
|
Gonzalez RR, Cherfils S, Escobar M, Yoo
JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A,
Serikawa T, et al: Leptin signaling promotes the growth of mammary
tumors and increases the expression of vascular endothelial growth
factor (VEGF) and its receptor type two (VEGF-R2). J Biol Chem.
281:26320–26328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gonzalez R Rene, Watters A, Xu Y, Singh
UP, Mann DR, Rueda BR and Penichet ML: Leptin-signaling inhibition
results in efficient anti-tumor activity in estrogen receptor
positive or negative breast cancer. Breast Cancer Res. 11:R362009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perera CN, Chin HG, Duru N and Camarillo
IG: Leptin-regulated gene expression in MCF-7 breast cancer cells:
Mechanistic insights into leptin-regulated mammary tumor growth and
progression. J Endocrinol. 199:221–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Shinawi M, Abdelwahab SF, Sobhy M, Nouh
MA, Sloane BF and Mohamed MM: Capturing and characterizing immune
cells from breast tumor microenvironment: An innovative surgical
approach. Ann Surg Oncol. 17:2677–2684. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hwang CS, Loftus TM, Mandrup S and Lane
MD: Adipocyte differentiation and leptin expression. Annu Rev Cell
Dev Biol. 13:231–259. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garofalo C, Koda M, Cascio S, Sulkowska M,
Kanczuga-Koda L, Golaszewska J, Russo A, Sulkowski S and Surmacz E:
Increased expression of leptin and the leptin receptor as a marker
of breast cancer progression: Possible role of obesity-related
stimuli. Clin Cancer Res. 12:1447–1453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nyante SJ, Gammon MD, Kaufman JS, Bensen
JT, Lin DY, Barnholtz-Sloan JS, Hu Y, He Q, Luo J and Millikan RC:
Common genetic variation in adiponectin, leptin, and leptin
receptor and association with breast cancer subtypes. Breast Cancer
Res Treat. 129:593–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jeong YJ, Bong JG, Park SH, Choi JH and Oh
HK: Expression of leptin, leptin receptor, adiponectin, and
adiponectin receptor in ductal carcinoma in situ and invasive
breast cancer. J Breast Cancer. 14:96–103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stattin P, Söderberg S, Biessy C, Lenner
P, Hallmans G, Kaaks R and Olsson T: Plasma leptin and breast
cancer risk: A prospective study in northern Sweden. Breast Cancer
Res Treat. 86:191–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Miyoshi Y, Funahashi T, Tanaka S, Taguchi
T, Tamaki Y, Shimomura I and Noguchi S: High expression of leptin
receptor mRNA in breast cancer tissue predicts poor prognosis for
patients with high, but not low, serum leptin levels. Int J Cancer.
118:1414–1419. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Harris HR, Tworoger SS, Hankinson SE,
Rosner BA and Michels KB: Plasma leptin levels and risk of breast
cancer in premenopausal women. Cancer Prev Res (Phila).
4:1449–1456. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Whiteman MK, Hillis SD, Curtis KM,
McDonald JA, Wingo PA and Marchbanks PA: Body mass and mortality
after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev.
14:2009–2014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grossmann ME, Ray A, Nkhata KJ, Malakhov
DA, Rogozina OP, Dogan S and Cleary MP: Obesity and breast cancer:
Status of leptin and adiponectin in pathological processes. Cancer
Metastasis Rev. 29:641–653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Razmkhah M, Jaberipour M, Hosseini A,
Safaei A, Khalatbari B and Ghaderi A: Expression profile of IL-8
and growth factors in breast cancer cells and adipose-derived stem
cells (ASCs) isolated from breast carcinoma. Cell Immunol.
265:80–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lim S, Honek J, Xue Y, Seki T, Cao Z,
Andersson P, Yang X, Hosaka K and Cao Y: Cold-induced activation of
brown adipose tissue and adipose angiogenesis in mice. Nat Protoc.
7:606–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Arita Y, Kihara S, Ouchi N, Takahashi M,
Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K,
et al: Paradoxical decrease of an adipose-specific protein,
adiponectin, in obesity. Biochem Biophys Res Commun. 257:79–83.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tessitore L, Vizio B, Jenkins O, de
Stefano I, Ritossa C, Argiles JM, Benedetto C and Mussa A: Leptin
expression in colorectal and breast cancer patients. Int J Mol Med.
5:421–426. 2000.PubMed/NCBI
|
|
45
|
Ostlund RE Jr..Yang JW, Klein S and
Gingerich R: Relation between plasma leptin concentration and body
fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol
Metab. 81:3909–3913. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hou WK, Xu YX, Yu T, Zhang L, Zhang WW, Fu
CL, Sun Y, Wu Q and Chen L: Adipocytokines and breast cancer risk.
Chin Med J (Engl). 120:1592–1596. 2007.PubMed/NCBI
|
|
47
|
Al Awadhi SA, Al Khaldi RM, Al Rammah T,
Kapila K and Mojiminiyi OA: Associations of adipokines &
insulin resistance with sex steroids in patients with breast
cancer. Indian J Med Res. 135:500–505. 2012.PubMed/NCBI
|
|
48
|
Mohammadzadeh G, Ghaffari MA, Bafandeh A
and Hosseini SM: Association of serum soluble leptin receptor and
leptin levels with breast cancer. J Res Med Sci. 19:433–438.
2014.PubMed/NCBI
|
|
49
|
Lorincz AM and Sukumar S: Molecular links
between obesity and breast cancer. Endocr Relat Cancer. 13:279–292.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Strong AL, Ohlstein JF, Biagas BA, Rhodes
LV, Pei DT, Tucker HA, Llamas C, Bowles AC, Dutreil MF, Zhang S, et
al: Leptin produced by obese adipose stromal/stem cells enhances
proliferation and metastasis of estrogen receptor positive breast
cancers. Breast Cancer Res. 17:1122015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang Y, Daquinag A, Traktuev DO,
Amaya-Manzanares F, Simmons PJ, March KL, Pasqualini R, Arap W and
Kolonin MG: White adipose tissue cells are recruited by
experimental tumors and promote cancer progression in mouse models.
Cancer Res. 69:5259–5266. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Leek RD: The prognostic role of
angiogenesis in breast cancer. Anticancer Res. 21:4325–4331.
2001.PubMed/NCBI
|
|
53
|
Somiari SB, Shriver CD, Heckman C, Olsen
C, Hu H, Jordan R, Arciero C, Russell S, Garguilo G, Hooke J and
Somiari RI: Plasma concentration and activity of matrix
metalloproteinase 2 and 9 in patients with breast disease, breast
cancer and at risk of developing breast cancer. Cancer Lett.
233:98–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Linderholm BK, Hellborg H, Johansson U,
Elmberger G, Skoog L, Lehtiö J and Lewensohn R: Significantly
higher levels of vascular endothelial growth factor (VEGF) and
shorter survival times for patients with primary operable
triple-negative breast cancer. Ann Oncol. 20:1639–1646. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Strong AL, Strong TA, Rhodes LV, Semon JA,
Zhang X, Shi Z, Zhang S, Gimble JM, Burow ME and Bunnell BA:
Obesity associated alterations in the biology of adipose stem cells
mediate enhanced tumorigenesis by estrogen dependent pathways.
Breast Cancer Res. 15:R1022013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nieman KM, Romero IL, Van Houten B and
Lengyel E: Adipose tissue and adipocytes support tumorigenesis and
metastasis. Biochim Biophys Acta. 1831:1533–1541. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Weisberg SP, McCann D, Desai M, Rosenbaum
M, Leibel RL and Ferrante AW Jr.: Obesity is associated with
macrophage accumulation in adipose tissue. J Clin Invest.
112:1796–1808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gnerlich JL, Yao KA, Fitchev PS,
Goldschmidt RA, Bond MC, Cornwell M and Crawford SE: Peritumoral
expression of adipokines and fatty acids in breast cancer. Ann Surg
Oncol. 20 Suppl 3:S731–S738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dehdashti F, Mortimer JE, Trinkaus K,
Naughton MJ, Ellis M, Katzenellenbogen JA, Welch MJ and Siegel BA:
PET-based estradiol challenge as a predictive biomarker of response
to endocrine therapy in women with estrogen-receptor-positive
breast cancer. Breast Cancer Res Treat. 113:509–517. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Catalano S, Marsico S, Giordano C, Mauro
L, Rizza P, Panno ML and Andò S: Leptin enhances, via AP-1,
expression of aromatase in the MCF-7 cell line. J Biol Chem.
278:28668–28676. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Garofalo C, Sisci D and Surmacz E: Leptin
interferes with the effects of the antiestrogen ICI 182,780 in
MCF-7 breast cancer cells. Clin Cancer Res. 10:6466–6475. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dubois V, Jardé T, Delort L, Billard H,
Bernard-Gallon D, Berger E, Geloen A, Vasson MP and Caldefie-Chezet
F: Leptin induces a proliferative response in breast cancer cells
but not in normal breast cells. Nutr Cancer. 66:645–655. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Khanal T, Kim HG, Do MT, Choi JH, Won SS,
Kang W, Chung YC, Jeong TC and Jeong HG: Leptin induces CYP1B1
expression in MCF-7 cells through ligand-independent activation of
the ERα pathway. Toxicol Appl Pharmacol. 277:39–48. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Alshaker H, Krell J, Frampton AE, Waxman
J, Blyuss O, Zaikin A, Winkler M, Stebbing J, Yagüe E and
Pchejetski D: Leptin induces upregulation of sphingosine kinase 1
in oestrogen receptor-negative breast cancer via Src family
kinase-mediated, janus kinase 2-independent pathway. Breast Cancer
Res. 16:4262014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qian Y, Shi D, Qiu J, Zhu F, Qian J, He S,
Shu Y, Yin Y and Chen X: ObRb downregulation increases breast
cancer cell sensitivity to tamoxifen. Tumour Biol. 36:6813–6821.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang L, Tang C, Cao H, Li K, Pang X, Zhong
L, Dang W, Tang H, Huang Y, Wei L, et al: Activation of IL-8 via
PI3K/Akt-dependent pathway is involved in leptin-mediated
epithelial-mesenchymal transition in human breast cancer cells.
Cancer Biol Ther. 16:1220–1230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Takeda K, Noguchi K, Shi W, Tanaka T,
Matsumoto M, Yoshida N, Kishimoto T and Akira S: Targeted
disruption of the mouse Stat3 gene leads to early embryonic
lethality. Proc Natl Acad Sci USA. 94:3801–3804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bromberg JF, Wrzeszczynska MH, Devgan G,
Zhao Y, Pestell RG, Albanese C and Darnell JE Jr.: Stat3 as an
oncogene. Cell. 98:295–303. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Giordano C, Chemi F, Panza S, Barone I,
Bonofiglio D, Lanzino M, Cordella A, Campana A, Hashim A, Rizza P,
et al: Leptin as a mediator of tumor-stromal interactions promotes
breast cancer stem cell activity. Oncotarget. 7:1262–1275.
2015.
|