Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
condition characterized by the accumulation of large fat droplets
in hepatocytes (hepatic steatosis) that usually appear in those
without a history of alcohol abuse or known liver disease (1).
NAFLD is a metabolic disorder affecting obese or
overweight individuals in particular, and is considered the main
cause of chronic liver disease with an increasing incidence
worldwide (2). NAFLD is considered
to be the hepatic component of metabolic syndrome, which includes
features such as obesity, hyperinsulinemia, peripheral insulin
resistance, diabetes, dyslipidemia, and hormonal disturbances
secondary to interaction between this syndrome and reproductive
axis (3). In women with ovarian
dysfunction, such us polycystic ovary syndrome (PCOS) and acne, the
possibility of NAFLD occurrence is much higher, and approximately
7% of obese patients with PCOS also develop NAFLD associated with
insulin resistance (4,5). Environmental pollutants, particularly
pesticides or solvents represent tangible NAFLD risk factors
(6–8). Studies have demonstrated that NAFLD is
an important risk factor for the development of primary liver
cancer, mostly due to NAFLD-associated cirrhosis (cryptogenic
cirrhosis) (9,10). A high prevalence of NAFLDS occurs in
hepatitis C virus infections where double antiviral therapy with
peginterferon and ribavirin can have a significant impact on the
progression of the disease (11,12).
Understanding the pathogenesis, biochemical parameters,
histological grading and staging, and the management of NAFLD, are
vital in clinical practice today.
The prevalence of NAFLD in the general population is
not yet completely understood. NAFLD is the most common liver
disorder in Western industrialized countries, affecting 20–40% of
the population. The major risk factors for NAFLD are abdominal
obesity, type 2 diabetes mellitus, dyslipidemia and metabolic
syndrome (13). Some studies also
demonstrate an association between cardiovascular disease and
development of NAFLD (14). NAFLD is
also associated with metabolic syndrome, insulin resistance being
considered as the key mechanism leading to hepatic steatosis
(15). Therefore, it is important to
actively search for NAFDL in other conditions associated with
insulin resistance, such as PCOS, acromegaly and psoriasis, and to
consider liver function when treating the primary disorder.
In the management of patients with NAFLD there is a
need for a multidisciplinary system, as apart from the standard
treatment for liver disease, and also a need for the specific
treatment of associated metabolic disturbances, such as obesity,
hyperlipidemia, insulin resistance and type 2 diabetes mellitus.
The aim of this planned, prospective and uncontrolled study was to
evaluate the efficacy of atorvastatin and pentoxifylline in
treating NAFLD.
Materials and methods
The present study included 98 patients with
histologically confirmed NAFLD, admitted between October 2012 and
January 2016 at the Department of Internal Medicine at Filantropia
University Hospital (Craiova, Romania) Upon admission, a
comprehensive medical history and full physical examination was
carried out, including the determination of body mass index (mean
BMI, 31.45±5.54 kg/m2). There were 2 groups of patients:
group I (57 dyslipidemic patients and group II (41 non-dyslipidemic
patients). No differences in terms of age and gender between the 2
groups were observed. The results of biochemical tests upon
admission are shown in Table I.
 | Table I.Mean values of biochemical parameters
and BMI in therapeutic groups at inclusion. |
Table I.
Mean values of biochemical parameters
and BMI in therapeutic groups at inclusion.
|
| Group I n=57 | Group II n=41 |
|
|---|
|
|
|
|
|
|---|
| Parameter | Mean | SD | Mean | SD | P-value |
|---|
| ALT UI/dl |
82.64 | 13.82 |
82.60 | 12.82 |
0.9884 |
| AST UI/dl |
83.13 | 20.36 |
91.47 | 14.17 |
0.0262 |
| GGT UI/dl |
52.99 | 15.27 |
55.27 | 17.55 |
0.4951 |
| TC mg/dl | 298.76 | 26.76 | 141.32 | 34.56 | <0.0001 |
| TG mg/dl | 260.09 | 62.36 |
95.41 | 27.12 | <0.0001 |
| ALP UI/dl | 209.20 | 61.23 | 165.61 | 52.45 |
0.0004 |
| G (mg/dl) | 117.40 | 27.78 | 103.85 | 19.95 |
0.0090 |
| BMI
(kg/m2) |
31.347 |
5.341 |
29.056 |
6.884 |
0.0667 |
This study was carried out in accordance with the
Helsinki Declaration of 1975, and was approved by the Review Ethics
Board of the University Medicine and Pharmacy of Craiova and of the
Filantropia University Hospital. All patients involved in this
study signed a full informed consent. Taking into consideration
ethical concerns and the overall poor consent to hepatic biopsies,
we decided not to use placebos or controls in the present study;
this is primarily due to the fact that it would involve a large
number of patients having to undergo two liver biopsies while
receiving no active treatment.
The patients were divided into 2 therapeutic groups
as follows: group I, 57 dyslipidemic patients receiving
atorvastatin 20 mg/day; and group II, 41 non-dyslipidemic patients
treated with pentoxifylline 800 mg/day (400 mg twice daily).
The average duration of drug administration was
32.8±3.4 weeks. According to the study design, the patients were
subjected to a medical examination upon admission (T0), two regular
medical examinations (T1 and T2) at 10 and 20 weeks after the
initial medical examination and one medical examination at the end
of treatment at week 30 (T3).
All patients (51 males/47 females) were Caucasians
with a mean age of 54/52 years and no active viral hepatitis and no
history of drug and/or alcohol abuse.
The study group was selected using inclusion and
exclusion criteria as follows: patients were included in the study
if they were able to provide written informed consent, had been
histologically confirmed to suffer from NAFLD and had no history of
drug and/or alcohol abuse. The exclusion criteria were represented
by the history of chronic intake or abuse of alcohol or active
viral hepatitis.
In monitoring alcohol intake, we used a
questionnaire (which was slightly modified) based on the Behavioral
Risk Factor Surveillance System 2006 Questionnaire (16) taken at each medical examination. The
infrequent consumption of small amounts of alcohol amounts was
permitted, in the condition that this did not exceed >2 drinks
per week, with each drink being defined as one standard US
alcoholic drink (approximately 14 g ethanol i.e., 12 oz of beer, 5
oz of wine, or 1.5 oz of liquor; 1 US oz = approximately 30
ml).
No restriction or modifications in lifestyle or diet
were enforced on any patient, apart from any current
recommendations made by their endocrinologist or cardiologist. BMI,
serum levels of alanine aminotransferase (ALT), aspartate
aminotransferase (AST), gamma-glutamyl transpeptidase (GGT),
alkaline phosphatase (ALP), total cholesterol (TC) and
triglycerides (TG), and blood glucose levels were determined for
all patients upon admission, at each visit and at the end of
treatment. The patients were sampled after at least 8 h of
overnight fasting by standard venipuncture.
The patients underwent liver biopsy at the beginning
and end of the present study; we deemed 2 weeks before or after the
first and last visit to be a respectable and acceptable time
interval for biopsy. Hepatic biopsy was carried out using the
Menghini technique, using Braun Melsungen Sonocan 20 G needles (B.
Braun Medical S.R.L., Timis, Romania), 0.8×160 mm and 21 G, 0.8×160
mm. Biopsy fragments with a minimum length of 12 mm were considered
adequate and delivered for pathological analysis.
Histological staining techniques were carried out to
determine inflammation, steatosis and hepatic fibrosis using the
NAFLD activity score (NAS or the Brunt score) for each case, upon
admission into the study and at the end of the treatment, as
previously described (17).
In this study, we used the NASH recognized lesion
evaluation system developed by Kleiner et al (17). Using these criteria, the NASH
Clinical Research Network Pathology Committee designed and
validated a histological system of scoring which addresses the
entire spectrum of NAFLD lesions and proposed a NAS to use in
clinical trials (17). The scoring
system is made up of 14 histological features, of which 4 were
evaluated semi-quantitatively: lobular inflammation (0–2),
steatosis (0–3), fibrosis (0–4) and hepatocellular ballooning
(0–2). NAS is the sum of lobular inflammation, steatosis and
hepatocellular ballooning scores, a NAS score >5 correlates with
a NASH diagnosis and scores of ≤3 are considered as ‘not NASH.’ The
NAS admission scores at T0 (score 1) and at the termination of the
study at T3 (score 2) were database-stored and compared between the
therapeutic groups.
The results were calculated as the means ± standard
deviation. For data processing, the program Microsoft Excel was
used (Microsoft Corp., Redmond, WA, USA), along with the XLSTAT
suite for MS Excel (Addinsoft SARL, Paris, France).
For statistical comparisons between the 2 groups at
admission (T0) and at the end of the treatment period (T3),
variance analysis (one way ANOVA) was used, and for comparisons
within the groups themselves, variant analysis and the
Kruskall-Wallis and Wilcoxon tests were used. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
During the analysis of our results, we noted that
the TG and TC levels were higher in group I compared with group II
at T0, while the ALT, AST, GGT and ALP values were comparable
between the groups (Table I). BMI
and blood glucose levels were also higher in the dyslipidemic
patients than in the patients with normal lipid levels (Table I).
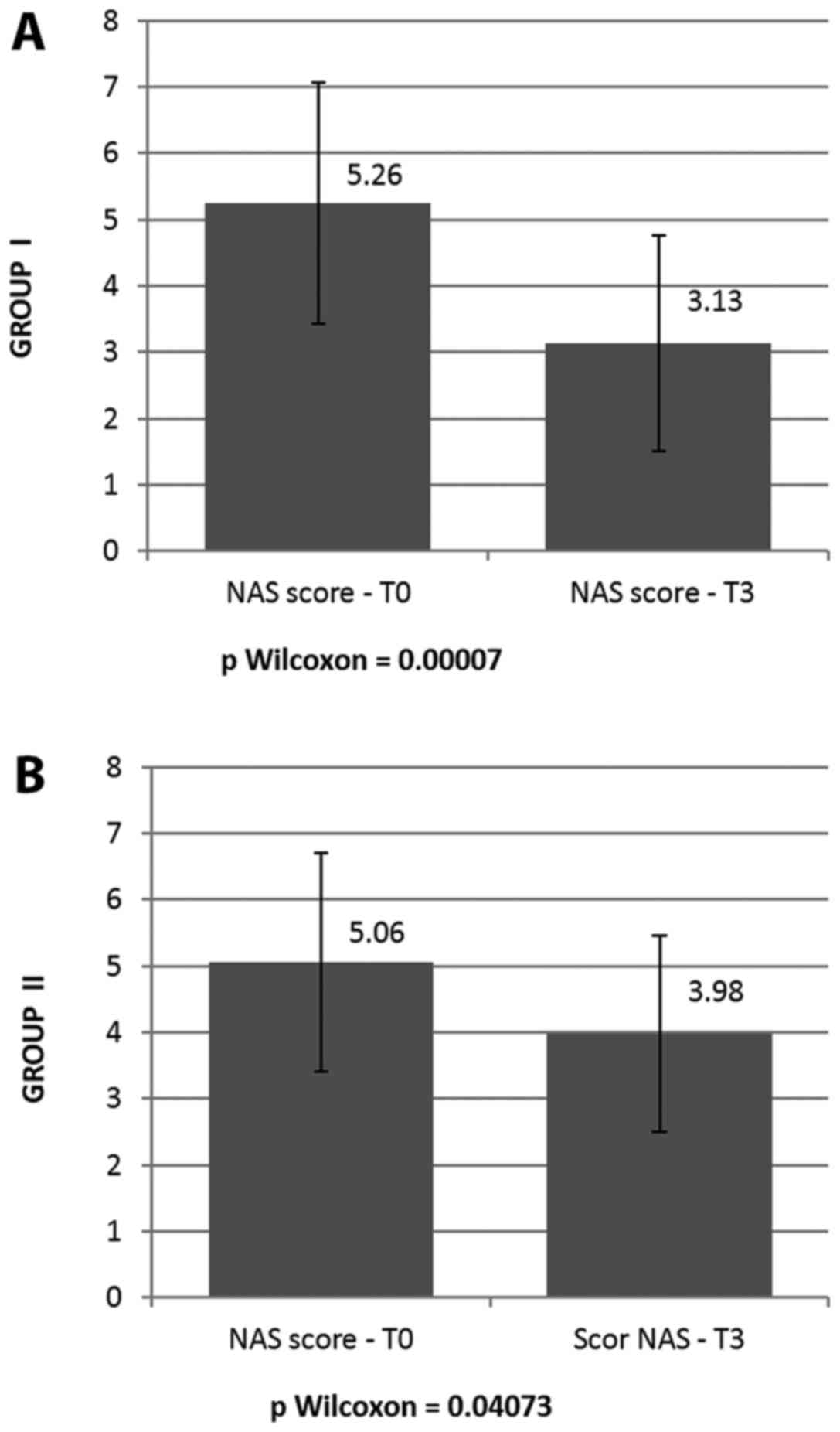
The mean NAS score upon admission (NAS score T0) was
5.26±1.82 in group I and 5.06±1.48 in group II, with no significant
differences between the 2 therapeutic groups (P=0.5639, P>0.05)
(Table II); likewise, there were no
significant differences observed between the 2 groups as regards
steatosis, necroinflammation, ballooning and fibrosis upon
admission (data not shown).
 | Table II.Mean values for NAS scores at T0 and
T3. |
Table II.
Mean values for NAS scores at T0 and
T3.
|
| Atorvastatin | Pentoxifylline |
|
|---|
|
|
|
|
|
|---|
|
| Mean | SD | Mean | SD | P-value |
|---|
| Score 1 | 5.26 | 1.82 | 5.06 | 1.65 | 0.5639 |
| Score 2 | 2.93 | 1.62 | 3.98 | 1.48 | 0.0093 |
| Mean diff. |
|
|
|
|
|
| Score 1/Score
2 | 2.33 | 1.08 |
|
|
|
| P-value | <0.001 | <0.05 |
|
|
|
The importance of evaluating patients at T1 and T2,
respectively, consisted of the information obtained on the direct
and comparative effects of administering atorvastatin or
pentoxifylline after 10 and 20 weeks following the different
treatments (Table III). During
treatment, an improvement in the liver biochemical parameters was
noted.
 | Table III.Mean values of biochemical parameters
and BMI in the therapeutic groups at the different time points in
the experiment. |
Table III.
Mean values of biochemical parameters
and BMI in the therapeutic groups at the different time points in
the experiment.
|
| Group I | Group II |
|---|
|
|
|
|
|---|
| Param | T0 | T1 | T2 | T3 | P-value | T0 | T1 | T2 | T3 | P-value |
|---|
| ALT | 82.64±13.82 | 75.12±11.89 | 69.53±10.20 | 43.63±10.32 | <0.0001 | 82.6±12.82 | 80.02±12.19 | 77.08±9.59 | 54.9±6.83 | <0.0001 |
| AST | 83.13±20.36 | 75.59±16.57 | 70.61±13.31 | 43.28±12.35 | <0.0001 | 91.47±14.17 | 88.87±11.62 | 86.91±9.11 | 58.59±8.45 | <0.0001 |
| GGT | 52.99±15.27 | 49.82±12.26 | 48.84±9.04 | 37.02±10.45 | <0.0001 | 55.27±17.55 | 53.04±13.46 | 51.23±11.34 | 42.95±8.95 | <0.0001 |
| TC | 298.76±26.76 | 275.60±24.24 | 269.72±20.77 | 243.7±16.07 | <0.0001 | 141.32±34.56 | 141.85±29.41 | 136.57±30.09 | 137.68±31.79 | 0.837 |
| TG | 260.09±62.36 | 257.31±55.08 | 249.96±46.13 | 239.04±40.41 | 0.002 | 95.41±27.12 | 95.81±26.27 | 91.09±22.19 | 88.44±18.35 | 0.435 |
| ALP | 209.2±61.23 | 218.32±60.45 | 178.93±54.89 | 142.5±40.2 | <0.0001 | 165.61±52.45 | 202.06±68.35 | 208.31±49.95 | 151.07±40.98 | <0.0001 |
| G | 117.4±27.78 | 115.91±26.18 | 111.35±25.24 | 112.68±19.8 | 0.542 | 103.85±19.95 | 101.85±17.48 | 103.88±15.42 | 119.39±21.12 | <0.0001 |
| BMI | 31.347±5.341 | 31.47±5.65 | 31.23±6.74 | 30.337±4.341 | 0.726 | 29.056±6.884 | 28.75±6.324 | 28.36±6.783 | 28.059±5.867 | 0.905 |
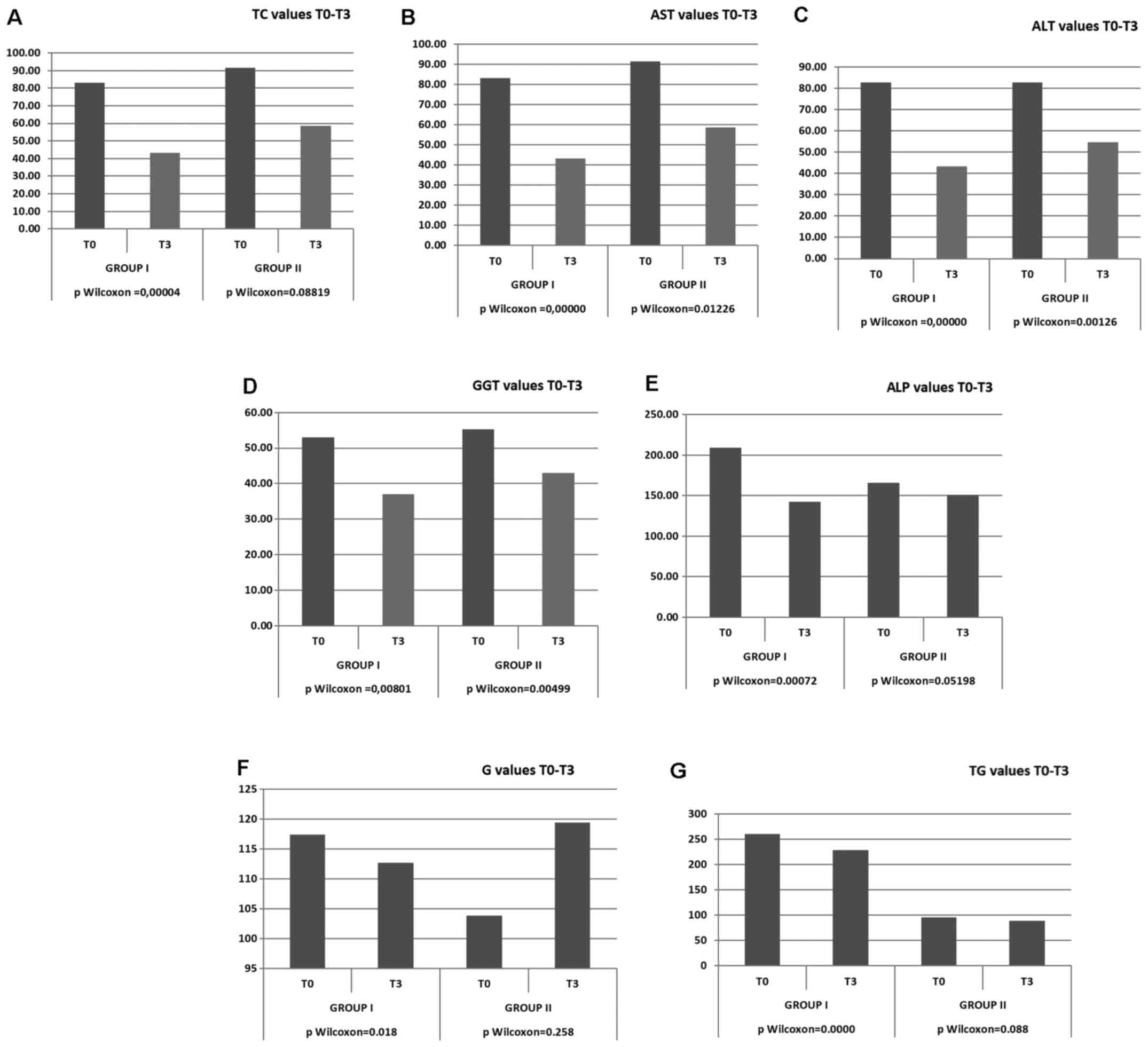
The results of biochemical parameters after 30 weeks
from the beginning of the present study revealed a significant
decrease (P=0.018) in the glucose levels in patients from study
group I and a non-significant decrease in patients from study group
II. A significant a decrease was observed in group I in the levels
of ALT (P<0.0001), AST (P<0.0001), GGT (P<0.0001), TC
(P<0.0001) and ALP (P<0.0001). In group II, the decrease in
the ALT, AST and GGT levels was similar to that of group I
(P<0.05); however, the levels of TC and TG in group II were only
slightly and non-significantly decreased (Tables III and IV, and Fig.
1)..
 | Table IV.Mean values for biochemical
parameters and BMI at study termination. |
Table IV.
Mean values for biochemical
parameters and BMI at study termination.
|
| Atorvastatin | Pentoxifylline |
|
|---|
|
|
|
|
|
|---|
| Parameter | Mean | SD | Mean | SD | P-value |
|---|
| ALT |
43.63 | 10.32 |
54.90 |
6.83 | <0.0001 |
| AST |
43.28 | 12.35 |
58.59 |
8.45 | <0.0001 |
| GGT |
37.02 | 10.45 |
42.95 |
8.95 | 0.0041 |
| TC | 243.70 | 16.07 | 137.68 | 31.79 | <0.0001 |
| TG | 239.04 | 40.41 |
88.44 | 18.35 | <0.0001 |
| ALP | 142.50 | 40.20 | 151.07 | 40.98 | 0.3044 |
| G | 112.68 | 19.80 | 119.39 | 21.12 | 0.1108 |
| BMI |
30.337 |
4.341 | 28.059 |
5.867 | 0.905 |
No significant alteration was noticed regarding the
levels of glucose and BMI within the groups. After the first 10
weeks of treatment, a significant decrease in the ALT levels in
both groups was observed (Table
III). In both groups, a significant decrease was observed in
the ALT and AST levels; the ALT and AST levels markedly decreased
after 20 weeks of treatment. For GGT values, a similar descending
trend was observed during treatment with the difference that lower
rates were noted between T2 and T3. Histopathological evaluation
for group I presented a mean NAS score at termination of 3.13±1.62,
which is highly significantly lower than score 1 (P=0.0007). Group
II exhibited a mean NAS score at termination of 3.98±1.48, which is
borderline significant compared to the initial NAS score
(P=0.04073). The lowest NAS score at termination was achieved by
the patients in group I treated with atorvastatin (Table III).
NAS components improvement (one or more) was noticed
only in group I; however, no attenuation of fibrosis was observed
in either group. The steatosis score was significantly decreased in
patients from group I, but only group II experienced an improvement
of necroinflammation that was statistically significant. A
comparison between the NAS scores at admission vs. termination in
both groups is shown in Fig. 2.
Discussion
The pharmacological treatment of NAFLD includes
vitamin E, insulin sensitisers and other metabolic agents aiming an
antioxidant or hypolipemic activity, such as atorvastatin, while
pentoxifylline inhibits the production of tumor necrosis factor-α
(TNF-α), which stimulates NASH development.
The results of the present study are in agreement
with those of other trials. In short, both drugs led to a
significant reduction in ALT and GGT levels. No significant weight
loss was observed in the current study (P>0.05). Atorvastatin
also led to a significant reduction in the levels of ALP
(P<0.0001), TC (P<0.0001) and TG (P=0.002).
The lack of a control group imposed limits on the
ability of determining the real pharmacotherapy impact, but when
considering that these parameters failed to improve treatment
previously, it is possible that the positive effects observed in
the present study were caused by pentoxifylline and
atorvastatin.
On the other hand, the histological evaluation
showed significant improvement in 2 of the 4 NAS components of both
groups. Neither group exhibited any changes in fibrosis. The
dyslipidemic patients exhibited a highly statistically significant
difference between the initial and final NAS scores, resulting in a
P-value of 0.00007. The difference in the initial and final NAS
scores of patients from the non-dyslipidemic group administered
pentoxifylline was of marginal significance (P=0.04073).
Atorvastatin is a HMG-CoA reductase inhibitor which
catalyzes the HMG-CoA conversion into mevalonate, an early and
rate-limiting step in the cholesterol biosynthesis, leading to low
cholesterol production by the liver, high LDL cholesterol plasmatic
clearance and up-regulation of hepatocyte LDL-receptors (18).
Kiyici et al observed that the use of
atorvastatin for 6 months on a number of 27 dyslipidemic patients,
for whom NASH diagnosis was histopathologicaly verified, was both
effective and safe (19), while
Balistreri observed that atorvastatin normalized serum ALT levels,
TC and TG levels in patients with NASH (20).
In 2011, as part of the Saint Francis Heart Study,
80 patients with NAFLD confirmed by computer tomography were
administered 20 mg/day atorvastatin combined with 1 g vitamin C,
and 1,000 IU vitamin E. After 4 years of therapy, the reduction of
hepatic steatosis was 71% (OR=0.29, P<0.001). However, the fact
that the patients received a combination of vitamins C and E along
with atorvastatin, and that the NAFLD diagnosis was based on
imaging and not on histology, limited the power of the conclusions
(21).
Gómez-Domínguez et al examined the way in
which atorvastatin in doses of 10–80 mg/day affects lipid
metabolism and transaminases levels in 22 patients for whom NAFLD
diagnosis was established through ultrasonography. Following 6
months of treatment, 36.3% of the patients presented normal levels
of transaminases and TC and after 12 months of treatment, a
statistically noticeable decrease in transaminases levels and TC
(from 268.5±44.2 to 186.8±14.4 mg/dl) were present, confirming the
effect of atorvastatin in NAFLD and dyslipidemic patients without
notable side-effects for daily doses of 10–80 mg (22).
Pentoxifylline is an inhibitor of TNF-α. It is
evident that TNF-α is associated with hepatic inflammatory cell
recruitment, which represents a key step in the initiation and
perpetuation of NASH liver injury (23). TNF-α also interferes with insulin
receptor signaling by impairing and reducing insulin
sensitivity.
Several pilot studies have demonstrated biochemical
improvement, and in some cases histological improvement following
the administration of pentoxifylline to patients with NASH
(24–26).
Satapathy et al observed in 18
histopathologicaly verified NAFLD patients with elevated
transaminases levels, that after 6 months of pentoxifylline
administration in 800 mg/day doses, the serum transaminases levels
were significantly reduced (AST: 66±29 to 33±11 IU/l, P<0.0001
and ALT: 109±44 vs. 47±20 IU/l, P<0.0001). Moreover, ALT was
normalized for 23% of the patients within the first month
(P=0.125), 35% after the second month and 60% of the patients after
6 months of treatment. The levels of cholesterol and TG were not
significantly altered (24).
The same authors, reported in 2007 of nine patients
that administered 800 mg pentoxifylline daily doses and after an
average of 12 months treatment, a decrease in transaminase values
was achieved (ALT: 111±53 to 45±19 IU/l, P=0.003 and AST: 61±27 to
33±12 IU/l, P=0.005) accompanied by steatosis and lobular
inflammation reduction in 55% of the cases, as well as decreased
histological fibrosis in 4 out of 9 patients with baseline fibrosis
(25).
In another study, on 55 patients with NASH confirmed
by biopsy who received 400 mg pentoxifylline 3 times per day or the
placebo for 1 year, patients treated with pentoxifylline were more
likely than those treated with the placebo to present a decrease in
histological NAFLD. The NAS score decreased by 2 points in 38.5% of
the patients treated with pentoxifylline compared to only 13.8% of
the patients receiving the placebo. In this study, the
administration of pentoxifylline improved the liver fibrosis
scores, lobular inflammation and steatosis. In 3 patients receiving
pentoxifylline, the dose of medication was decreased from 3 times
daily to twice daily due to nausea, which resulted in adequate
symptom control (26).
In another study by Van Wagner et al, in 30
patients treatesd with pentoxifylline, in doses of 1,200 mg/day, or
placebo, for a period of 12 months. At the completion of the study,
decreases in the transaminase levels (ALT: 92±12 to 67±13 IU/l and
AST: 67±6 to 47±6 IU/l, P<0.05), as well as in the degree of
steatosis and lobular inflammation (P<0.05) were observed in the
group administed pentoxifylline (27).
In other studies, pentoxifylline at doses of 400
mg/day twice daily, administered to 20 patients for 12 months, was
associated with the normalization of serum levels of ALT and AST
(84±64 vs. 138±76, P=0.002 and 58±37 vs. 102±62, P=0.003,
respectively). A total of 9 patients withdrew from the study, due
to nausea (28) and in another pilot
study, similar biochemical improvements under pentoxifylline
treatment were demonstated (29).
Neuner et al (30) studied the mechanisms of
pentoxifylline action in NASH. Αll patients studied presented
elevated levels of TNF-α. Hepatic damage is associated with TNF-α
production that triggers the production of various cytokines
(31), recruiting inflammatory
cells, that affect hepatocytes and induce fibrogenesis (32,33). The
main mechanism through which pentoxifylline improves hepatic
histology (decreasing steatosis and necroinflammation) is the
reduction of lipopolysaccharide stimulated TNF-α production.
In conclusion, statins are well known for their
lipid lowering properties, but they may have the potential to
diminish some of the histological features of NAFLD. Dyslipidemia
is common among patients with NAFLD and atorvastatin proved to be
efficient in the treatment of both disorders, by improving
biochemical parameters and steatosis. Pentoxifylline was
well-tolerated and showed similar efficacy in patients with
non-dyslipidemia by decreasing the degree of steatosis/lobular
inflammation and improving liver function.
References
|
1
|
Guturu P and Duchini A: Etiopathogenesis
of nonalcoholic steatohepatitis: Role of obesity, insulin
resistance and mechanisms of hepatotoxicity. Int J Hepatol.
2012:2128652012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bala C, Crăciun AE and Hâncu N: Updating
the concept of metabolically healthy obesity. Acta Endo.
12:197–205. 2016. View Article : Google Scholar
|
|
3
|
Badiu C: Endocrine management in
Prader-Willi Syndrome. Acta Endo. 8:99–106. 2012. View Article : Google Scholar
|
|
4
|
Ianoşi S, Ianoşi G, Neagoe D, Ionescu O,
Zlatian O, Docea AO, Badiu C, Sifaki M, Tsoukalas D, Tsatsakis AM,
et al: Age-dependent endocrine disorders involved in the
pathogenesis of refractory acne in women. Mol Med Rep.
14:5501–5506. 2016.PubMed/NCBI
|
|
5
|
Rehm JL, Connor EL and Reeder SB:
Non-alcoholic fatty liver disease in an adolescent with polycystic
ovary syndrome. J Pediatr Adolesc Gynecol. 24:e61–e66. 2011.
View Article : Google Scholar
|
|
6
|
Arciello M, Gori M, Maggio R, Barbaro B,
Tarocchi M, Galli A and Balsano C: Environmental pollution: A
tangible risk for NAFLD pathogenesis. Int J Mol Sci.
14:22052–22066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hernández AF, Gil F, Lacasaña M,
Rodríguez-Barranco M, Tsatsakis AM, Requena M, Parrón T and Alarcón
R: Pesticide exposure and genetic variation in
xenobiotic-metabolizing enzymes interact to induce biochemical
liver damage. Food Chem Toxicol. 61:144–151. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsitsimpikou C, Tzatzarakis M, Fragkiadaki
P, Kovatsi L, Stivaktakis P, Kalogeraki A, Kouretas D and Tsatsakis
AM: Histopathological lesions, oxidative stress and genotoxic
effects in liver and kidneys following long term exposure of
rabbits to diazinon and propoxur. Toxicology. 307:109–114. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duan XY, Zhang L, Fan JG and Qiao L: NAFLD
leads to liver cancer: do we have sufficient evidence? Cancer Lett.
345:230–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baffy G, Brunt EM and Caldwell SH:
Hepatocellular carcinoma in non-alcoholic fatty liver disease: an
emerging menace. J Hepatol. 56:1384–1391. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Marconi A, Candido S, Talamini R, Libra M,
Nicoletti F, Spandidos DA, Stivala F and Proietti L: Prevalence of
hepatitis C virus infection among health-care workers: A 10-year
survey. Mol Med Rep. 3:561–564. 2010.PubMed/NCBI
|
|
12
|
Docea AO, Gofiță E, Călina D, Zaharie SI,
Vâlcea DI and Mitruț P: Autoimmune disorders due to double
antiviral therapy with Peginterferon and Ribavirin in patients with
hepatitis C virus infection. Farmacia. 64:605–611. 2016.
|
|
13
|
Chitturi S, Farrell GC, Hashimoto E,
Saibara T, Lau GK and Sollano JD: Asia-Pacific Working Party on
NAFLD: Non-alcoholic fatty liver disease in the Asia-Pacific
region: Definitions and overview of proposed guidelines. J
Gastroenterol Hepatol. 22:778–787. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lizardi-Cervera J and Aguilar-Zapata D:
Nonalcoholic fatty liver disease and its association with
cardiovascular disease. Ann Hepatol. 8 Suppl 1:S40–S43.
2009.PubMed/NCBI
|
|
15
|
Almeda-Valdés P, Cuevas-Ramos D and
Aguilar-Salinas CA: Metabolic syndrome and non-alcoholic fatty
liver disease. Ann Hepatol. 8:S18–S24. 2009.PubMed/NCBI
|
|
16
|
Centers for Disease Control and
Prevention: Behavioral Risk Factor Surveillance System. National
Center for Chronic Disease Prevention and Health Promotion,
Division of Population Health; Atlanta, GA: 2014, http://apps.nccd.cdc.gov/brfss/index.asp
|
|
17
|
Kleiner DE, Brunt EM, van Natta M, Behling
C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS,
Unalp-Arida A, et al: Nonalcoholic Steatohepatitis Clinical
Research Network: Design and validation of a histological scoring
system for nonalcoholic fatty liver disease. Hepatology.
41:1313–1321. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taylor F, Ward K, Moore TH, Burke M, Smith
G Davey, Casas JP and Ebrahim S: Statins for the primary prevention
of cardiovascular disease. Cochrane Database Syst Rev.
19:CD0048162011.
|
|
19
|
Kiyici M, Gulten M, Gurel S, Nak SG, Dolar
E, Savci G, Adim SB, Yerci O and Memik F: Ursodeoxycholic acid and
atorvastatin in the treatment of nonalcoholic steatohepatitis. Can
J Gastroenterol. 17:713–718. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balistreri WF: Nonalcoholic fatty liver
disease - insights and controversies. CME Digestive Disease Week.
Medscape. 2006.
|
|
21
|
Foster T, Budoff MJ, Saab S, Ahmadi N,
Gordon C and Guerci AD: Atorvastatin and antioxidants for the
treatment of nonalcoholic fatty liver disease: The St Francis Heart
Study randomized clinical trial. Am J Gastroenterol. 106:71–77.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gómez-Domínguez E, Gisbert JP,
Moreno-Monteagudo JA, García-Buey L and Moreno-Otero R: A pilot
study of atorvastatin treatment in dyslipemid, non-alcoholic fatty
liver patients. Aliment Pharmacol Ther. 23:1643–1647. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chitturi S and Farrell GC: TNF-alpha as
therapeutic target in NASH: Tried, but not yet proven. J
Gastroenterol Hepatol. 22:613–614. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Satapathy SK, Garg S, Chauhan R, Sakhuja
P, Malhotra V, Sharma BC and Sarin SK: Beneficial effects of tumor
necrosis factor-alpha inhibition by pentoxifylline on clinical,
biochemical, and metabolic parameters of patients with nonalcoholic
steatohepatitis. Am J Gastroenterol. 99:1946–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Satapathy SK, Sakhuja P, Malhotra V,
Sharma BC and Sarin SK: Beneficial effects of pentoxifylline on
hepatic steatosis, fibrosis and necroinflammation in patients with
non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 22:634–638.
2007.PubMed/NCBI
|
|
26
|
Zein CO, Yerian LM, Gogate P, Lopez R,
Kirwan JP, Feldstein AE and McCullough AJ: Pentoxifylline improves
nonalcoholic steatohepatitis: A randomized placebo-controlled
trial. Hepatology. 54:1610–1619. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Wagner LB, Koppe SW, Brunt EM,
Gottstein J, Gardikiotes K, Green RM and Rinella ME: Pentoxifylline
for the treatment of non-alcoholic steatohepatitis: A randomized
controlled trial. Ann Hepatol. 10:277–286. 2011.PubMed/NCBI
|
|
28
|
Adams LA, Zein CO, Angulo P and Lindor KD:
A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am
J Gastroenterol. 99:2365–2368. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Comar KM and Sterling RK: Review article:
Drug therapy for non-alcoholic fatty liver disease. Aliment
Pharmacol Ther. 23:207–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Neuner P, Klosner G, Schauer E, Pourmojib
M, Macheiner W, Grünwald C, Knobler R, Schwarz A, Luger TA and
Schwarz T: Pentoxifylline in vivo down-regulates the release of
IL-1 beta, IL-6, IL-8 and tumour necrosis factor-alpha by human
peripheral blood mononuclear cells. Immunology. 83:262–267.
1994.PubMed/NCBI
|
|
31
|
Warne JP: Tumour necrosis factor alpha: A
key regulator of adipose tissue mass. J Endocrinol. 177:351–355.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wigg AJ, Roberts-Thomson IC, Dymock RB,
McCarthy PJ, Grose RH and Cummins AG: The role of small intestinal
bacterial overgrowth, intestinal permeability, endotoxaemia, and
tumour necrosis factor alpha in the pathogenesis of non-alcoholic
steatohepatitis. Gut. 48:206–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ungureanu A, Gaman AE, Drocas AI, Tieranu
E and Dobrițoiu M: The influence of steatosis and conjugate factors
of response to antiviral treatment in cronic hepatitis C, The null.
Research and Science Today. 11:147–155. 2016.
|