Introduction
Breast cancer, as one of the most common malignant
tumor types affecting women worldwide, severely threatens their
physiological and psychological health (1). In 2008, almost 1.4 million women were
diagnosed with breast cancer worldwide, and ~459,000 instances of
breast cancer-associated mortality were recorded (2). In China, the incidence rate and
mortality rate of breast cancer rank first and fifth, respectively,
among all types of cancer (3). As
with other types of cancer, breast cancer involves multiple genes
and factors, including hormonal and reproductive factors such as
progesterone and estrogen receptors (4). Therefore, it is important to determine
appropriate markers in order to determine the molecular mechanism
for its onset and progression. Krüppel-like factor 4 (KLF4), which
is a member of the KLF family, is a transcription element-binding
protein that is present across eukaryotes (5). Three continuous zinc finger domains at
the C-terminus are bound to GC-rich sequences in the promoter
region of the target gene, regulating the transcription of KLF4
(6). Besides being associated with
the growth, differentiation and apoptosis of normal tissue cells,
KLF4 may function as an oncogene in liver cancer or as a tumor
suppressor in renal cell carcinoma by interacting with different
target genes (7–9). The role of KLF4 in breast cancer
remains controversial. Therefore, KLF4 gene expression was
determined in tissue from patients with breast cancer and analyzed
the correlation with clinical pathological parameters. In addition,
an expression vector, pcDNA3.1-KLF4, was constructed and expressed
by transient transfection into the breast cancer cell line
MDA-MB-231, in order to observe the effects of the KLF4 gene in
cell proliferation.
Materials and methods
Sample sources
A total of 239 cancerous tissue samples were
collected from 239 patients with breast cancer who received radical
mastectomy in The Second Hospital of Shanxi Medical University
between January 2009 and October 2014 to prepare tissue microarrays
that contained primary foci. In addition, 40 samples of
paracancerous tissues were harvested from randomly selected
patients in this group. Patients did not receive chemotherapy or
radiotherapy prior to the surgery, and the results of postoperative
pathological examination were confirmed by at least two
pathologists. The clinical medical records were all complete. The
present study was approved by the Ethics Committee of The Second
Hospital of Shanxi Medical University (Taiyuan, China), and written
consent was obtained from all patients.
Materials
The human breast cancer cell line MDA-MB-231 was
purchased from the Cell Bank/Stem Cell Bank, Shanghai Institute for
Biological Sciences, CAS (Shanghai, China). Rabbit anti-human KLF4
monoclonal antibody (cat. no. ab72543) was purchased from Abcam
(Cambridge, UK). Streptavidin-peroxidase (SP) conjugate
immunohistochemical assay kit (cat. no. SA-5004) was purchased from
Vector Laboratories, Inc., (Burlingame, CA, USA) and the
3,3′-diaminobenzidine color development kit (cat. no. ab94665) was
purchased from Abcam (Cambridge, UK). Lipofectamine®
2000 (cat. no. 11668019), the western blot detecting
chemiluminescent kit (cat. no. WB7106) and the bicinchoninic acid
assay (BCA) protein determination kit (cat. no. 23225) were
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
TRIzol and the reverse transcription (RT) kit (PrimeScript RT
reagent kit; cat. no. RR037A) were purchased from Takara Bio, Inc.
(Otsu, Japan), and primers were synthesized by Sangon Biotech Co.,
Ltd. (Shanghai, China).
Detection of KLF4 protein expression
by immunohisto-chemistry
All samples were stained using the SP method
according to the manufacturer's protocol, and the primary antibody
was replaced with PBS as a negative control. In total, 5 high-power
fields were randomly selected for each sample, and the staining
results were analyzed according to the percentages of positive
cells and the staining intensities, as described previously
(10). The positive cells were
counted based on the proportions of their numbers to the total
number in the 5 high-power fields, as follows: <5%, 0 point;
5–25%, 1 point; 26–50%, 2 points; 51–75%, 3 points and 76–100%, 4
points. The scoring based on staining intensities was as follows:
pale yellow, 1 point; yellow or dark yellow, 2 points and brown or
sepia, 3 points. In addition, the multiplication of the two results
were considered to be positive if ≥1 and negative if <1.
Estrogen and progesterone receptors were also detected in cells
using hematoxylin and eosin staining as described previously
(11).
Culture of breast cancer cells
Proliferating MDA-MB-231 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum in a 37°C incubator with 5% CO2 atmosphere and
saturated humidity, and the cells were then passaged. Cells in the
logarithmic growth phase were selected for subsequent
experiments.
Cell transfection and grouping
Eukaryotic expression vector pcDNA3.1-KLF4 was
constructed by Tiandz Gene Technology Co., Ltd. (Beijing, China).
An MDA-MB-231 single-cell suspension (1×104 cells/ml) was
inoculated onto 6-well plates and cultured for 24 h at 37°C in an
atmosphere containing 5% CO2 after addition of DMEM containing 10%
fetal bovine serum. The cells were divided into three groups: An
experimental group (transfected with pcDNA3.1-KLF4 plasmid), a
negative control group (transfected with empty plasmid pcDNA3.1)
and a blank control group (untransfected cells). The cells were
transfected according to the protocol of the
Lipofectamine® 2000 kit. After 8 h of transfection, DMEM
containing 10% fetal bovine serum was replaced to culture the cells
for another 48 h.
Detection of KLF4 mRNA expression by
RT-polymerase chain reaction (PCR)
Cells were collected after 48 h of transfection,
from which total mRNA was extracted using TRIzol. A total of 100 ng
cDNA was synthesized from 1 µg total mRNA. mRNA was denatured at
65°C for 5 min and RT was performed with the PrimeScript RT reagent
kit at 50°C for 50 min. The reaction was stopped by denaturing the
enzyme at 70°C for 50 min and cDNA was stored at −20°C. PCR was
performed at 95°C for 30 sec, 95°C for 3 sec and 60°C for 30 sec,
for 40 cycles. Primers were annealed at 62°C for 40 sec. The PCR
reaction mixture contained the following: cDNA, 1 µl; reverse
primer, 1 µl; forward primer; 1 µl; dNTPs, 1 µl; MasterMix, 10 µl;
DMSO, 1 µl; and water, 5 µl. GAPDH was used as the internal
reference. The PCR reagent kit (cat. no. RR036Q) was purchased from
Takara Bio, Inc. (CA, USA).
This experiment was performed in triplicate for each
sample. Following the reaction, the products were resolved on a
1.5% agarose gel by electrophoresis. The sequences of the KLF4
primer were as follows: forward, 5′-ACCAGGCACTACCGTAAACACA-3′ and
reverse, 5′-GGTCCGACCTGGAAAATGCT-3′. In addition, the sequences of
the GAPDH primer were: forward, 5′-GAAGGTGAAGGTCGAAGT-3′ and
reverse, 5′-GAAGATGGTGATGGGATTT-3′.
Detection of KLF4 protein expression
by western blotting
After 48 h of transfection, the cells were collected
in order to extract the total protein, the concentration of which
was determined by the BCA method. The protein samples were loaded,
separated by 10% SDS-PAGE, and transferred to a nitrocellulose
membrane at 250 mA for 90 min. These membranes were blocked in 5%
skimmed milk for 1 h and incubated overnight with a primary
antibody against KLF4 (1:1,000) at 4°C. After this, the membrane
was washed three times with Tris-buffered saline and Tween-20
(TBST) (10 min each time), incubated with TBST-diluted horseradish
peroxidase (HRP)-labeled secondary antibody (1:6,000) at room
temperature for 1 h and washed three more times with TBST (5 min
each time). Subsequently, the membranes were incubated with
Luminata Forte Western HRP Substrate (EMD Millipore, Billercia, MA,
USA) for 3 min or Western Bright (Advansta) in 1:1 dilution with
water for 30 sec. Under red safelight, the membranes were evaluated
with an X-ray Film (Super RX; Fujifilm, Tokyo, Japan) on an X-ray
developing unit (Agfa-Gevaert, Mortsel, Belgium) for 10 min.
β-actin was used as an internal reference, with an antibody
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA;
1:1,500; cat. no. ab8227).
Detection of cell proliferation by the
MTT assay
Following 48 h of transfection the cells were
collected and prepared into a single-cell suspension that was
inoculated onto 96-well plates at a density of 1×104 cells/well.
The experiment was performed in triplicate for each sample. The
cells were thereafter cultured in a 37°C incubator with a 5% CO2
atmosphere and saturated humidity, and 10 µl of 5 mg/ml MTT was
added 24, 48 and 72 h later. After another 4 h of culture, the
supernatant was removed, and 150 µl of dimethyl sulfoxide was added
in each well. Next, the plates were oscillated for 10 min. The
optical density (OD) of each well was measured by a microplate
reader (Biotek China, Beijing, China) at 492 nm and cell growth
curves were plotted, using time as the x-axis and the mean of OD
values from three wells as the y-axis.
Statistical analysis
All data were analyzed using SPSS 18.0 (SPSS, Inc.,
Chicago, IL, USA). The numerical data were compared by the χ2 test.
For the categorical data, intergroup comparisons were performed by
univariate analysis of variance, and further comparisons were
conducted using Student's t-test or Fisher's Least Significant
Difference test. P<0.05 was considered to represent a
statistically significant difference.
Results
KLF4 protein expression in breast
cancer and paracancerous tissues
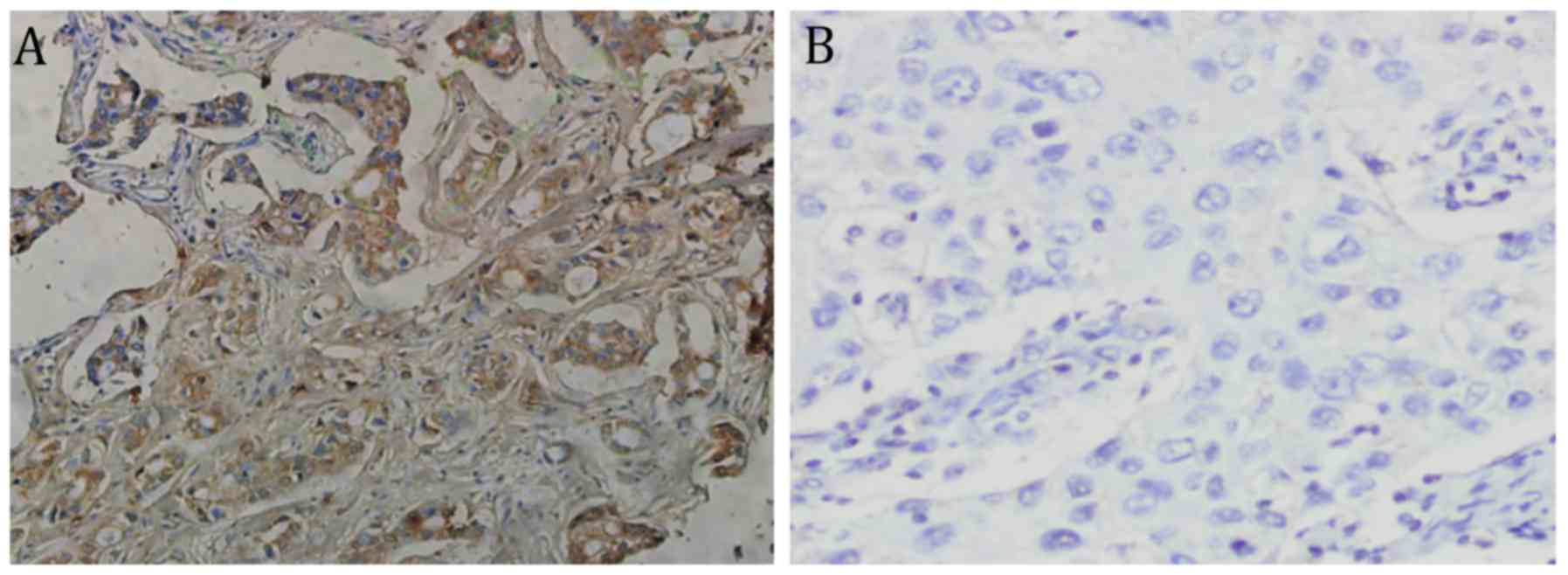
The tissues in which KLF4 protein was positively
expressed were stained pale yellow to sepia. As shown in Fig. 1, the KLF4 protein expression
assessment was predominantly negative in the majority of breast
cancer tissues and positive in most paracancerous tissues. In
addition, the positive expression rates were 39.0 (93/239 tissue
samples) and 77.5% (31/40 tissue samples), respectively, which
represented a significant difference (χ2=20.462, P<0.05).
Correlation between KLF4 protein
expression and clinical pathological parameters
Positive expression of the KLF4 protein was
significantly associated with pathological type, histological grade
and lymphatic metastasis (P<0.05) but was not significantly
associated with age, tumor size, estrogen and progesterone receptor
presence (P>0.05) (Table I).
 | Table I.Correlation between positive KLF4
protein expression and clinical pathological parameters. |
Table I.
Correlation between positive KLF4
protein expression and clinical pathological parameters.
| Clinical pathological
parameter | Subcategories | Total samples | Positive KLF4
expression, n (%) | χ2 value | P-value |
|---|
| Age, years | ≤51 | 133 | 58 (43.6) | 2.944 | 0.086 |
|
| >51 | 106 | 35 (33.0) |
|
|
| Tumor size, cm | ≤2 | 71 | 26 (36.6) | 1.290 | 0.525 |
|
| 2–5 | 109 | 45 (41.3) |
|
|
|
| >5 | 59 | 22 (37.3) |
|
|
| Tumor type | Early invasive
carcinoma | 21 | 10 (47.6) | 6.539 | 0.039 |
|
| Infiltrating ductal
carcinoma | 49 | 26 (53.1) |
|
|
|
| Invasive carcinoma of
no special type | 169 | 57 (33.7) |
|
|
| Histological
grade | I | 32 | 16 (50.0) | 7.210 | 0.026 |
|
| II | 142 | 60 (42.3) |
|
|
|
| III | 65 | 17 (26.2) |
|
|
| Lymphatic
metastasis | No | 143 | 64 (44.8) | 5.315 | 0.019 |
|
| Yes | 96 | 29 (30.2) |
|
|
| Estrogen
receptor | Negative | 127 | 50 (39.4) | 0.042 | 0.837 |
|
| Positive | 112 | 43 (38.4) |
|
|
| Progesterone
receptor | Negative | 137 | 55 (40.1) | 0.155 | 0.695 |
|
| Positive | 102 | 38 (37.3) |
|
|
Effects of KLF4 gene expression on the
proliferation of MDA-MB-231 cells
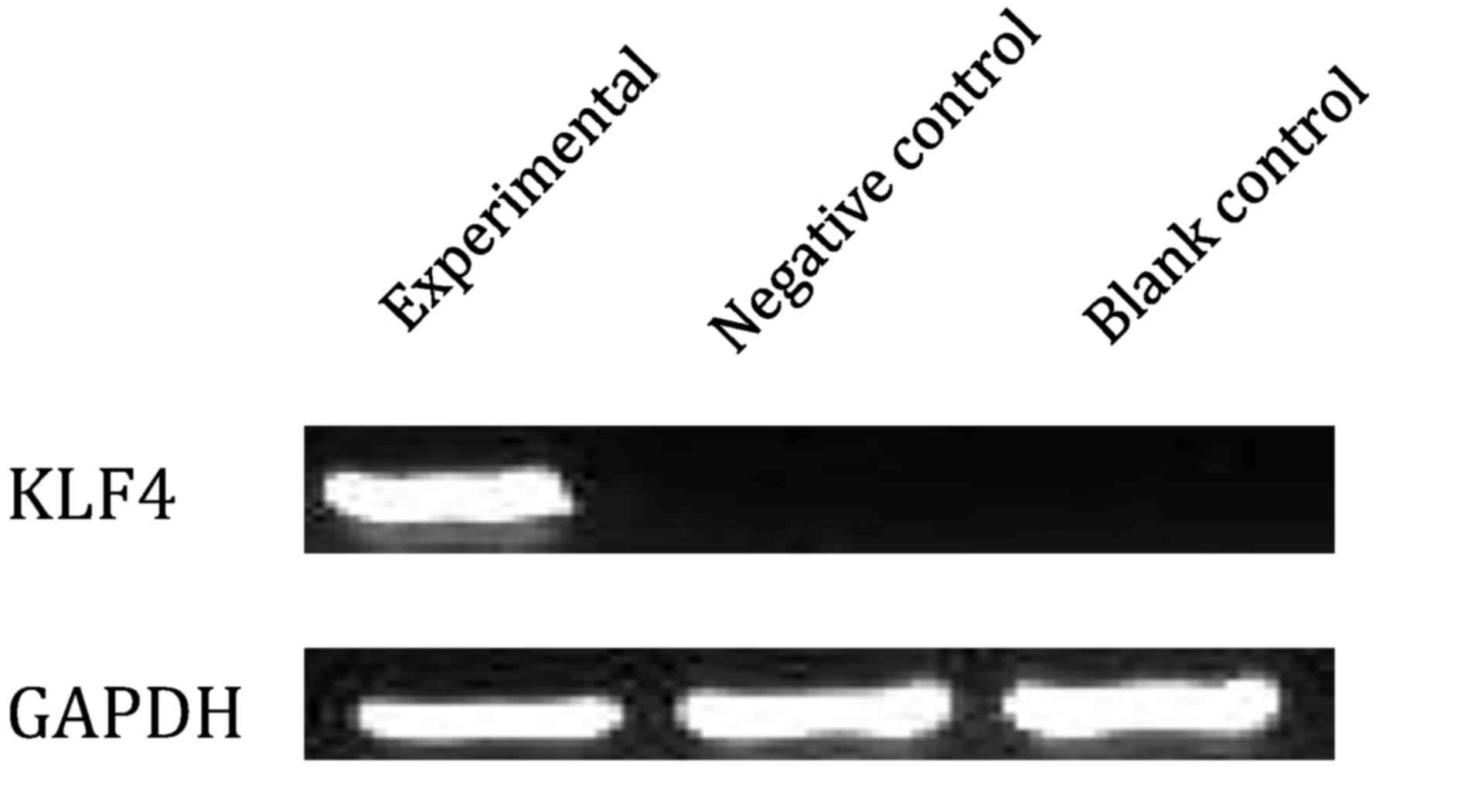
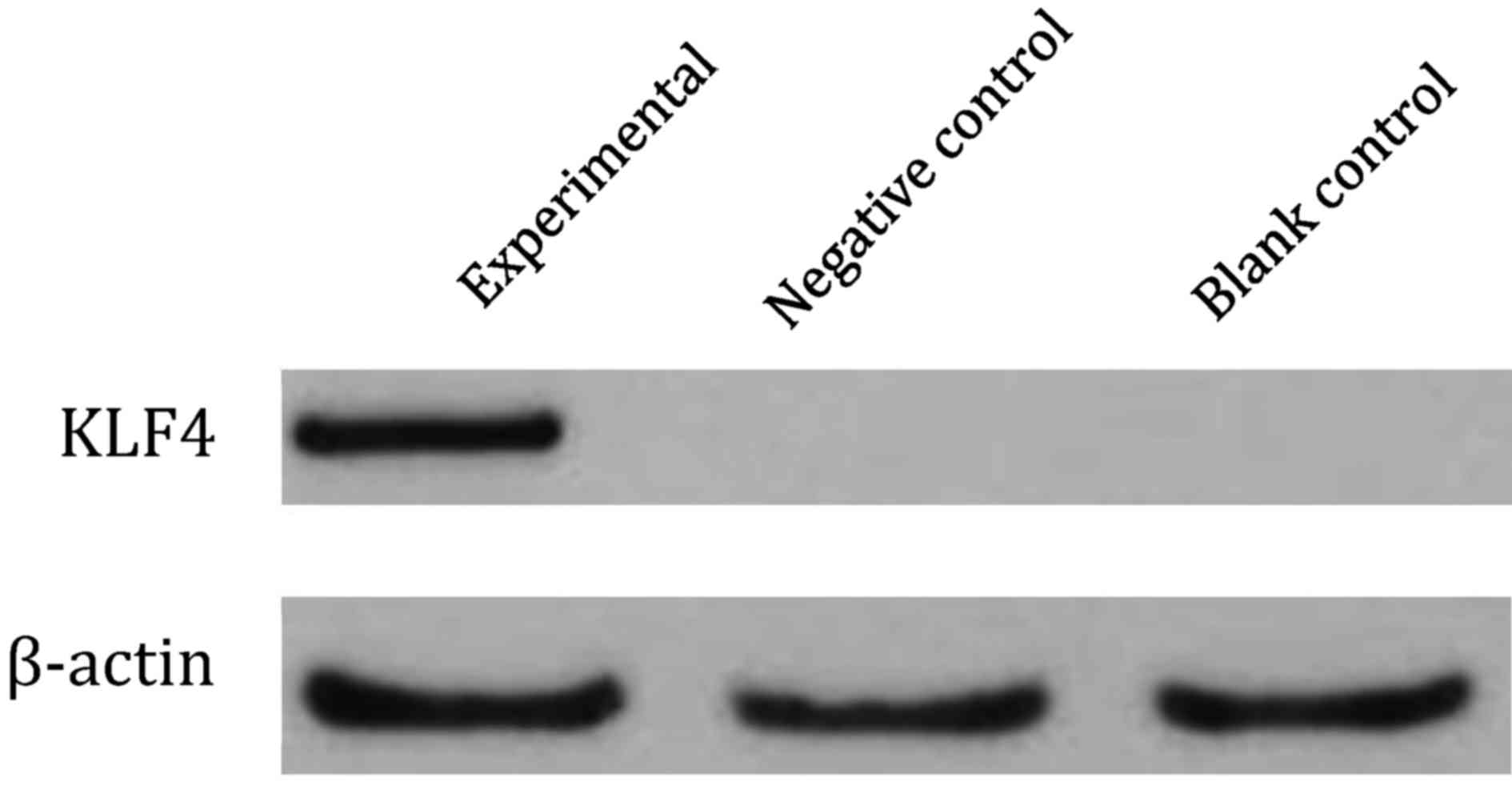
KLF4 mRNA and protein expression, as indicated by
RT-PCR (Fig. 2) and western blotting
(Fig. 3), were expressed in the
experimental group but not in the negative control or the blank
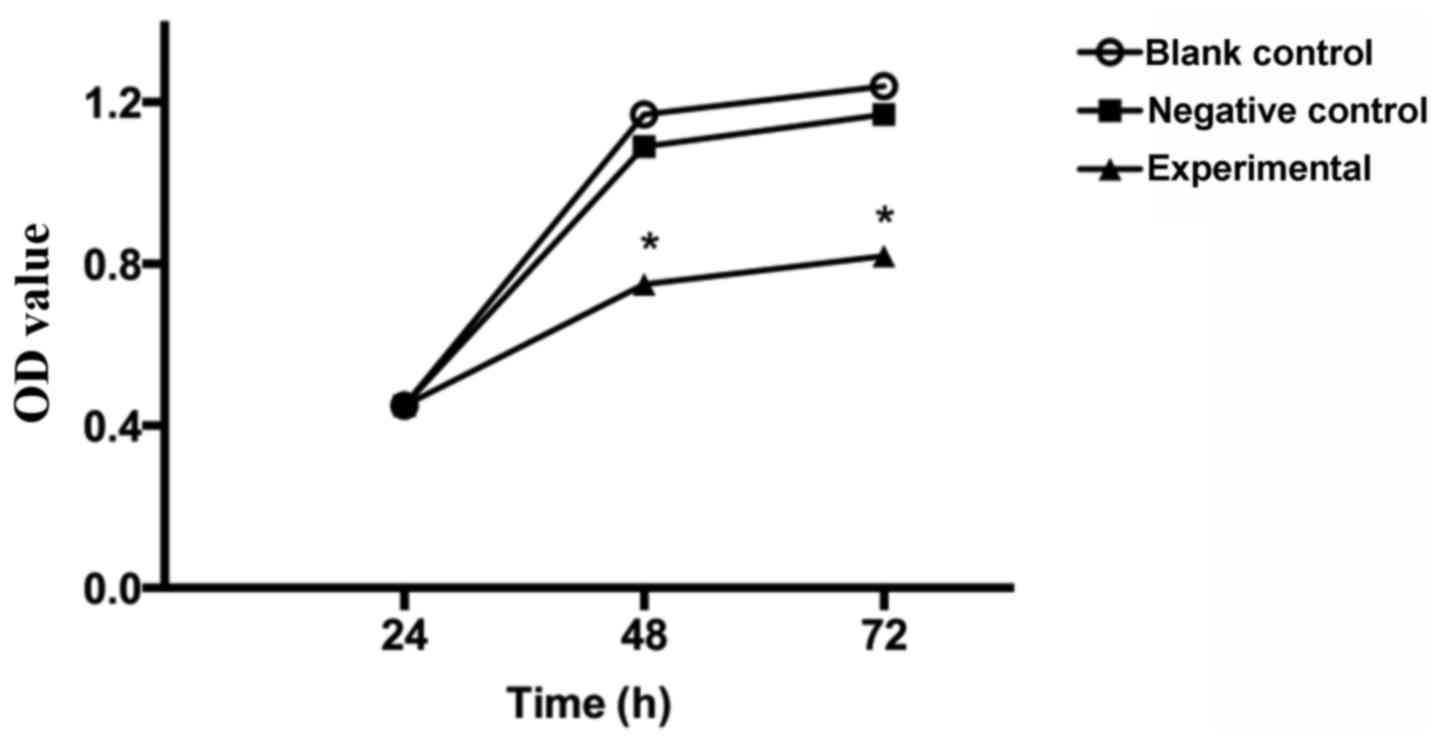
control groups. The MTT assay revealed that after 24 h of culture,
the experimental group grew similarly to the negative and blank
control groups (P>0.05). After 48 and 72 h of culture, however,
the growth of the experimental group significantly decreased
compared with both groups (P<0.05), but growth of the other two
groups did not differ significantly from each other (P>0.05)
(Fig. 4). Therefore, overexpression
of the KLF4 gene was able to inhibit the growth of breast cancer
cells.
Discussion
The human KLF4 gene, which is 5,631 bp long and is
located on chromosome 9q31, has five exons. The KLF4 mRNA is larger
than this by ~3.5 kb, and its sequence contains 1,876 nucleotides
(12). In addition, the KLF4
protein, which weighs 54,671 Da and comprises 513 amino acid
residues, contains three C2H2 zinc-finger motifs (13). By directly activating or inhibiting
the transcription of downstream genes, KLF4 is involved in cell
cycle regulation, apoptosis, metabolism and stem cell self-renewal.
As a regulatory factor for cell proliferation, KLF4 both induces
and inhibits tumor formation. KLF4 is expressed at a low level in
many types of human malignant tumors accompanied by
hypermethylation and loss of heterozygosity (14,15) and
has inhibitory effects on gastric (16), colorectal (17), bladder (18) and lung (19) cancer. However, it is highly expressed
in ductal carcinoma in situ and in oral squamous cell
carcinoma compared with those in normal tissues (20,21). For
example, KLF4 overexpression leads to squamous cell carcinoma by
inducing hyperplasia and dysplasia (22). Wei et al (23) identified that the expression of KLF4
mRNA and protein were upregulated in metastatic pancreatic and
human pancreatic cancer tissues. Thus, differences in the
expression of the KLF4 gene in various tumors may be associated
with tissue specificity.
In the present study, low levels of the KLF4 protein
were expressed in breast cancer tissues (39.0%, 93/239) but high
levels were expressed in paracancerous tissues (77.5%, 31/40). The
KLF4 protein expression in breast cancer tissues was negatively
correlated with histological grade and lymphatic metastasis.
Furthermore, the expression rates of KLF4 protein in early invasive
carcinoma and infiltrating ductal carcinomas invasive carcinoma of
special type were significantly higher than those in other types of
carcinoma, which accounts for ~80% of all breast cancer cases, with
low degree of differentiation and poor prognosis (24). Hence, the KLF4 gene was negatively
correlated with malignant behaviors of breast cancer, implying that
this gene participated in several intracellular events and markedly
suppressed the onset and progression of this type of cancer.
In order to clarify the role of the KLF4 gene in
breast cancer, MDA-MB-231 cells were transiently transfected with a
constructed eukaryotic expression vector called pcDNA3.1-KLF4.
Western blotting demonstrated that the KLF4 protein, similar to
KLF4 mRNA, was only expressed in the experimental group, suggesting
that transcription of this gene enhanced target gene transcription
in addition to protein translation. Additionally, the MTT assay
demonstrated that the growth of the experimental group was
significantly inhibited, indicating that KLF4 gene expression
suppressed the proliferation of breast cancer cells. Given that the
two control groups had similar outcomes, the vector did not affect
cell proliferative capacity per se. Similarly to the
inhibitory effects of the KLF4 gene on breast cancer progression,
Yori et al (15) revealed
that KLF4 overexpression in human breast cancer MDA-MB-231 cells
upregulated the protein and mRNA levels of E-cadherin, which were
decreased by interfering with its expression. Yori et al
(25) also reported that KLF4
inhibited the invasion and distal metastasis of breast cancer by
suppressing epithelial-mesenchymal transition.
Regardless, the influence of KLF4 on breast cancer
remains controversial. Foster et al (22) demonstrated that KLF4 expression was
increased in 6 out of 8 breast cancer cell lines, and in 70% of
breast cancer tissue samples. Meanwhile, KLF4 was similarly
expressed in ductal carcinoma in situ and invasive
carcinoma, indicating that it may be associated with the early
onset of breast cancer. Furthermore, KLF4 can maintain a high
glycolytic metabolism through transcriptional activation of human
platelet phosphofructokinase, thereby predominantly controlling the
proliferation of breast cancer cells (26). It is likely that KLF4 also functions
as an oncogene; whether KLF4 is an oncogene or a tumor suppressor
may depend on the histological type and microenvironment. Serum
starvation (27), oxidative stress
(28) and interferon-γ (29) may induce the production of KLF4, the
expression of which can be downregulated by the hypermethylation
and loss of heterozygosity in the promoter region (30). Accordingly, the role of KLF4 requires
additional in-depth studies.
In summary, the positive expression rates of the
KLF4 protein in breast cancer tissues significantly decreased, and
transfection of the KLF4 gene significantly inhibited the
proliferation of breast cancer cells, revealing that the KLF4 gene
was crucial in the onset and progression of this type of cancer.
The observations herein may allow the KLF4 gene to act as a novel
target for the molecular diagnosis and gene therapy used to treat
breast cancer.
References
|
1
|
Zeng H, Zheng R, Zhang S, Zou X and Chen
W: Female breast cancer statistics of 2010 in China: Estimates
based on data from 145 population-based cancer registries. J Thorac
Dis. 6:466–470. 2014.PubMed/NCBI
|
|
2
|
Youlden DR, Cramb SM, Dunn NA, Muller JM,
Pyke CM and Baade PD: The descriptive epidemiology of female breast
cancer: An international comparison of screening, incidence,
survival and mortality. Cancer Epidemiol. 36:237–248. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen WQ, Zheng RS, Zhang SW, Li N, Zhao P,
Li GL, Wu LY and He J: Report of incidence and mortality in china
cancer registries, 2008. Chin J Cancer Res. 24:171–180. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galvão ER, Martins LM, Ibiapina JO,
Andrade HM and Monte SJ: Breast cancer proteomics: A review for
clinicians. J Cancer Res Clin Oncol. 137:915–925. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue YK, Tan J, Dou DW, Chen D, Chen LJ,
Ren HP, Chen LB, Xiong XG and Zheng H: Effect of Kruppel-like
factor 4 on Notch pathway in hepatic stellate cells. J Huazhong
Univ Sci Technolog Med Sci. 36:811–816. 2016.PubMed/NCBI
|
|
6
|
Mahatan CS, Kaestner KH, Geiman DE and
Yang VW: Characterization of the structure and regulation of the
murine gene encoding gut-enriched Krüppel-like factor (Krüppel-like
factor 4). Nucleic Acids Res. 27:4562–4569. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rowland BD and Peeper DS: KLF4, p21 and
context-dependent opposing forces in cancer. Nat Rev Cancer.
6:11–23. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin ZS, Chu HC, Yen YC, Lewis BC and Chen
YW: Correction: Krüppel-like factor 4, a tumor suppressor in
hepatocellular carcinoma cells reverts epithelial mesenchymal
transition by suppressing slug expression. PLoS One.
11:e01541682016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Wang J, Xiao W, Xia D, Lang B, Yu G,
Guo X, Guan W, Wang Z, Hu Z, et al: Epigenetic alterations of
Krüppel-like factor 4 and its tumor suppressor function in renal
cell carcinoma. Carcinogenesis. 34:2262–2270. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao F, Wang K, Zhu R, Hu YW, Fang WZ and
Ding HZ: Clinicopathological significance of reduced SPARCL1
expression in human breast cancer. Asian Pac J Cancer Prev.
14:195–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bauer KR, Brown M, Cress RD, Parise CA and
Caggiano V: Descriptive analysis of estrogen receptor
(ER)-negative, progesterone receptor (PR)-negative, and
HER2-negative invasive breast cancer, the so-called triple-negative
phenotype: A population-based study from the California cancer
registry. Cancer. 109:1721–1728. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Evans PM and Liu C: Roles of Krüpel-like
factor 4 in normal homeostasis, cancer and stem cells. Acta Biochim
Biophys Sin (Shanghai). 40:554–564. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yet SF, McA'Nulty MM, Folta SC, Yen HW,
Yoshizumi M, Hsieh CM, Layne MD, Chin MT, Wang H, Perrella MA, et
al: Human EZF, a Krüppel-like zinc finger protein, is expressed in
vascular endothelial cells and contains transcriptional activation
and repression domains. J Biol Chem. 273:1026–1031. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoon HS, Chen X and Yang VW: Kruppel-like
factor 4 mediates p53-dependent G1/S cell cycle arrest in response
to DNA damage. J Biol Chem. 278:2101–2105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yori JL, Johnson E, Zhou G, Jain MK and
Keri RA: Kruppel-like factor 4 inhibits epithelial-to-mesenchymal
transition through regulation of E-cadherin gene expression. J Biol
Chem. 285:16854–16863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei D, Gong W, Kanai M, Schlunk C, Wang L,
Yao JC, Wu TT, Huang S and Xie K: Drastic down-regulation of
Krüppel-like factor 4 expression is critical in human gastric
cancer development and progression. Cancer Res. 65:2746–2754. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Choi BJ, Cho YG, Song JW, Kim CJ, Kim SY,
Nam SW, Yoo NJ, Lee JY and Park WS: Altered expression of the KLF4
in colorectal cancers. Pathol Res Pract. 202:585–589. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohnishi S, Ohnami S, Laub F, Aoki K,
Suzuki K, Kanai Y, Haga K, Asaka M, Ramirez F and Yoshida T:
Downregulation and growth inhibitory effect of epithelial-type
Krüppel-like transcription factor KLF4, but not KLF5, in bladder
cancer. Biochem Biophys Res Commun. 308:251–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu W, Hofstetter WL, Li H, Zhou Y, He Y,
Pataer A, Wang L, Xie K, Swisher SG and Fang B: Putative
tumor-suppressive function of Kruppel-like factor 4 in primary lung
carcinoma. Clin Cancer Res. 15:5688–5695. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen YJ, Wu CY, Chang CC, Ma CJ, Li MC and
Chen CM: Nuclear Krüppel-like factor 4 expression is associated
with human skin squamous cell carcinoma progression and metastasis.
Cancer Biol Ther. 7:777–782. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tai SK, Yang MH, Chang SY, Chang YC, Li
WY, Tsai TL, Wang YF, Chu PY and Hsieh SL: Persistent Krüppel-like
factor 4 expression predicts progression and poor prognosis of head
and neck squamous cell carcinoma. Cancer Sci. 102:895–902. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Foster KW, Liu Z, Nail CD, Li X,
Fitzgerald TJ, Bailey SK, Frost AR, Louro ID, Townes TM, Paterson
AJ, et al: Induction of KLF4 in basal keratinocytes blocks the
proliferation-differentiation switch and initiates squamous
epithelial dysplasia. Oncogene. 24:1491–1500. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei D, Wang L, Kanai M, Jia Z, Le X, Li Q,
Wang H and Xie K: KLF4α up-regulation promotes cell cycle
progression and reduces survival time of patients with pancreatic
cancer. Gastroenterology. 139:2135–2145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rakha EA, Reis-Filho JS, Baehner F, Dabbs
DJ, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani
SR, et al: Breast cancer prognostic classification in the molecular
era: The role of histological grade. Breast Cancer Res. 12:2072010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yori JL, Seachrist DD, Johnson E, Lozada
KL, Abdul-Karim FW, Chodosh LA, Schiemann WP and Keri RA:
Krüppel-like factor 4 inhibits tumorigenic progression and
metastasis in a mouse model of breast cancer. Neoplasia.
13:601–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moon JS, Kim HE, Koh E, Park SH, Jin WJ,
Park BW, Park SW and Kim KS: Krüppel-like factor 4 (KLF4) activates
the transcription of the gene for the platelet isoform of
phosphofructokinase (PFKP) in breast cancer. J Biol Chem.
286:23808–23816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen ZY, Wang X, Zhou Y, Offner G and
Tseng CC: Destabilization of Krüppel-like factor 4 protein in
response to serum stimulation involves the ubiquitin-proteasome
pathway. Cancer Res. 65:10394–10400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cullingford TE, Butler MJ, Marshall AK, el
L Tham, Sugden PH and Clerk A: Differential regulation of
Krüppel-like factor family transcription factor expression in
neonatal rat cardiac myocytes: Effects of endothelin-1, oxidative
stress and cytokines. Biochim Biophys Acta. 1783:1229–1236. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen ZY, Shie J and Tseng C: Up-regulation
of gut-enriched krüppel-like factor by interferon-gamma in human
colon carcinoma cells. FEBS Lett. 477:67–72. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei D, Kanai M, Huang S and Xie K:
Emerging role of KLF4 in human gastrointestinal cancer.
Carcinogenesis. 27:23–31. 2006. View Article : Google Scholar : PubMed/NCBI
|