Introduction
Diabetic nephropathy (DN), one of the most serious
microvascular complications of diabetes mellitus, is the leading
cause of end-stage renal disease in developed countries and major
cause of diabetes-related death (1,2). It is
characterized by kidney hypertrophy, glomerulus and tubular
basement membrane thickening, tubular interstitial fibrosis and
arteriosclerosis (3,4), which is largely due to mesangial cell
proliferation, extracellular matrix (ECM) deposition. Therefore,
searching for effective methods of inhibiting mesangial cell
proliferation and ECM accumulation may be of great clinical
importance for intervention in DN.
Hyperglycemia plays a central role in the
development and progression of DN (5). High glucose (HG) promotes mesangial
cell proliferation and fibronectin expression in vitro. HG
induces renal mesangial cell proliferation and fibronectin
expression through JNK/nuclear factor (NF)-κB/NADPH oxidase/ROS
pathway (6). HG induced mesangial
cell proliferation and arrested cell cycle progress through
upregulation of p-p38MAPK (7).
Mesangial cells also play a role in the synthesis, as well as the
degradation of the ECM, which is mediated by proteinases such as
matrix metalloproteinases (MMPs), including type IV collagen,
fibronectin and proteoglycans (8).
Increased levels of both MMP-2 and MMP-9 have been demonstrated in
serum from patients with type 1 diabetes (9), and the level of urine MMP-9 was
correlated with the degree of albuminuria (10).
ATP-sensitive potassium (KATP) channels were first
identified in cardiac cell (11),
inhibited by intracellular ATP and activated by MgADP (12), and coupled cellular energy status to
electrical activity playing a critical role in regulating numerous
cellular functions, such as cardiac preconditioning,
vasodilatation, neuroprotection (13) as well as glucose homeostasis by
regulating insulin secretion (14).
High glucose conditions have led to the recruitment of KATP
channels to the β-cell plasma membrane in a Ca2+ and
PKA-dependent manner, resulting in an increase in KATP currents
(15), whereas a protein kinase C
activator facilitated endocytic trafficking of KATP, resulting in
decreased KATP currents (16). At
molecular level, the KATP channel is composed of subunits from a
combination of inwardly rectifying K+ channels (Kir6.1
or Kir6.2) and sulfonylurea receptors (SUR1, SUR2A and SUR2B). KATP
channel closure occur by binding to the pore-forming subunit
Kir6.2, yet activate channel opening by interacting with the
regulatory subunit SUR in a Mg2+-dependent manner
(17). It is believed that different
Kir6 combine with different SUR to form the various native KATP
channels (18). Different KATP
channels exhibit different pharmacological profiles dictated by the
SUR subtypes they are composed of. However, the existence subtypes
and role of KATP channels in DN is still not well defined.
In the present study, we investigated the effects of
HG on cell proliferation and release of MMP-2 and FN in rat
mesangial cells, and elucidated the role of KATP channels in
HG-induced DN.
Materials and methods
Isolation and culture of mesangial
cells
The experiments were performed in accordance with
the Animal Ethics Committee of the Second Military Medical
University. Primary rat mesangial cells were harvested from male
Sprague-Dawley rat (150–200 g) (the Second Military Medical
University Animal Center, Shanghai, China) by filtration with
ice-cold 0.9% NaCl solution through a 200-, 120- and 80-µm nylon
mesh. Those retained on the sieve were collected, washed by
centrifugation (4°C; 800 × g; 6 min), and incubated with 4 mg/ml
collagenase type IV for 10 min at 37°C under constant, gentle
shaking. Mesangial cells were placed on 25-cm2 culture
flasks in Dulbecco's modified Eagle's medium (DMEM)/F12
(Hyclone-Thermo Fisher Scientific, Inc., San Jose, CA, USA) at 37°C
in humidified 5% CO2-95% air. The culture media was not
changed for the first 7 days. Thereafter, the medium was changed
every other day until confluence and passages 11 to 17 were used in
this study. Mesangial cells at ~80% confluence were cultured in 1%
fetal bovine serum (FBS) DMEM for 24 h for synchronization, and
then were exposed to low glucose (5.6 mM), high glucose (30 mM),
with or without the pretreatment of diazoxide (DZX) (50 µM) or
5-hydroxydecanoate (5-HD) (200 µM). The cell phenotypes were
determined by light microscopy.
Quantitative real-time PCR
Total RNA was extracted and purified from
1×106 cells using QIAzol reagent (Qiagen, Valencia, CA,
USA). Total RNA (1 µg) was reverse-transcribed into cDNA according
to the instructions of PrimeScript RT Master Mix (Takara Bio Inc.,
Shiga, Japan). Real-time PCR was performed to measure the mRNA
levels of Kir6.1, Kir6.2, SUR1, SUR2A, SUR2B and GAPDH, an internal
control, for each sample in separate wells in duplicate on an
ABI-7300 Sequence Detection system using 2 µl of cDNA, 300 nM
primers and SYBR-Green PCR Master Mix (all from Applied Biosystems,
Foster City, CA, USA). The primers were: Kir6.1 forward,
5′-TTGGAGGGAGAATGATGAC-3′ and reverse, 5′-CATTACGGACCGCAATTAC-3′;
Kir6.2 forward, 5′-CCACGACAGGATAAGTTTACC-3′ and reverse,
5′-TCTCAGTGTTTGCCCAATG-3′; SUR1 forward, 5′-GGTTCGGTCCACTGTCAAG-3′
and reverse, 5′-GTTGTCAGCGTCTCCATCC-3′; SUR2A forward,
5′-GGAGCAATCCAGACCAAGAT-3′ and reverse, 5′-AGCCAGCAGATGATGACA-3′;
SUR2B forward, 5′-ACCTGCTCCAGCACAAGAAT-3′ and reverse,
5′-TCTCTTCATCACATTGACCAGG-3′; GAPDH forward,
5′-GTCGGTGTGAACGGATTTG-3′ and reverse, 5′-TCCCATTCTCAGCCTTGAC-3′.
The relative gene expression levels are presented as
2−ΔΔCq (19).
Subcellular localization of SUR
subunit of KATP
Cell slices were washed three times with
phosphate-buffered saline (PBS) and fixed with 4% methanol for 30
min prior to PBS washed twice, and then cell were cultured in
serum-free DMEM medium containing 1 µM BODIPY-glibenclamide at 37°C
for 30 min in the dark and 5 µM DiIC18(3) at 37°C for 10 min in the dark. After
incubation, cells were washed three times with PBS and DAPI
Fluoromount-G was added at 37°C for 5 min in the dark. Laser
scanning confocal microscopy was used to determine the fluorescence
signal, magnification, ×600.
Cell proliferation assay
Cells were seeded at 104 cells/well in
96-well plates. After 24-h incubation, the media was removed and
replaced with serum-free medium for 24-h incubation. Plates were
then treated with high glucose (10, 20, 30 or 40 mM) for another 12
or 24 h. Then 10-µl Cell Counting Kit-8 (CCK-8) solution was added
to each well and incubation continued at 37°C for another 1 h. Cell
proliferation was determined by scanning with a microplate reader
(model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 450
nm.
ELISA
Cell culture supernatants from different treatment
groups were harvested and centrifuged at 800 × g for 20 min. After
centrifugation, the supernatants were then assayed for MMP-2 and
fibronectin using the enzyme-linked immunosorbent assay (ELISA)
kits (Wuhan Boster Biological Engineering Co., Ltd., Wuhan, China).
The absorbance was read at 450 nm with an ELx800 microplate (BioTek
Instruments, Inc., Winooski, VT, USA).
Statistical analysis
All investigations were performed in triplicate. The
data were assessed by SPSS version 11.5 (IBM SPSS, Armonk, NY,
USA). All values were expressed as mean ± SD. Statistical analyses
of data were performed by one-way analysis of variance (ANOVA) and
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
KATP subunit expression and
subcellular localization
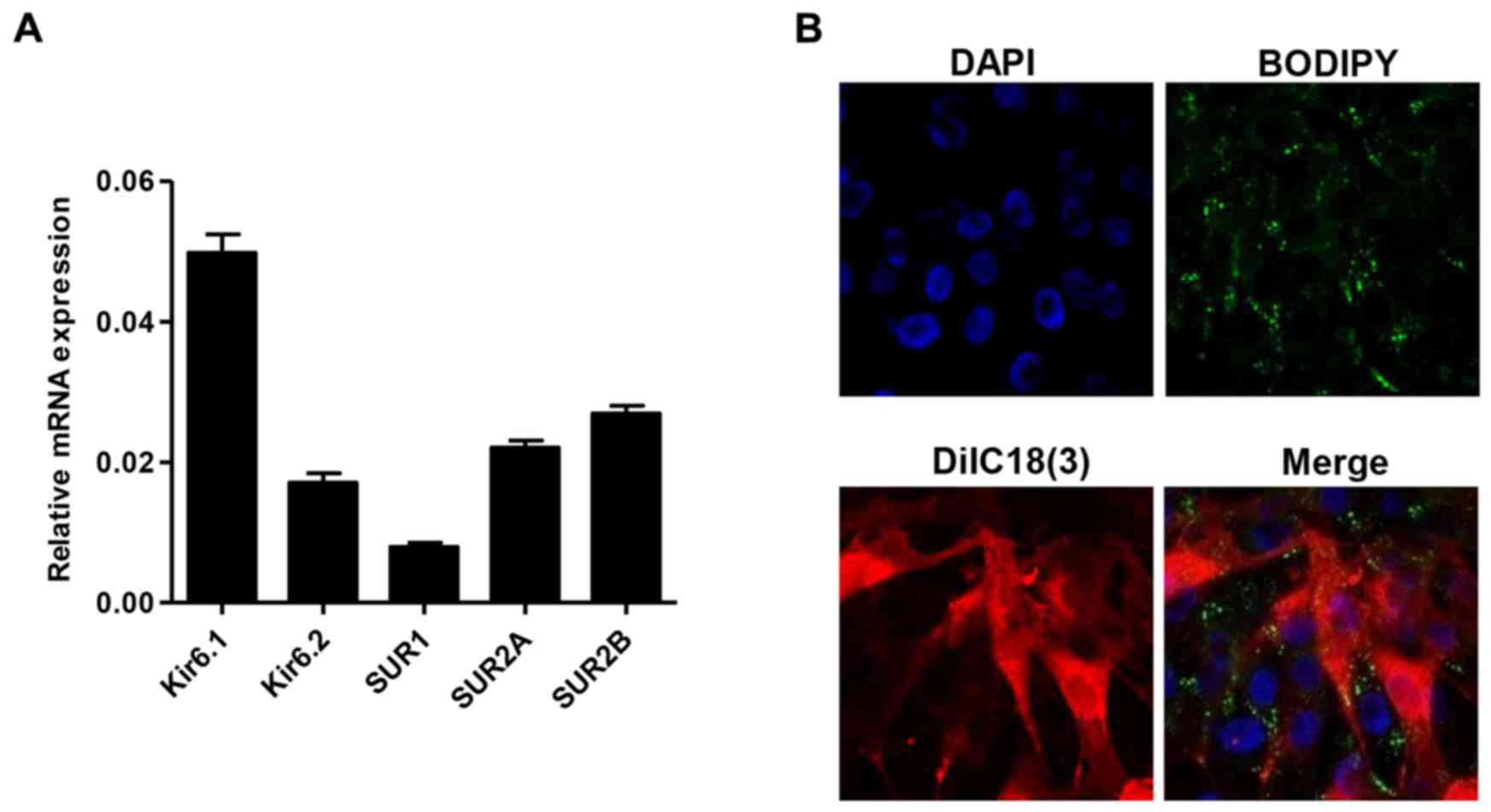
To investigate the role of KATP in mesangial cells,
the expression of KATP subunit, including Kir6.1, Kir6.2, SUR1,
SUR2A and SUR2B, was measured by quantitative real-time PCR. As
shown in Fig. 1A, in all detected
KATP subunit, the highest mRNA expression was detected in Kir6.1
and the lowest mRNA expression was detected in SUR1. Moreover, the
expression of SUR in plasma membranes was also found using a laser
scanning confocal microscopy incubated with BODIPY-glibenclamide
and DiIC18(3) (Fig. 1B).
High glucose increases mesangial cell
proliferation and release of MMP-2 and fibronectin
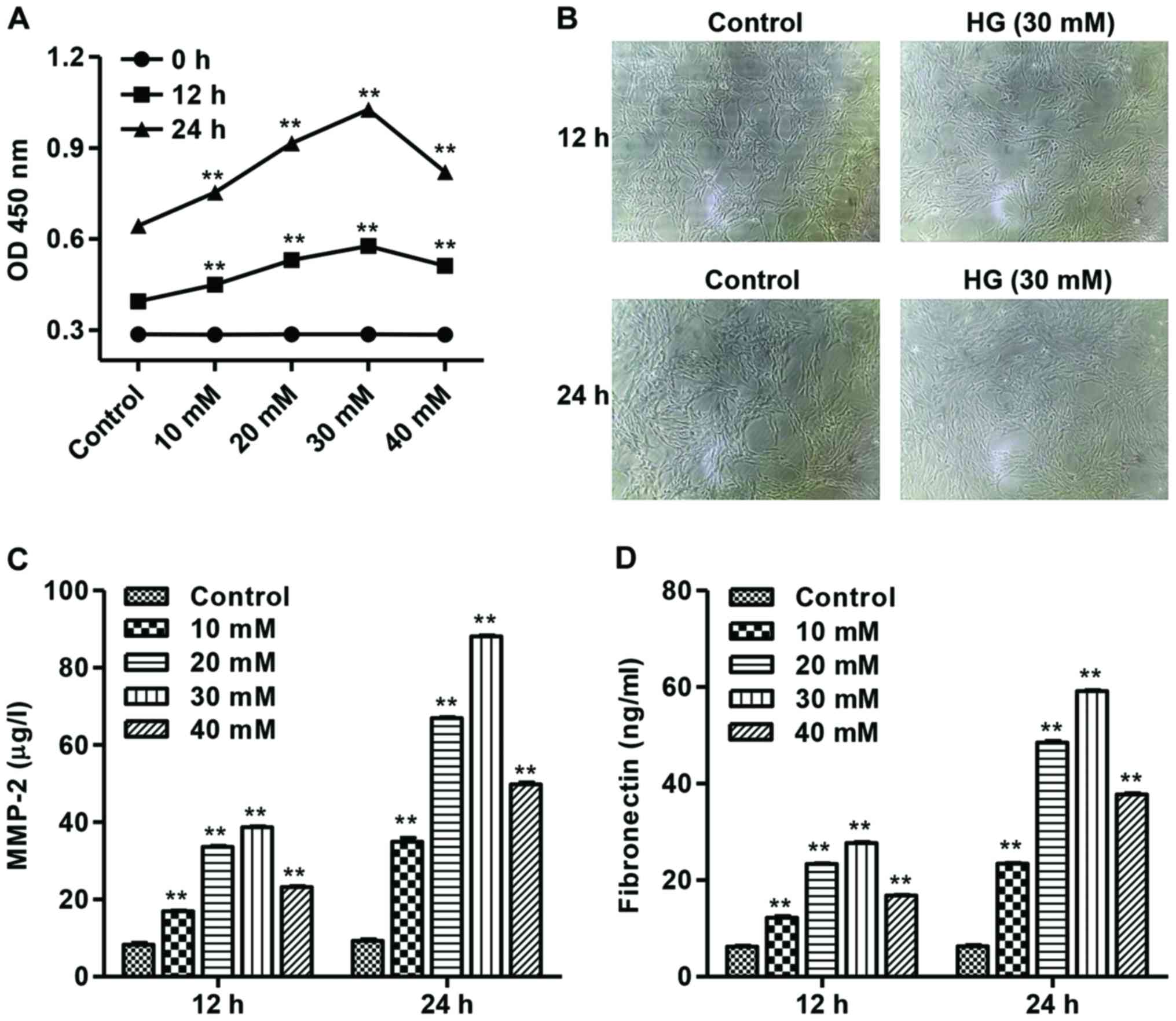
After different concentrations of high glucose (HG,
10, 20, 30 or 40 mM) treatment in mesangial cells, the cell
proliferation was measured by CCK-8 assay. As shown in Fig. 2A, HG with concentration ranges from
10 to 30 mM significantly increased mesangial cell proliferation in
a dose-dependent manner in 12 and 24 h, while 40 mM HG inhibits
mesangial cell proliferation, compared with control mesangial
cells. The phenotypes of mesangial cells in response to 30 mM HG
for 12 and 24 h are shown in Fig.
2B.
Furthermore, to investigate the effect of HG on
matrix components, the production of MMP-2 and fibronectin was
measured by ELISA. Our results showed that HG with concentration
ranges from 10 to 30 mM significantly increased MMP-2 and
fibronectin production in a dose-dependent manner, while 40 mM HG
inhibits MMP-2 and fibronectin production, compared with control
mesangial cells (Fig. 2C and D).
DXZ inhibits high glucose-induced KATP
subunit expression
To investigate the effect of HG on KATP subunit, the
expression of Kir6.1, Kir6.2, SUR1, SUR2A and SUR2B was detected.
We found that 30 mM HG significantly decreased the mRNA expression
of Kir6.1, SUR2A and SUR2B, but had no effect on Kir6.2 and SUR1
expression compared with controls (Fig.
3A). These results suggest that HG induced mesangial cell
proliferation and MMP-2 and fibronectin production may be through
inhibiting KATP channel activity. Therefore, a selective opener of
KATP (DZX) and a selective inhibitor of KATP (5-HD) were also
introduced in our study. As shown in Fig. 3B, DZX significantly decreased the
mRΝA expression of SUR1, SUR2A and SUR2B in HG-induced mesangial
cells. However, the changes in SUR1, SUR2A and SUR2B mRNA
expression were significantly reversed by 5-HD. Whereas, DZX or
5-HD treatment had no effect on the mRNA expression of Kir6.2 KATP
subunit (data not shown).
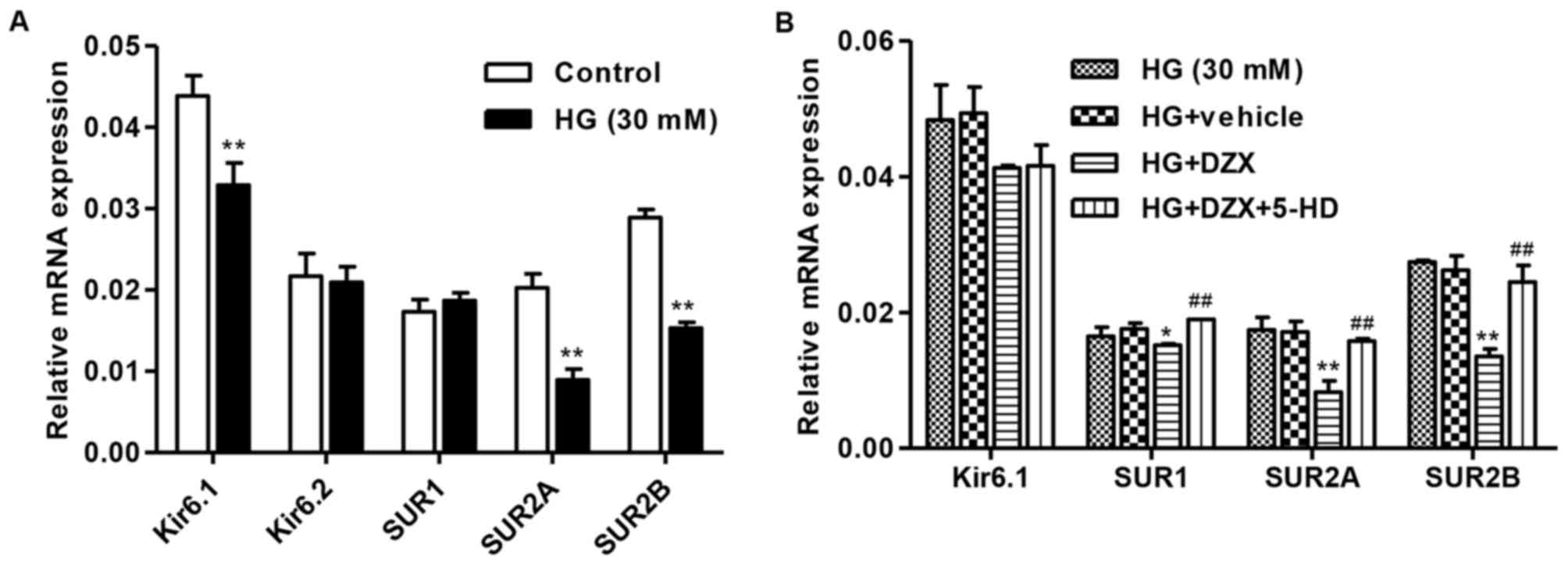 | Figure 3.DZX inhibits HG-induced KATP subunit
expression. Rat mesangial cells were cultured with 30 mM HG for 24
h. (A) The mRNA expression of KATP subunit, including Kir6.1,
Kir6.2, SUR1, SUR2A and SUR2B, was measured by quantitative
real-time PCR. Rat mesangial cells were cultured with DZX or/and
5-HD prior to 30 mM HG for 24 h. **P<0.01 compared with the
control. (B) The mRNA expression of KATP subunit, including Kir6.1,
SUR1, SUR2A and SUR2B, was measured by quantitative real-time PCR.
*P<0.05, **P<0.01 compared with HG; ##P<0.01
compared with HG+DZX. DZX, diazoxide; KATP, ATP-sensitive
potassium. |
DXZ inhibits high glucose-induced
mesangial cell proliferation and MMP-2 and fibronectin
production
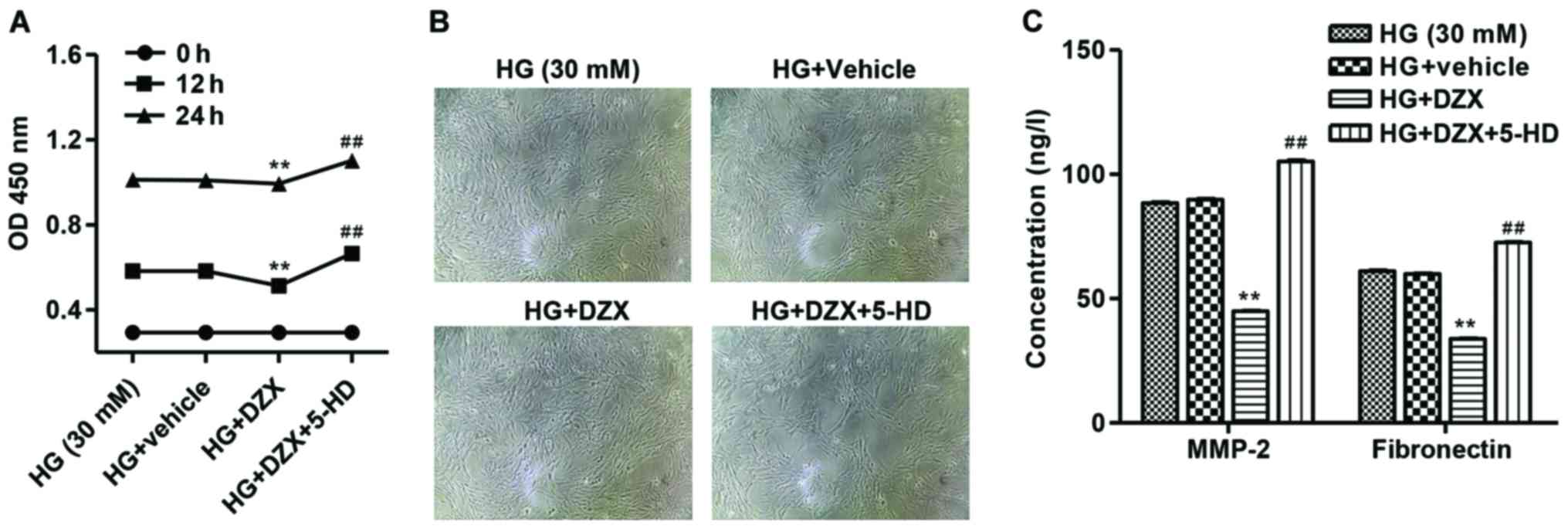
As shown in Fig. 4A and
B, DZX significantly decreased mesangial cell proliferation,
while 5-HD increased mesangial cell proliferation under HD
condition at 12 and 24 h with DZX pretreatment. Additionally, DZX
significantly decreased the production of MMP-2 and fibronectin in
HG-induced mesangial cells (Fig.
4C). However, the changes in MMP-2 and fibronectin production
were significantly reversed by 5-HD.
Discussion
The KATP channel serves as a metabolic sensor,
coupling cellular metabolism to electrical activity in a wide range
of tissues. Opening of KATP channels under conditions of low
metabolism leads to membrane hyperpolarization and switches off
cellular functions, and close when metabolism increases, producing
a membrane depolarization that leads to cellular responses such as
hormonal secretion, neurotransmitter release and contraction,
conversely (14). A previous result
has indicated that KATP channels seem to play an essential role in
murine myometrial motility via activation of SUR2B and Kir6.2
(20). Moreover, the main KATP
channel in cardiomyocytes is composed of Kir6.2/SUR2A, and the
primary role of KATP channels in heart is cardioprotection under
metabolic stress, such ischemia, anoxia or metabolic inhibition
(21,22). Despite the large amount of evidence
confirming the role of KATP channels in cardioprotection during
metabolic stress, the SUR1−/− and SUR2−/−
knockout mice were found to be more tolerant of global
ischemia-reperfusion than control mice (23,24).
However, the roles of KATP channels in HG-induced DN are not
elucidated. In the present study, we found that activating KATP
channels significantly suppressed HG-induced cell proliferation and
release of MMP-2 and fibronectin in cultured mesangial cells.
Several clinical studies have demonstrated that
elevated blood glucose level was a risk for the development of
microvascular complications of diabetes, including DN. Increased
mesangial cell proliferation and excessive accumulation of ECM
proteins synthesized by mesangial cells are major pathologic
features in the early stage of DN (25,26). Our
results showed that HG enhanced mesangial cell proliferation and
release of MMP-2 and fibronectin in a dose-dependent manner range
from 10 to 30 mM. As one of the most important ECM proteins,
glucose-induced fibronectin expression in MCs results in the
accelerated progression of glomerulosclerosis (27). Previous studies have implied that
MMPs play an important role in regulating physiological homeostasis
and pathological disorders of the kidney through modulating the
decomposition of ECM components, including fibronectin (28).
Most studies have focused on direct regulation, such
as changes in the open probability or the ATP sensitivity of the
channels (29), but little is known
about how channel numbers at the surface membrane are regulated.
Our results demonstrated that all the subunits of KATP were
expressed in rat mesangial cell, and the SUR subunit was observed
in plasma membranes using a laser scanning confocal microscopy
incubated with BODIPY-glibenclamide and DiIC18 (3). A previous study demonstrated that KATP
channels play a role in the cardioprotective effects of
H2S against HG-induced injury, in which treatment of the
cells with HG markedly decreased the expression level of KATP
channels (30). Our results showed
that the expression of KATP subunits, including Kir6.1, SUR2A and
SUR2B, was also decreased by HG stimulation. Thus, we hypothesized
that the inhibition of KATP channels is a critical mechanism which
underlies HG-induced renal injury. To confirm this hypothesis, we
observed the influence of KATP channel activation on HG-induced
injury. One promising strategy is the use of DZX (a mitochondrial
KATP channel opener) and a mitochondrial KATP channel blocker
(5-HD). We found that DZX pretreatment inhibited HG-induced SUR1,
SUR2A and SUR2B expression and 5-HD pretreatment inhibited
DZX-induced decreased expression of SUR1, SUR2A and SUR2B.
Importantly, DZX decreased cell proliferation and the release of
MMP-2 and fibronectin, while 5-HD inhibited the effects of DZX in
HG-induced mesangial cells.
In conclusion, the present study provides novel
evidence that the impairment of KATP channels is associated with
HG-induced multiple renal injuries, including increased mesangial
cell proliferation and excessive accumulation of ECM proteins.
Taken together, we could conclude that one of the molecular
mechanisms involved in renal protective effects was activation of
KATP channels. Our results also provided a potential target in
treatment of DN.
References
|
1
|
Aghadavod E, Khodadadi S, Baradaran A,
Nasri P, Bahmani M and Rafieian-Kopaei M: Role of oxidative stress
and inflammatory factors in diabetic kidney disease. Iran J Kidney
Dis. 10:337–343. 2016.PubMed/NCBI
|
|
2
|
John S: Complication in diabetic
nephropathy. Diabetes Metab Syndr. 10:247–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kolset SO, Reinholt FP and Jenssen T:
Diabetic nephropathy and extracellular matrix. J Histochem
Cytochem. 60:976–986. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ruster C and Wolf G: The role of
chemokines and chemokine receptors in diabetic nephropathy. Front
Biosci. 13:944–955. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kikkawa R, Koya D and Haneda M:
Progression of diabetic nephropathy. Am J Kidney Dis. 41 Suppl
1:S19–S21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang L, Pang S, Deng B, Qian L, Chen J,
Zou J, Zheng J, Yang L, Zhang C, Chen X, et al: High glucose
induces renal mesangial cell proliferation and fibronectin
expression through JNK/NF-κB/NADPH oxidase/ROS pathway, which is
inhibited by resveratrol. Int J Biochem Cell Biol. 44:629–638.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Liu W, Wang Q, Liu P, Deng Y, Lan T,
Zhang X, Qiu B, Ning H and Huang H: Emodin suppresses cell
proliferation and fibronectin expression via p38MAPK pathway in rat
mesangial cells cultured under high glucose. Mol Cell Endocrinol.
307:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan RJ and Liu Y: Matrix
metalloproteinases in kidney homeostasis and diseases. Am J Physiol
Renal Physiol. 302:F1351–F1361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gharagozlian S, Svennevig K, Bangstad HJ,
Winberg JO and Kolset SO: Matrix metalloproteinases in subjects
with type 1 diabetes. BMC Clin Pathol. 9:72009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson J, Wilson P, Brandewie K, Taneja
D, Schaefer L, Mitchell B and Tannock LR: Renal accumulation of
biglycan and lipid retention accelerates diabetic nephropathy. Am J
Pathol. 179:1179–1187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burke MA, Mutharasan RK and Ardehali H:
The sulfonylurea receptor, an atypical ATP-binding cassette
protein, and its regulation of the KATP channel. Circ Res.
102:164–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wheeler A, Wang C, Yang K, Fang K, Davis
K, Styer AM, Mirshahi U, Moreau C, Revilloud J, Vivaudou M, et al:
Coassembly of different sulfonylurea receptor subtypes extends the
phenotypic diversity of ATP-sensitive potassium (KATP) channels.
Mol Pharmacol. 74:1333–1344. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Teramoto N, Zhu HL, Shibata A, Aishima M,
Walsh EJ, Nagao M and Cole WC: ATP-sensitive K+ channels in pig
urethral smooth muscle cells are heteromultimers of Kir6.1 and
Kir6.2. Am J Physiol Renal Physiol. 296:F107–F117. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McTaggart JS, Clark RH and Ashcroft FM:
The role of the KATP channel in glucose homeostasis in health and
disease: more than meets the islet. J Physiol. 588:3201–3209. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang SN, Wenna ND, Yu J, Yang G, Qiu H, Yu
L, Juntti-Berggren L, Köhler M and Berggren PO: Glucose recruits
K(ATP) channels via non-insulin-containing dense-core granules.
Cell Metab. 6:217–228. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu K, Huang CS, Jan YN and Jan LY:
ATP-sensitive potassium channel traffic regulation by adenosine and
protein kinase C. Neuron. 38:417–432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen PC, Kryukova YN and Shyng SL: Leptin
regulates KATP channel trafficking in pancreatic β-cells by a
signaling mechanism involving AMP-activated protein kinase (AMPK)
and cAMP-dependent protein kinase (PKA). J Biol Chem.
288:34098–34109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Masia R, Enkvetchakul D and Nichols CG:
Differential nucleotide regulation of KATP channels by SUR1 and
SUR2A. J Mol Cell Cardiol. 39:491–501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hong SH, Kyeong KS, Kim CH, Kim YC, Choi
W, Yoo RY, Kim HS, Park YJ, Ji IW, Jeong EH, et al: Regulation of
myometrial contraction by ATP-sensitive potassium (KATP) channel
via activation of SUR2B and Kir 6.2 in mouse. J Vet Med Sci.
78:1153–1159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang H, Flagg TP and Nichols CG: Cardiac
sarcolemmal K(ATP) channels: latest twists in a questing tale! J
Mol Cell Cardiol. 48:71–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pu JL, Ye B, Kroboth SL, McNally EM,
Makielski JC and Shi NQ: Cardiac sulfonylurea receptor short
form-based channels confer a glibenclamide-insensitive KATP
activity. J Mol Cell Cardiol. 44:188–200. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Elrod JW, Harrell M, Flagg TP, Gundewar S,
Magnuson MA, Nichols CG, Coetzee WA and Lefer DJ: Role of
sulfonylurea receptor type 1 subunits of ATP-sensitive potassium
channels in myocardial ischemia/reperfusion injury. Circulation.
117:1405–1413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lefer DJ, Nichols CG and Coetzee WA:
Sulfonylurea receptor 1 subunits of ATP-sensitive potassium
channels and myocardial ischemia/reperfusion injury. Trends
Cardiovasc Med. 19:61–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z,
Ni J, Yu C, Yao T, Huang Y, et al: Rescue of mesangial cells from
high glucose-induced over-proliferation and extracellular matrix
secretion by hydrogen sulfide. Nephrol Dial Transplant.
26:2119–2126. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rysz J, Banach M, Stolarek RA, Pasnik J,
Cialkowska-Rysz A, Koktysz R, Piechota M and Baj Z: Serum matrix
metalloproteinases MMP-2 and MMP-9 and metalloproteinase tissue
inhibitors TIMP-1 and TIMP-2 in diabetic nephropathy. J Nephrol.
20:444–452. 2007.PubMed/NCBI
|
|
27
|
Wang J, Huang H, Liu P, Tang F, Qin J,
Huang W, Chen F, Guo F, Liu W and Yang B: Inhibition of
phosphorylation of p38 MAPK involved in the protection of
nephropathy by emodin in diabetic rats. Eur J Pharmacol.
553:297–303. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hopps E and Caimi G: Matrix
metalloproteinases in metabolic syndrome. Eur J Intern Med.
23:99–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tarasov AI, Girard CA and Ashcroft FM: ATP
sensitivity of the ATP-sensitive K+ channel in intact and
permeabilized pancreatic beta-cells. Diabetes. 55:2446–2454. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang W, Chen J, Mo L, Ke X, Zhang W,
Zheng D, Pan W, Wu S, Feng J, Song M, et al: ATP-sensitive K+
channels contribute to the protective effects of exogenous hydrogen
sulfide against high glucose-induced injury in H9c2 cardiac cells.
Int J Mol Med. 37:763–772. 2016.PubMed/NCBI
|