Introduction
Acute cerebral ischemia/reperfusion injury (ACIR) is
a major disease that threatens health of the patients by causing
excessive release of oxygen-free radicals and inducing oxidative
stress and inflammatory response to aggravate the neurological
injuries (1,2). Besides neurological injuries,
oxygen-free radicals affect vascular endothelium and delay the
repair of neurological tissues (3).
Spermine is a polyamine with the ability to bind to
DNA, RNA and proteinase molecules and regulate cell proliferation,
differentiation and apoptosis. Spermine is believed to be an
indispensable factor in cellular metabolism (4). In this study, using on ACIR animal
model, we investigated the effect of spermine on oxidative stress
and apoptosis and obtained excellent results.
Materials and methods
Experimental animals
Sixty healthy and clean male Sprague-Dawley rats
(provided by the Experimental Animal Center in Medical School of
Xiamen University) with a gestational age ranging from 6 to 8 weeks
and weight ranging from 250 to 270 g were used in this study.
Before starting the experiments, the rats were kept and fed for one
week in order for the animals to become acclimatized to their new
environment. Rats were housed in a temperature controlled room
(21±2°C) on a 12:12-h light/dark cycle (lights on at 6:00 a.m.).
All rats had free access to water and food. The rats were randomly
divided into 3 groups: The sham-operated group (n=12), the model
group (n=12) and the experiment group (n=36). The experiment group
was further divided into 3 subgroups: The SPE-10 group (n=12), the
SPE-25 group (n=12) and the SPE-50 group (n=12). Rats in the
experimental sub-groups SPE-10, SPE-25 and SPE-50 were injected
with 10, 25 and 50 mg/kg of spermine, respectively, one week before
the establishment of rat models. Rats in the sham-operated and
model groups were injected with 0.9% NaCl solution. Ethics approval
was obtained from the Animal Ethics Committee of Xuzhou Medical
University.
Reagents
We used Spermine (Sigma-Aldrich, St. Louis, MO,
USA); malondialdehyde (MDA) and superoxide dismutase (SOD) ELISA
kits for rats (Shanghai Gefan Biotechnology Co., Ltd., Shanghai,
China); 1% pentobarbital, 10% paraformaldehyde, 0.9% NaCl solution
(Tianjin Chemreagent Co., Ltd., Tianjin, China), rat anti-Bax and
anti-Bcl-2 monoclonal antibody, DAB coloration kits (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA).
Establishment of ACIR animal
models
Rats received an intraperitoneal injection of 1%
pentobarbital for anesthesia, and were fixed on the operating
table. The common carotid artery, internal and external carotid
arteries were isolated by a midline linear skin incision in the
neck. External carotid artery and the end part of the common
carotid artery proximal to the heart were all ligatured and the
internal carotid artery was occluded by carotid clips. After the
removal of carotid clips from internal carotid artery, the common
carotid artery was sutured. Suture thread was advanced slowly (~2.0
cm) towards the head, and suture thread and the ligatured internal
carotid artery were fixed and seamed layer by layer. After 2 h, the
suture thread was removed to establish the reperfusion model. In
the sham-operated group we only isolated the relevant vessels.
Neurological function scale
Neurological function scale assessment was performed
at 24, 48 and 72 h after establishment of the rat models according
to the Longa 5-point scale of 0–4. In this scale, 0 point was given
for normal condition without any symptoms of neurological injury; 1
point, for cases incapable of extending upper limbs; those cases
incapable of accomplishing contralateral forelimb internal rotation
received 2 points; cases incapable of accomplishing contralateral
bending motion received 3 points and those who are in coma or
unconscious received 4 points.
Effect of spermine on MDA level and
SOD activity
Seventy-two hours after establishment of the models,
the spinal cords of the rats were extracted and MDA level as well
as SOD activity were measured using ELISA kits. We closely followed
the instructions provided by the kit manufacturer to realize these
measurements. We used a microplate reader with the wavelength set
as 450 nm.
Bax and Bcl-2 protein expression
levels
Seventy-two hours after establishment of the models,
brain tissue was extracted. Tissue was ground and treated with 3
ml/g of protein lysis buffer. Samples were then centrifuged at
1×104 g for 40 min. Total protein concentration was
measured using BCA assay. For western blot analysis, protein
samples were subjected to SDS-PAGE followed by transfer to a
membrane. Each membrane was blocked and incubated with rabbit
monoclonal Bax antibody (dilution, 1:500; cat. no. ab32503) and
rabbit monoclonal Bcl-2 antibody (dilution, 1:500; cat. no.
ab32124) (Abcam, Cambridge, MA, USA) overnight. After rinsing, the
membrane was incubated with secondary goat anti-rabbit (HRP) IgG
antibody (dilution, 1:2,000; cat. no. ab6721; Abcam) for 3 h.
Finally, color developer was added and results were compared with
reference GAPDH. We used a GEL imaging system and the gray value
was calculated using ImageJ software (version X; Media Cybernetics,
Silver Springs, MD, USA). The relative gray value was calculated
as: (assayed gray value of protein/internal reference gray value of
GAPDH) × 100%.
Statistical analysis
SPSS 20.0 (IBM, Armonk, NY, USA) was used for our
statistical analysis. Measurement data are presented as mean ±
standard deviation. For intergroup comparisons we used single
factor ANOVA analysis. LSD test was used for paired comparisons and
variance analysis of repeated measurement was performed for
intragroup comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Significant increases in neurological function
scores were detected in different groups after the onset of ACIR
injuries and when compared with the sham-operated group,
differences were statistically significant (P<0.05).
Neurological function scores in all the rats decreased over time;
however rats in the experimental groups experienced a more
significant decrease in these scores. Our results revealed that
higher doses of spermine intensified the score reduction.
Differences were statistically significant (P<0.05) (Table I).
 | Table I.Neurological function scale after
onset of ACIR injuries in each group. |
Table I.
Neurological function scale after
onset of ACIR injuries in each group.
| Group | n | 24 h | 48 h | 72 h |
|---|
| Sham-operated
group | 12 | 0 | 0 | 0 |
| Model group | 12 |
3.71±0.32a |
2.84±0.30a |
2.33±0.24a |
| SPE (10 mg/kg) | 12 |
3.14±0.35b |
2.42±0.28b |
1.87±0.21b |
| SPE (25 mg/kg) | 12 |
2.85±0.31b |
2.31±0.26b |
1.74±0.22b |
| SPE (50 mg/kg) | 12 |
2.77±0.29b |
2.10±0.23b |
1.52±0.19b |
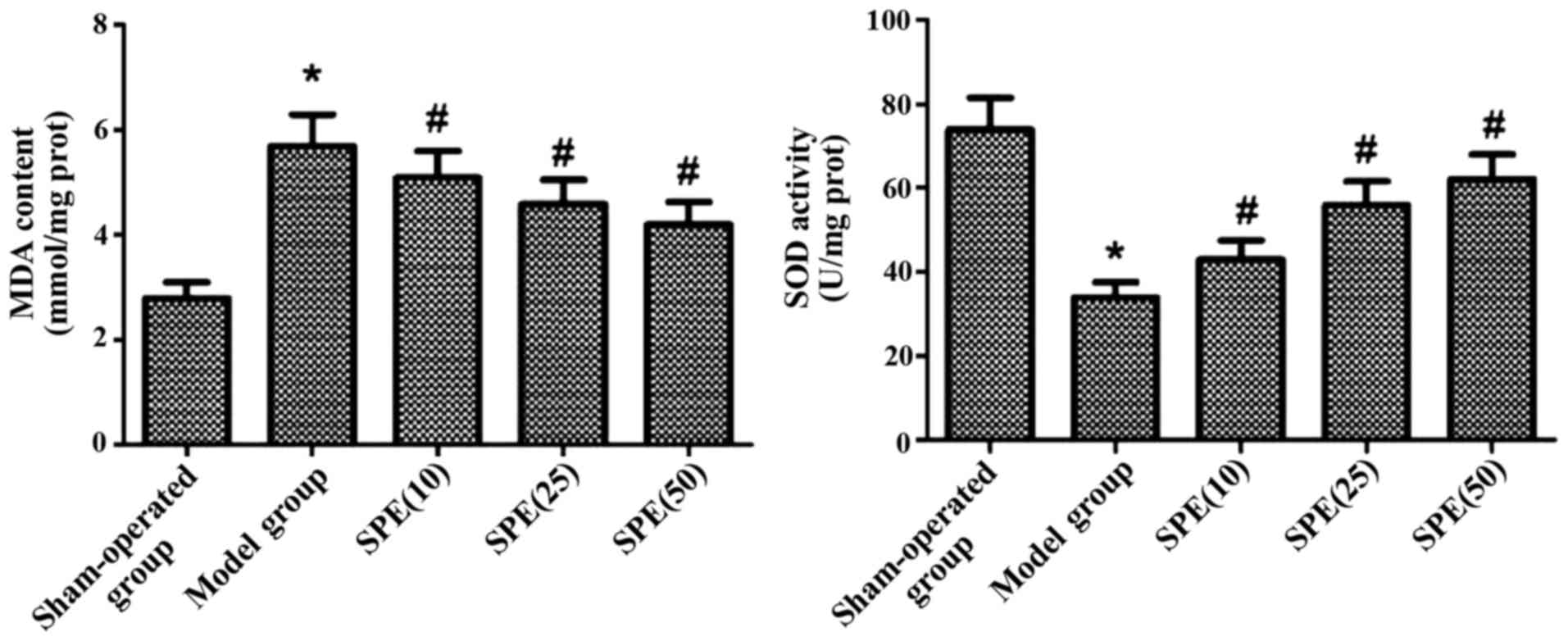
After the onset of ACIR injuries, we detected a
significant increase in MDA levels in all the rats. At the same
time, a significant decrease in SOD activity was observed in all
the rats (P<0.05). After spermine injection, MDA levels
dcereased significantly while SOD activity increased significantly.
Differences between the experimental groups and model group were
statistically significant (P<0.05). In rats injected with
different doses of spermine, the variations in MDA levels and SOD
activity were dose-dependent (P<0.05) (Fig. 1).
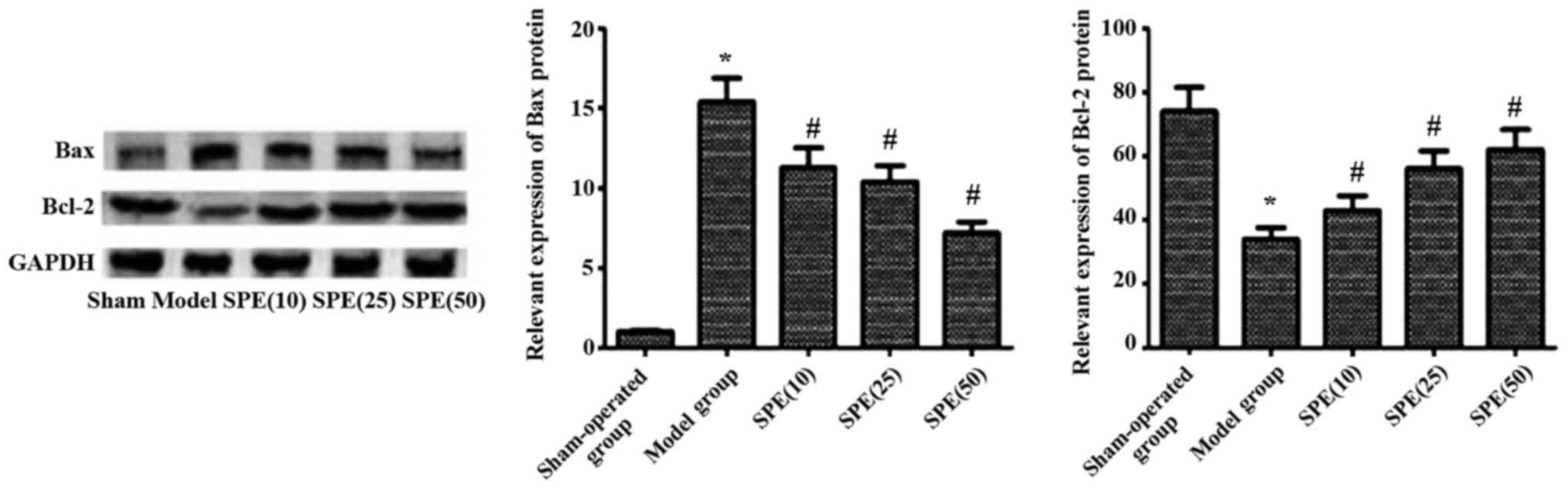
Bax protein levels increased significantly, while
Bcl-2 levels decreased significantly after the onset of ACIR
injuries (P<0.05). After spermine injection, there was a
significant drop in Bax levels, while the Bcl-2 levels increased
obviously in these rats. Differences between the experimental
groups and model group were statistically significant (P<0.05).
In rats injected with different doses of spermine, the observed
decrease in Bax levels and the increase in Bcl-2 were also
dose-dependent (P<0.05) (Fig.
2).
Discussion
Pathological and physiological mechanisms involved
in acute cerebral ischemia/reperfusion injuries are quite complex.
Results obtained from previous studies suggested that a shortage in
oxygen supply caused by acute cerebral ischemia, increased
apoptosis as well as necrosis rate in neurons (5). This condition could trigger the release
of substances such as proteolytic enzymes and reactive oxygen
species (ROS). These substances could trigger the focal oxidative
stress response. It has been shown that after the onset of
ischemia, oxidative stress response aggravates neuron injuries
(6). This is deemed to be a major
mechanism for injuries secondary to the ACIR (6). Activated ROS facilitates the oxidative
stress response induced by ACIR and further increases the
expression of neuron-related apoptosis proteins in cerebral tissues
(7). Prior studies showed that
spermine played an important role in the pathological and
physiological processes in tumors, diabetes and Alzheimer's disease
(8). Amyloid β-peptide, usually
found in brain tissues of Alzheimer patients, can activate the ROS
in neurons and induce an oxidative stress response. However,
spermine suppresses the generation of amyloid β-peptide and
protects nerves and exerts an antioxidant effect (9). Additional findings suggested that
spermine level in the ischemic region of brain was significantly
low, therefore cells in this region were more susceptible to
oxidative stress (10). Spermine can
also increase the stability of chromosomes by eradicating the
intracellular free radicals (11).
Oxidative stress response is an important pathogenesis of ACIR. MDA
level can directly reflect the level of internal oxygen radicals
and indirectly reflect the injuries caused by these free radicals
(12). SOD can protect cells from
injuries caused by oxygen-free radicals and SOD level can indicate
the activity of the oxygen-free radical endogenous scavenging
system (13).
In this study, we showed that spermine improved the
neuron condition by inhibiting the oxidative stress response and
alleviated injuries caused by cerebral ischemia-reperfusion. Bcl-2
protein family has been proven to be involved in apoptosis
(14). Bcl-2 is an important
anti-apoptosis protein in Bcl family that can protect neurons and
inhibit cell proliferation. Bcl-2 also suppresses cytochrome
c (Cyt C) and caspase by acting on the mitochondrial
membrane (15). As a major apoptosis
protein, Bax can facilitate cell apoptosis by suppressing Bcl-2
activity. It is believed that a dynamic balance exists between Bax
and Bcl-2. Bcl-2 as an oncogene-derived protein, confers negative
control in the apoptosis pathway, while Bax, promotes cell death by
competing with Bcl-2 (16). In this
study, we showed that in rats pre-treated with spermine, Bax level
increased significantly while Bcl-2 level decreased significantly.
This suggested that spermine could protect nerve tissues in rats
with ACIR injuries by inhibiting apoptosis.
We concluded that in rats with ACIR, spermine
protected nerve tissues by decreasing the MDA level, increasing the
activity of SOD, and modifying the balance between Bax and Bcl-2
proteins.
References
|
1
|
McBryde FD, Malpas SC and Paton JF:
Intra-cranial mechanisms for preserving brain blood flow in health
and disease. Acta Physiol (Oxf). 12:46–47. 2016.
|
|
2
|
Liu H, Wei X, Kong L, Liu X, Cheng L, Yan
S, Zhang X and Chen L: NOD2 is involved in the inflammatory
response after cerebral ischemia-reperfusion injury and triggers
NADPH oxidase 2-derived reactive oxygen species. Int J Biol Sci.
11:525–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Katayama Y, Inaba T, Nito C, Ueda M and
Katsura K: Neuroprotective effects of erythromycin on cerebral
ischemia reperfusion-injury and cell viability after oxygen-glucose
deprivation in cultured neuronal cells. Brain Res. 1588:159–167.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dong H, Wang S, Zhang Z, Yu A and Liu Z:
The effect of mitochondrial calcium uniporter opener spermine on
diazoxide against focal cerebral ischemia - reperfusion injury in
rats. J Stroke Cerebrovasc Dis. 23:303–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui D, Wang L, Qi A, Zhou Q, Zhang X and
Jiang W: Propofol prevents autophagic cell death following oxygen
and glucose deprivation in PC12 cells and cerebral
ischemia-reperfusion injury in rats. PLoS One. 7:e353242012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen CM, Wu CT, Yang TH, Chang YA, Sheu ML
and Liu SH: Green tea catechin prevents hypoxia/reperfusion-evoked
oxidative stress-regulated autophagy-activated apoptosis and cell
death in microglial cells. J Agric Food Chem. 64:4078–4085.
2016.PubMed/NCBI
|
|
7
|
Wang T, Gu J, Wu PF, Wang F, Xiong Z, Yang
YJ, Wu WN, Dong LD and Chen JG: Protection by tetrahydroxystilbene
glucoside against cerebral ischemia: involvement of JNK, SIRT1, and
NF-kappaB pathways and inhibition of intracellular ROS/RNS
generation. Free Radic Biol Med. 47:229–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ragnarsson L, Mortensen M, Dodd PR and
Lewis RJ: Spermine modulation of the glutamate(NMDA) receptor is
differentially responsive to conantokins in normal and Alzheimer's
disease human cerebral cortex. J Neurochem. 81:765–779. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yatin SM, Yatin M, Varadarajan S, Ain KB
and Butterfield DA: Role of spermine in amyloid
beta-peptide-associated free radical-induced neurotoxicity. J
Neurosci Res. 63:395–401. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang L, Gao X, Yuan X, Dong H, Zhang Z
and Wang S: Mitochondrial calcium uniporter opener spermine
attenuates the cerebral protection of diazoxide through apoptosis
in rats. J Stroke Cerebrovasc Dis. 23:829–835. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
He Y, Yang J, Li H, Shao H, Wei C, Wang Y,
Li M and Xu C: Exogenous spermine ameliorates high glucose-induced
cardiomyocytic apoptosis via decreasing reactive oxygen species
accumulation through inhibiting p38/JNK and JAK2 pathways. Int J
Clin Exp Pathol. 8:15537–15549. 2015.PubMed/NCBI
|
|
12
|
Lorente L, Martín MM, Pérez-Cejas A,
Abreu-González P, Ramos L, Argueso M, Cáceres JJ, Solé-Violán J and
Jiménez A: Association between total antioxidant capacity and
mortality in ischemic stroke patients. Ann Intensive Care.
6:392016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye N, Liu S, Lin Y and Rao P: Protective
effects of intraperitoneal injection of TAT-SOD against focal
cerebral ischemia/reperfusion injury in rats. Life Sci. 89:868–874.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei H, Wei W, Bredesen DE and Perry DC:
Bcl-2 protects against apoptosis in neuronal cell line caused by
thapsigargin-induced depletion of intracellular calcium stores. J
Neurochem. 70:2305–2314. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao H, Yenari MA, Cheng D, Sapolsky RM
and Steinberg GK: Bcl-2 overexpression protects against neuron loss
within the ischemic margin following experimental stroke and
inhibits cytochrome c translocation and caspase-3 activity. J
Neurochem. 85:1026–1036. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yun Q, Jiang M, Wang J, Cao X, Liu X, Li S
and Li B: Overexpression Bax interacting factor-1 protects cortical
neurons against cerebral ischemia-reperfusion injury through
regulation of ERK1/2 pathway. J Neurol Sci. 357:183–191. 2015.
View Article : Google Scholar : PubMed/NCBI
|