Introduction
Intestinal ischemia/reperfusion (I/R) injury usually
occurs following acute mesenteric ischemia, severe trauma or burns,
hemorrhagic or septic shock, and major surgical procedures
(1). Intestinal I/R causes excessive
inflammation, oxidative response and cell apoptosis, and
subsequently leads to the disruption of the intestinal barrier and
distant organ damage (1). The
development of intestinal I/R is associated with multiple organ
dysfunction syndrome and a high probability of mortality (2,3). Indeed,
a recent multicenter study determined that the mortality rate from
acute mesenteric ischemia in intensive care units (ICU) was as high
as 58% (4). In clinical settings,
vasopressors including norepinephrine and dobutamine are essential
to maintain blood pressure and systemic perfusion in critically ill
patients with intestinal I/R injury.
Vasopressin is generally used for cardiovascular
support in the ICU (5) and is
recommended for the treatment of severe sepsis and septic shock
(6). However, it remains unclear
whether vasopressin aggravates intestinal I/R injury. Previous
studies have suggested that vasopressin decreases intestinal
mucosal perfusion and diminishes the beneficial effect of
norepinephrine due to its potent vasoconstriction (7–9). This
suggests that the use of vasopressin may further deteriorate
intestinal mucosal ischemia in critically ill patients with
intestinal I/R. However, other studies have indicated that
vasopressin does not compromise gut mucosal microcirculation and
oxygen supply (10,11). To the best of our knowledge, there
have been no studies investigating the effects of vasopressin on
intestinal mucosal epithelial cells, the key component of the
intestinal barrier, under I/R conditions. Thus, it is of clinical
relevance to clarify the effect of vasopressin and its analogue on
intestinal epithelial tissues in patients at risk of intestinal
ischemia.
Performing oxygen and glucose deprivation and
re-oxygenation (OGD/R) in vitro is a popular method to
simulate organic I/R in vivo. Therefore, the present study
was designed to assess the effects of terlipressin, a highly
selective vasopressin V1 receptor agonist, on the production of
inflammatory and oxidative cytokines in rat intestinal epithelial
cells (IEC-6), and on cell viability and proliferation in the OGD/R
model.
Materials and methods
IEC-6 cell culture
Rat IEC-6 intestinal epithelial cells (CRL-1592;
passage 16–20; American Type Culture Collection, Manassas, VA, USA)
were cultured as previously described (12). Briefly, IEC-6 cells were cultured in
Dulbecco's modified Eagle's medium (DMEM) containing 4.5 g/l
D-glucose, 10% v/v fetal bovine serum (all from Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and 1% penicillin/streptomycin
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
maintained under standard cell culture conditions of 37°C, 5% CO2
and 21% O2 for 24 h.
OGD/R
OGD intervention was conducted as previously
described (12). Briefly, after the
IEC-6 cells were grown under normal conditions up to 80%
confluence, the DMEM was replenished with D-Hanks buffer
(Sigma-Aldrich; Merck KGaA) and cells were incubated in a modular
incubator chamber filled with a 95% N2 and 5% CO2 gas mixture for 4
h at 37°C. Following completion of OGD, medium was changed back to
normoxic medium and the cells were incubated under normal
conditions for 4 h (re-oxygenation).
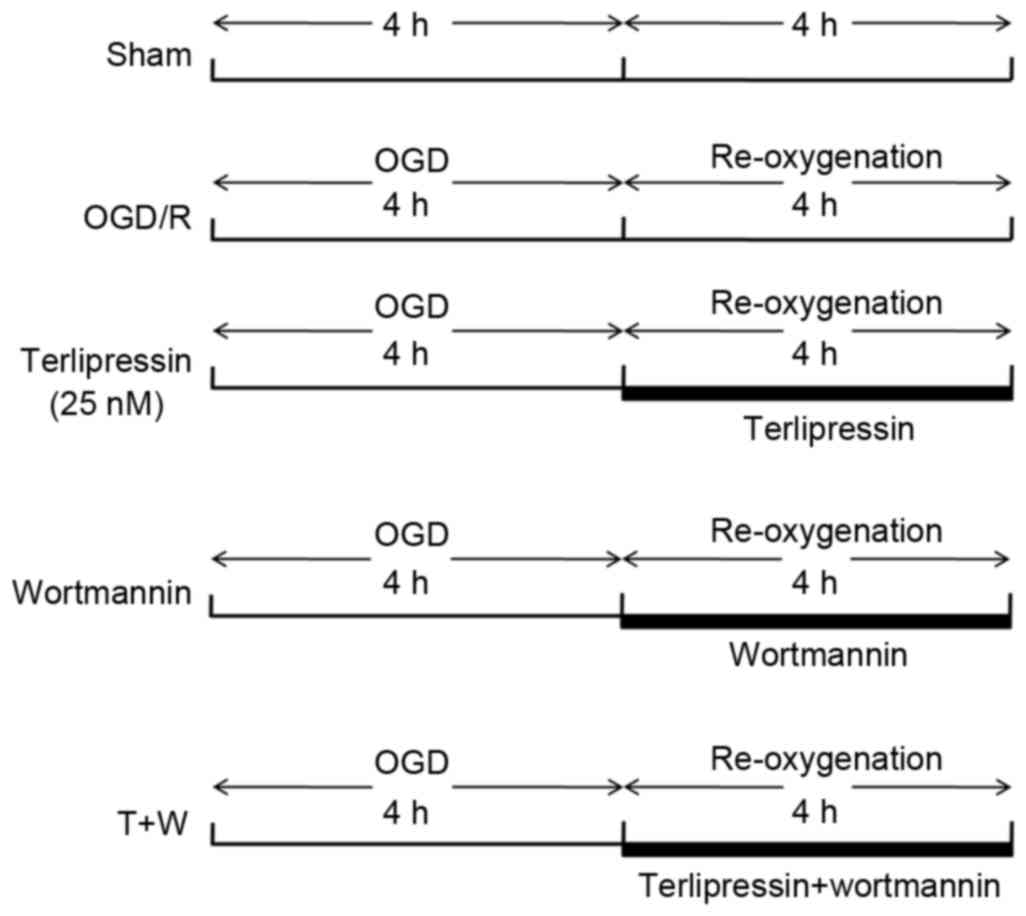
Study groups and experimental
protocol
IEC-6 cells were randomly assigned to receive
different concentrations (1, 5, 25 or 125 nM) terlipressin (Hybio
Pharmaceutical Co., Ltd., Shenzhen, China) following OGD, and then
an appropriate concentration of terlipressin (25 nM) was selected
according to the results of the cell viability test. Subsequently,
cells were incubated with the chosen concentration of terlipressin
and/or phosphatidylinositol 3-kinase (PI3K) inhibitor (Wortmannin;
Beyotime Institute of Biotechnology, Haimen, China). Cells were
randomly assigned to one of 5 groups (5 samples per group) as
follows: i) Sham group: IEC-6 cells were incubated in normoxic
medium for 8 h; ii) OGD/R group: IEC-6 cells were incubated for 4 h
OGD followed by 4 h re-oxygenation; iii) terlipressin group:
Following 4 h OGD, IEC-6 cells were incubated with 25 nM
terlipressin for 4 h; iv) wortmannin group: Following 4 h OGD,
IEC-6 cells were incubated with 2 µM wortmannin, a specific PI3K
inhibitor, for 4 h; or v) T+W group: 2 µM wortmannin and 25 nM
terlipressin were simultaneously administrated at the beginning of
re-oxygenation. The detailed experimental protocol is presented in
Fig. 1.
Cell viability assay
IEC-6 cells were seeded into 96-well plates
(1×105 cells/well) and incubated overnight at 37°C in 5%
CO2. Cells were then treated according to the aforementioned
experimental protocol, with a sham cell group as a control. An MTT
assay was used to determine cell viability as previously described
(12). Briefly, MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazoliumbromide; 5
mg/ml in phosphate-buffered saline) reagent (Sigma-Aldrich; Merck
KGaA) was added to each well and incubated for 4 h at 37°C. Medium
was then replaced with 150 µl dimethyl sulfoxide. Optical density
(OD) was recorded using a microplate reader at the wavelength of
490 nm. Cell viability was expressed as a percentage of the
Sham.
Cell cycle analysis
Cell cycle distribution was measured by a flow
cytometry assay with propidium iodide (PI) DNA staining. Briefly,
IEC-6 cells were seeded into 96-well plates (1×105
cells/well) and treated according to the aforementioned
experimental protocol, with a sham cell group as a control. Cells
were harvested and digested with 0.25% trypsin solution without
EDTA (Sigma-Aldrich; Merck KGaA) for 15 min at 37°C, then isolated
by centrifugation at 200 × g for 2 min at 4°C and washed twice with
cold phosphate-buffered saline (PBS). Following 100 µm mesh sieve
screening; the cell suspension was fixed with 75% ethanol at 4°C
for 24 h. Cells were harvested by centrifugation at 200 × g for 10
min at 4°C and two washes in cold PBS, followed by centrifugation
at 200 × g for 5 min at 4°C and cell collection by discarding of
the supernatant. The cell suspension was then treated with 100 µl
RNase (0.01 mol/l, Sigma-Aldrich; Merck KGaA) at 37°C for 30 min in
a water bath, followed by treatment with PI staining solution (0.5
mg/l; Beyotime Institute of Biotechnology). Following 30 min of
gentle mixing, cells were stored at 4°C in the dark for 30 min,
then analyzed with a FACSCalibur™ Cell Analyzer (BD Biosciences,
CA, USA). Red fluorescence at 488 nm was recorded using a
microplate reader (Thermo Fisher Scientific, Inc.) and results were
analyzed using Flowjo 7.6.3 software (Tree Star, Inc., OR,
USA).
Cell proliferation assay
IEC-6 cell proliferation was measured using a cell
counting kit-8 (CCK-8, Beyotime Institute of Biotechnology). At
baseline (the start of experiment), and 24, 48 and 72 h after
baseline, 5×103 cells/well were propagated in a 5% CO2
atmosphere at 37°C. Then, CCK-8 reagent was applied to the DMEM,
incubated for 1 h and absorbance at 450 nm was measured using a
multiwell spectrophotometer.
Apoptosis assay
IEC-6 cell apoptosis was measured by flow cytometry,
following a previously described procedure (12). Cells were washed twice with cold PBS
and stained with fluorescein isothiocyanate (FITC) Annexin V and PI
using the Annexin V-FITC Apoptosis Detection kit I (BD Biosciences)
for 15 min at room temperature in the dark. Stained cells were
analyzed using flow cytometry within 1 h. The Annexin
V+/PI− and Annexin
V+/PI+ cell populations were considered to
represent apoptotic cells. The apoptosis index was calculated as:
(Apoptotic cells/total cells) ×100.
Tumor necrosis factor (TNF)-α and
isoprostant assay
Following re-oxygenation, the culture medium was
centrifuged at 600 × g for 5 min at 4°C, and the supernatant was
collected. 15-F2t-isoprostane is a representative index of
oxidative stress-induced lipid peroxidation (13). The concentrations of TNF-α and
15-F2t-isoprostane in the supernatant were determined using
commercial kits (TNF-α: RTA00; R&D System, Inc., MN, USA and
15-F2t-isoprostane: 500431; Cayman Chemical Company, MI, USA),
according to the manufacturer's procedure, as described previously
(14,15).
Statistical analysis
Results were analyzed using SPSS 15.0 software (SPSS
Inc, Chicago, IL, USA). Data were expressed as mean ± standard
deviation. Data concerning cell proliferation were analyzed by
two-way analysis of variance (ANOVA) with repeated measures. Other
data were analyzed by one-way ANOVA with Tukey's post-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
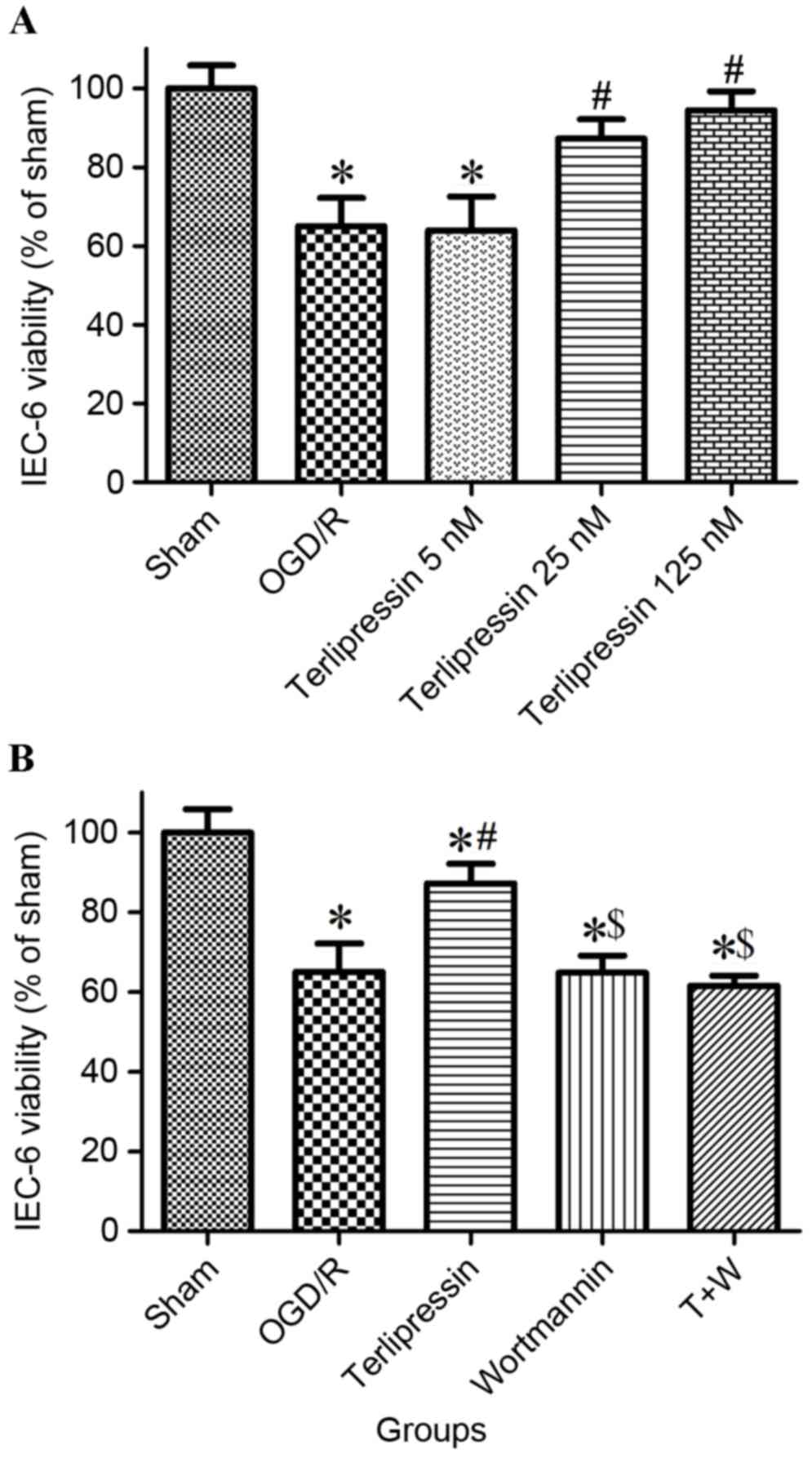
Effect of terlipressin on IEC-6 cell
viability following OGD/R
As presented in Fig.
2A, cell viability significantly decreased in the OGD/R group
(P<0.01 vs. Sham). Incubation with 25 nM and 125 nM terlipressin
increased cell viability (both P<0.01 vs. OGD/R), and cell
viability in 125 nM group was similar to that in 25 nM group
(P=0.756). Therefore, 25 nM terlipressin was selected to use in
subsequent experiments. As depicted in Fig. 2B, cell viability significantly
decreased in the OGD/R, Wortmannin and T+W groups (all P<0.01
vs. Sham). In addition, cell viability in the terlipressin group
was significantly higher than that in the OGD/R, Wortmannin and T+W
groups (all P<0.01), and was significantly lower than that in
the Sham group (P<0.01).
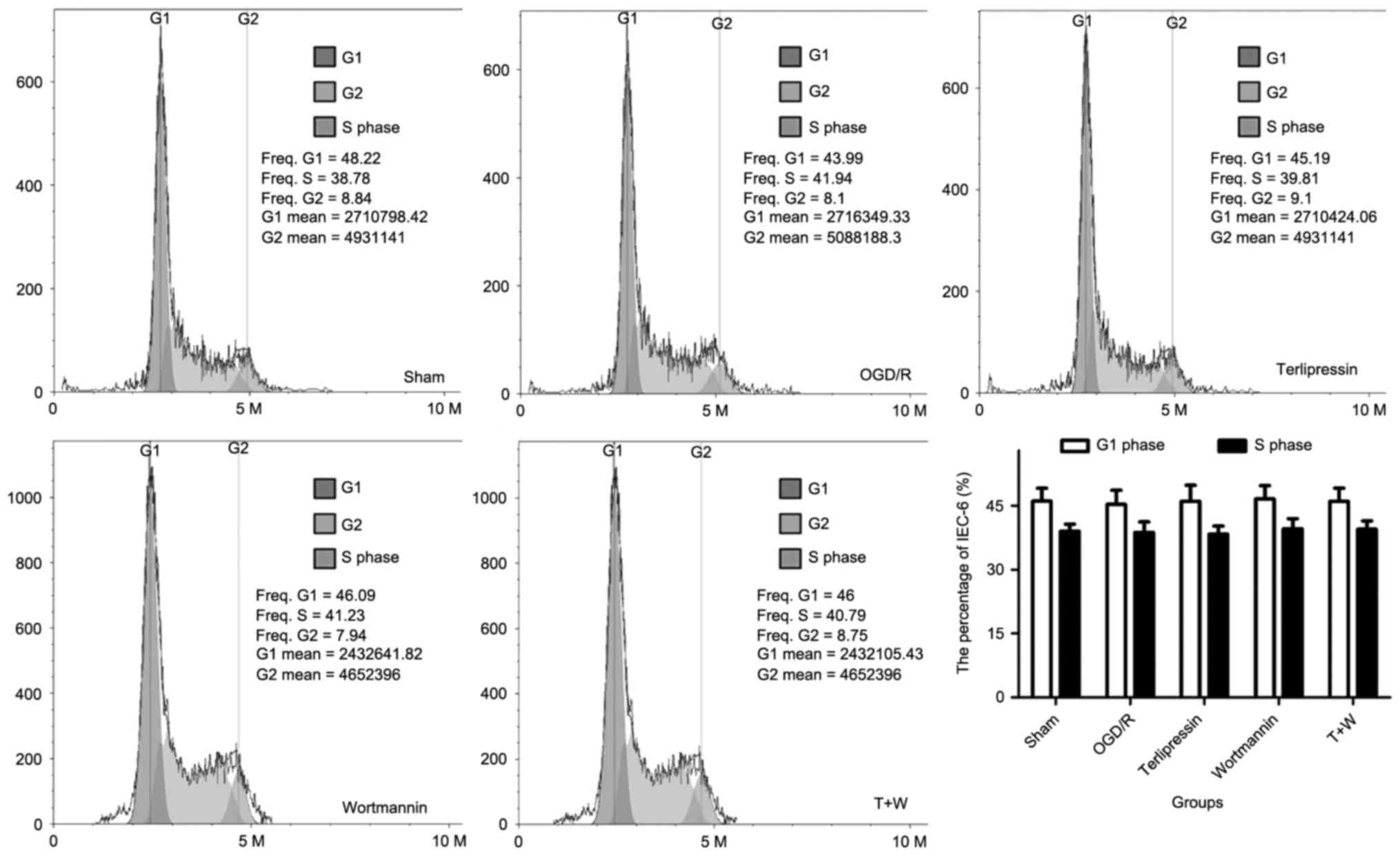
Effect of terlipressin on IEC-6 cell
cycle dynamics following OGD/R
As shown in Fig. 3,
OGD/R intervention did not significantly alter cell cycle dynamics
4 h after re-oxygenation (all P>0.05 vs. Sham). Furthermore,
terlipressin did not significantly influence the percentage of
IEC-6 cells in G1 phase and in S-phase (all P>0.05 vs. OGD/R).
Wortmannin and T+W did not significantly alter the percentage of
IEC-6 cells in different phases of the cell cycle (P>0.05;
Fig. 3).
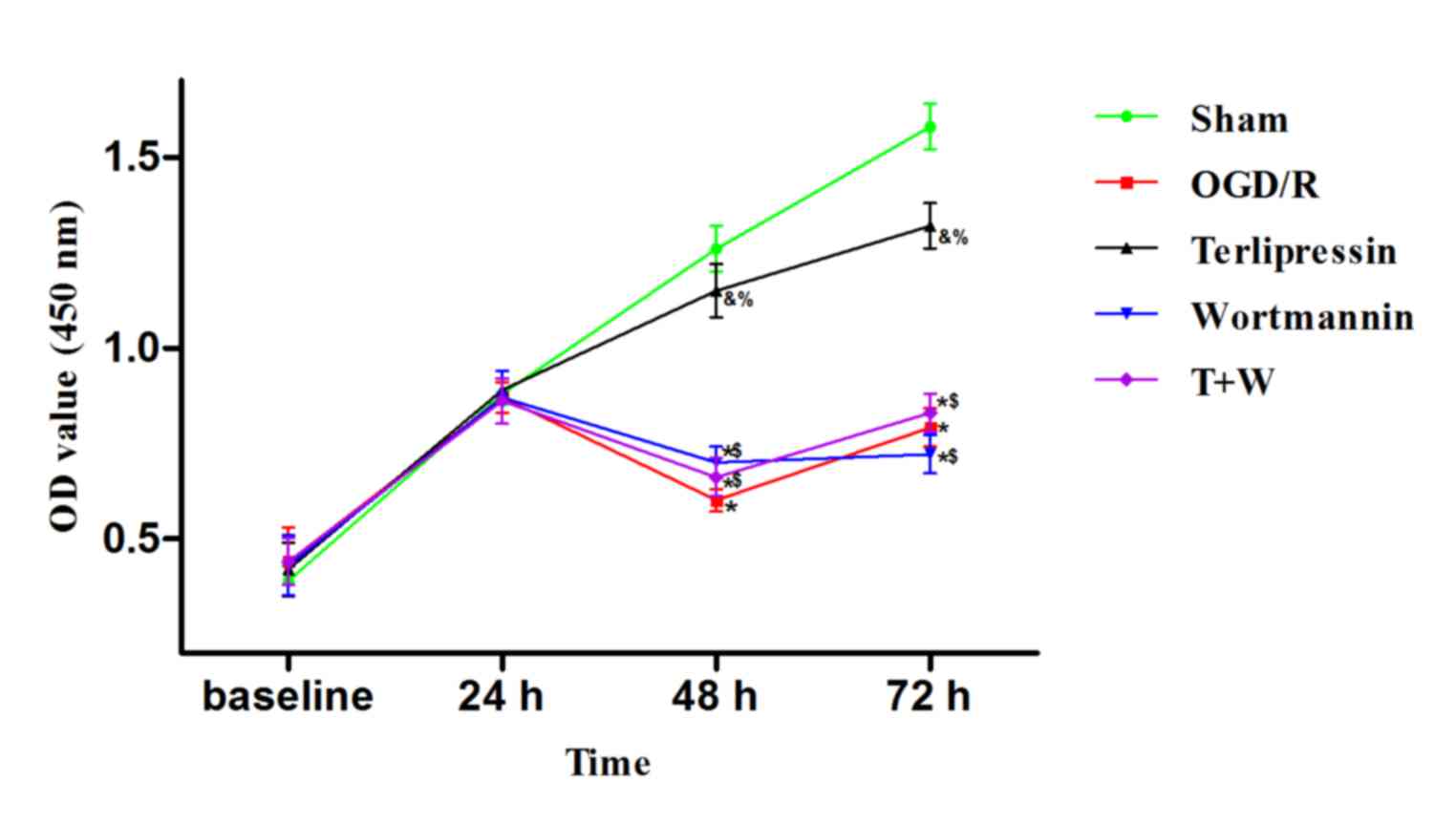
Effect of terlipressin on IEC-6 cell
proliferation following OGD/R
As indicated in Fig.
4, 24 h following the beginning of experiment (baseline), the
OD value in the OGD/R group was similar to that in the Sham group
(P=0.997). OD values at 48 and 72 h were significantly lower in the
OGD/R group compared with the control (both P<0.01 vs. Sham).
However, incubation with 25 nM terlipressin significantly increased
the OD values at 48 and 72 h (P=0.034 and P=0.035 vs. OGD/R,
respectively).
Effect of terlipressin on IEC-6 cell
apoptosis following OGD/R
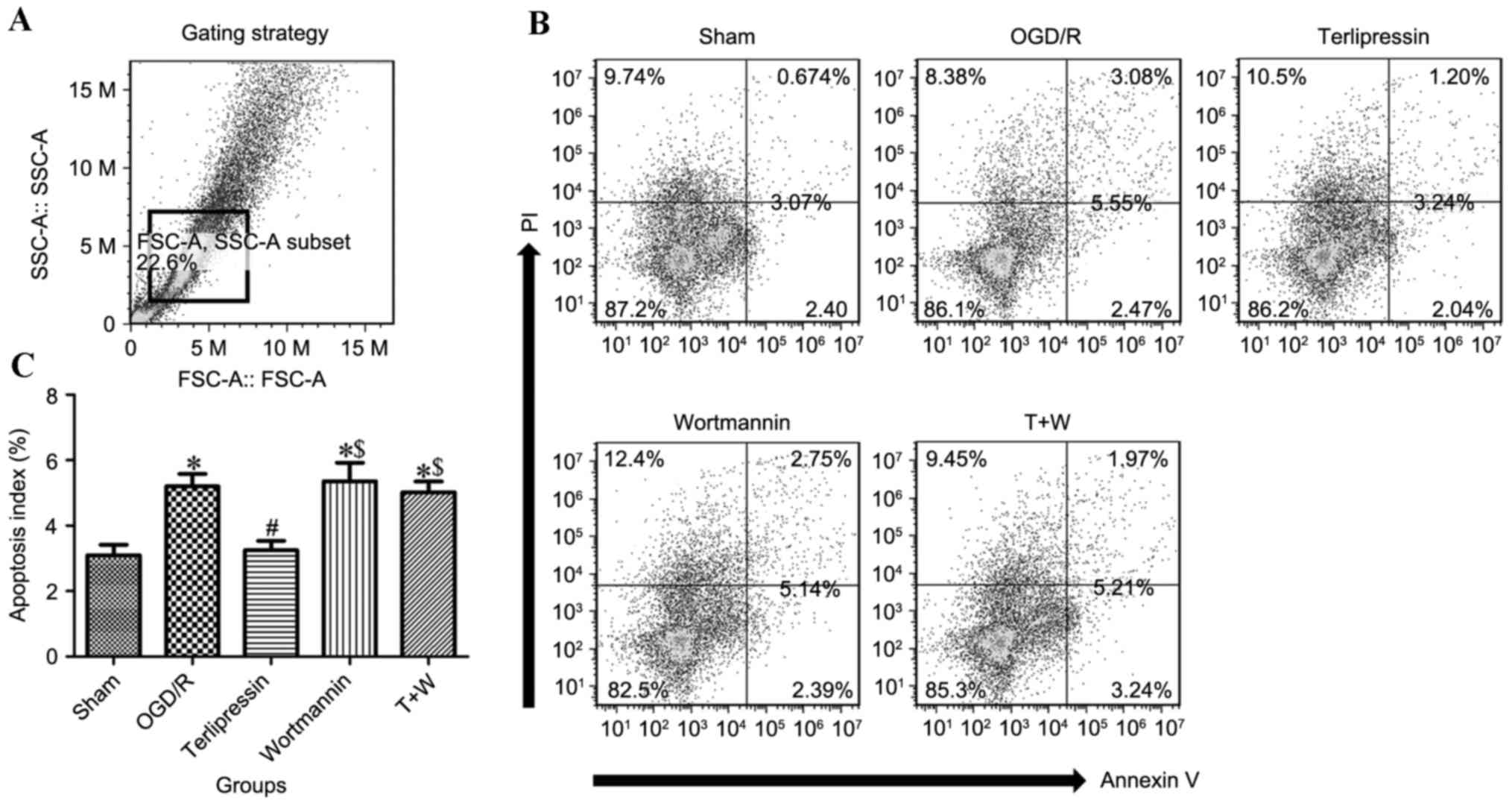
Apoptosis in IEC-6 cells was measured following
OGD/R (Fig. 5). The flow cytometry
gating strategy is depicted in Fig.
5A and representative histograms of each group are presented in
Fig. 5B. The apoptosis index in the
OGD/R group was significantly higher than that in the Sham group
(P<0.01; Fig. 5C). However,
terlipressin attenuated OGD/R-induced cell apoptosis (P<0.01 vs.
OGD/R; Fig. 5C).
Effect of terlipressin on the
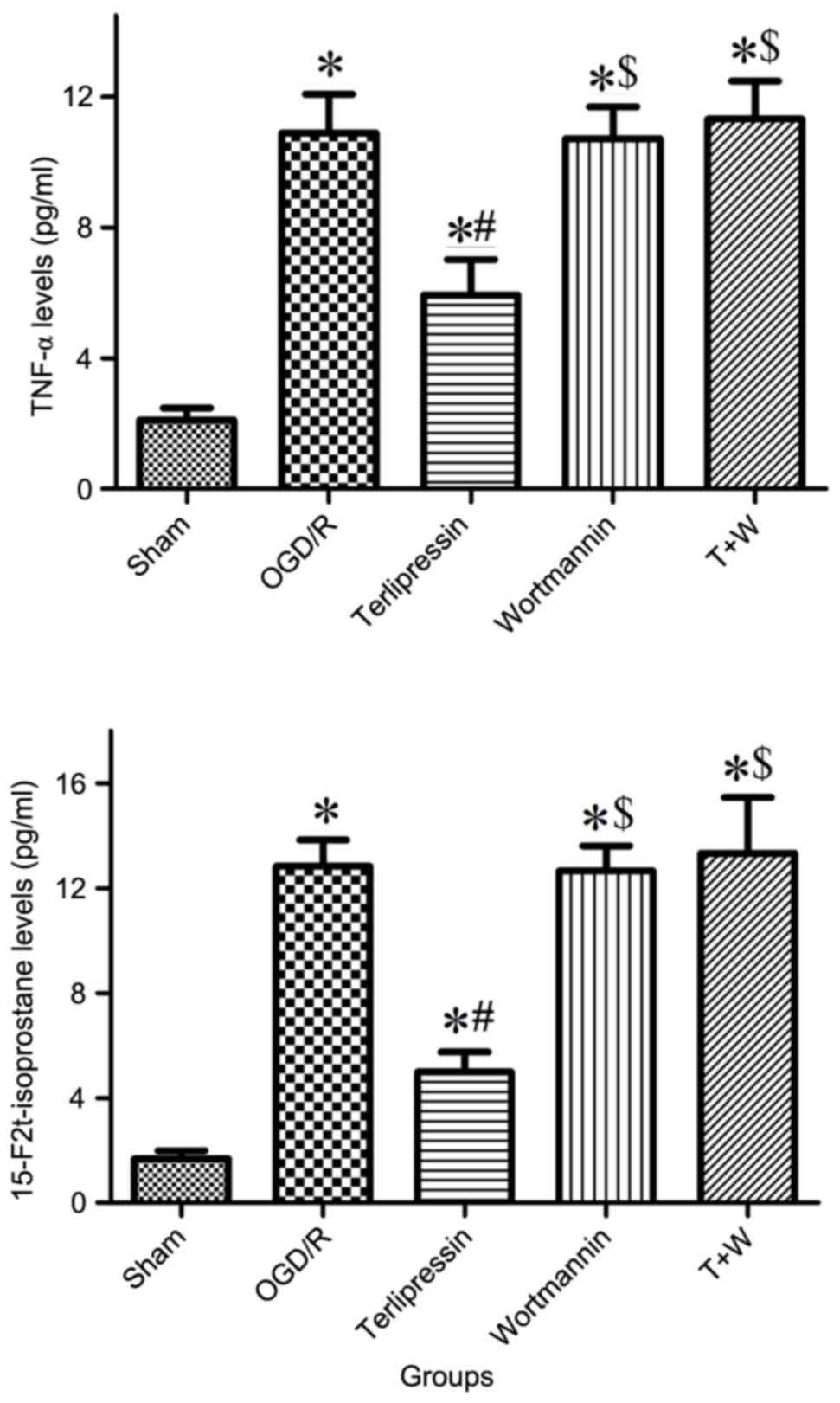
production of TNF-α and isoprostane following OGD/R
TNF-α and 15-F2t-isoprostane levels increased
following OGD/R (both P<0.01 vs. Sham; Fig. 6). Furthermore, terlipressin
significantly inhibited the production of TNF-α and
15-F2t-isoprostane from IEC-6 cells (both P<0.01 vs. OGD/R;
Fig. 6).
Effect of wortmannin on
terlipressin-induced cell protection following OGD/R
Wortmannin alone (Wortmannin group) produced no
significant impacts on the aforementioned variables compared with
the OGD/R group (all P>0.05; Figs.
2–6). In the T+W group,
wortmannin completely attenuated the effects of terlipressin on
cell viability, proliferation and apoptosis (Figs. 2B, 4
and 5C). Moreover, the increased
secretion of TNF-α and 15-F2t-isoprostane induced by terlipressin
was also abolished by wortmannin (Fig.
6).
Discussion
In clinical settings, intestinal I/R injury is a
serious condition and usually occurs prior to treatment with
vasopressors. Therefore, in the present study, terlipressin was
administrated following OGD to assess its effects on intestinal
epithelial cells. The results of the current study demonstrated
that terlipressin decreased the production of TNF-α and isoprostane
in IEC-6 cells and improved cellular viability and proliferation,
and decreased rates of apoptosis during OGD/R. Furthermore, the
aforementioned protective effects of terlipressin were abolished by
the specific PI3K inhibitor wortmannin.
Previous studies have indicated that vasopressin
reduces neuronal death and apoptosis via the vasopressin V1a
receptor (16,17). Similarly, Higashiyama et al
(18) determined that arginine
vasopressin inhibits the serum deprivation-induced apoptosis of
glomerular mesangial cells by activating the V1a receptor. It has
been demonstrated that in animals, apoptosis is the predominant
mechanism of mucosal epithelial cell death during destruction of
the intestinal epithelial barrier induced by intestinal I/R injury
(19). Furthermore, a recent study
performed by the current authors indicated that OGD/R significantly
enhanced IEC-6 apoptosis in vitro and that remifentanil, a
commonly used analgesic in general anesthesia, inhibited cell
apoptosis and improved cell viability (12). To date however, no studies have
investigated the effects of vasopressin on epithelial cell damage
conferred by OGD/R. To the best of our knowledge, the current study
is the first to demonstrate that terlipressin, an analogue of
vasopressin, not only promoted IEC-6 viability but also decreased
cellular apoptosis during OGD/R (Figs.
2 and 5), indicating that
terlipressin management in vivo may provide direct
protection for intestinal epithelial cells against I/R injury.
Interestingly, incubation with terlipressin did not affect cell
cycle and cell proliferation in the early period (<48 h)
following re-oxygenation (Fig. 3)
but significantly increased IEC-6 proliferation 48 and 72 h
following OGD (Fig. 4). This delayed
increase of cell proliferation induced by terlipressin may promote
intestinal epithelial repair following intestinal I/R injury.
A previous study by the current authors indicated
that overactivation of inflammatory response and oxidative stress
was involved in the pathogenesis of intestinal I/R injury (15). Uncontrolled production of reactive
oxygen species and inflammatory cytokines, including TNF-α and
interleukin 1, is closely associated with the disruption of
intestinal epithelia (20–21). A number of studies have determined
that OGD/R increases the release of inflammatory cytokines or
oxidative molecules in neural, renal tubular and vascular
endothelial cells (22–25). The results of the present study
demonstrated that OGD/R increased secretion of TNF-α and
isoprostane from intestinal epithelial cells (Fig. 6). Ferrier et al (26) suggested that vasopressin increased
TNF-α and myeloperoxidase levels through the V1b receptor in gut
inflammatory disease. However, in septic animals, several studies
have demonstrated that vasopressin may attenuate tissue
inflammation and oxidative stress by activating the V1a receptor
(27–29). Data from the current study indicate
that terlipressin exerts anti-inflammatory and anti-oxidative
effects during OGD/R attack (Fig.
6), suggesting that terlipressin may principally act on the V1a
receptor of IEC-6 cells. Further investigations are required to
determine the definite impacts of terlipressin, in vivo, on
intestinal inflammation and oxidation during intestinal I/R
insult.
Previous studies have indicated that vasopressin V1
receptor agonists deliver their effects through the PI3K signaling
pathway (16,30). V1 receptors couple to Gq protein and
Gq subsequently activates phospholipase C and PI3K (31). Notably, the PI3K pathway is involved
in inflammation, oxidation and cell apoptosis under ischemic
conditions (32–34). Therefore, a PI3K specific inhibitor,
wortmannin, was used to explore the potential signaling pathway of
terlipressin in this study. The results indicated that the
protective effects of terlipressin were completely abolished by
wortmannin (Figs. 2B and 4–6),
indicating that terlipressin reduced OGD-induced IEC-6 cell damage
via the PI3K pathway in vitro.
The current study had several limitations. Firstly,
the effects of terlipressin on IEC-6 cells were investigated using
an in vitro OGD/R model. Further analyses should be
conducted to clarify the effects and mechanisms of telipressin on
intestinal I/R injury in animals and in patients. Secondly,
although terlipressin has a high binding affinity to V1 receptor
agonist, a specific V1 receptor antagonist should be applied to
verify that IEC-6 cell protection is mediated by V1 receptors.
Finally, the downstream molecules of PI3K pathway were not measured
in this study. An exact signal pathway may clearly interpret the
protective effect of terlipressin following OGD injury.
In conclusion, the results of the current study
demonstrate that, in vitro, OGD/R attack increases the
production of TNF-α and isoprostane, as well as the apoptosis of
IEC-6 cells, and decreases cell viability and proliferation.
Moreover, the current results indicate for the first time that
terlipressin reduces inflammation, oxidative stress and apoptosis,
and directly protects IEC-6 cells against OGD/R-induced damage via
the PI3K pathway. These important findings may help medical
researchers and clinicians to better understand the pharmacological
characteristics of terlipressin, and provide useful information for
clinical trials and applications relevant to vasopressin.
Acknowledgements
The authors wish to express their gratitude to
Ke-Xuan Liu (Department of Anesthesiology, The First Affiliated
Hospital, Sun Yat-sen University) for the technical support given.
The present study was supported by grants from the Natural Science
Foundation of Guangdong Province, China (grant nos. 2014A030313210
and S2013010015398), and by a grant from the Major Science and
Technology Projects of Guangdong province, China (grant no.
2012A080204018).
References
|
1
|
Mallick IH, Yang W, Winslet MC and
Seifalian AM: Ischemia-reperfusion injury of the intestine and
protective strategies against injury. Dig Dis Sci. 49:1359–1377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Feinman R, Deitch EA, Watkins AC, Abungu
B, Colorado I, Kannan KB, Sheth SU, Caputo FJ, Lu Q, Ramanathan M,
et al: HIF-1 mediates pathogenic inflammatory responses to
intestinal ischemia-reperfusion injury. Am J Physiol Gastrointest
Liver Physiol. 299:G833–G843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Martin B: Prevention of gastrointestinal
complications in the critically ill patient. AACN Adv Crit Care.
18:158–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Leone M, Bechis C, Baumstarck K, Ouattara
A, Collange O, Augustin P, Annane D, Arbelot C, Asehnoune K,
Baldési O, et al: Outcome of acute mesenteric ischemia in the
intensive care unit: A retrospective, multicenter study of 780
cases. Intensive Care Med. 41:667–676. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Holmes CL and Walley KR: Vasoactive drugs
for vasodilatory shock in ICU. Curr Opin Crit Care. 15:398–402.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dellinger RP, Levy MM, Rhodes A, Annane D,
Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke
R, et al: Surviving sepsis campaign: International guidelines for
management of severe sepsis and septic shock: 2012. Crit Care Med.
41:580–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Knotzer H, Pajk W, Maier S, Ladurner R,
Kleinsasser A, Wenzel V, Dünser MW, Ulmer H and Hasibeder WR:
Arginine vasopressin reduces intestinal oxygen supply and mucosal
tissue oxygen tension. Am J Physiol Heart Circ Physiol.
289:H168–H173. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maier S, Hasibeder W, Pajk W, Hengl C,
Ulmer H, Hausdorfer H, Wurzinger B and Knotzer H:
Arginine-vasopressin attenuates beneficial norepinephrine effect on
jejunal mucosal tissue oxygenation during endotoxinaemia. Br J
Anaesth. 103:691–700. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nygren A, Thorén A and Ricksten SE:
Vasopressin decreases intestinal mucosal perfusion: A clinical
study on cardiac surgery patients in vasodilatory shock. Acta
Anaesthesiol Scand. 53:581–588. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiu X, Huang Y, Xu J, Qiu H and Yang Y:
Effects of terlipressin on microcirculation of small bowel
mesentery in rats with endotoxic shock. J Surg Res. 188:503–509.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wafa K, Lehmann C, Wagner L, Drzymulski I,
Wegner A and Pavlovic D: Desmopressin improves intestinal
functional capillary density and decreases leukocyte activation in
experimental endotoxemia. Microvasc Res. 97:98–104. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen JT, Li YS, Xia ZQ, Wen SH, Yao X,
Yang WJ, Li C and Liu KX: Remifentanil preconditioning protects the
small intestine against ischemia/reperfusion injury via intestinal
δ- and μ-opioid receptors. Surgery. 159:548–559. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Milatovic D, Montine TJ and Aschner M:
Measurement of isoprostanes as markers of oxidative stress. Methods
Mol Biol. 758:195–204. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wen SH, Li Y, Li C, Xia ZQ, Liu WF, Zhang
XY, Lei WL, Huang WQ and Liu KX: Ischemic postconditioning during
reperfusion attenuates intestinal injury and mucosal cell apoptosis
by inhibiting JAK/STAT signaling activation. Shock. 38:411–419.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XY, Liu ZM, Wen SH, Li YS, Li Y, Yao
X, Huang WQ and Liu KX: Dexmedetomidine administration before, but
not after, ischemia attenuates intestinal injury induced by
intestinal ischemia-reperfusion in rats. Anesthesiology.
116:1035–1046. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen J, Liu Y, Soh JW and Aguilera G:
Antiapoptotic effects of vasopressin in the neuronal cell line H32
involve protein kinase Calpha and beta. J Neurocheml. 10:1310–1320.
2009. View Article : Google Scholar
|
|
17
|
Chen J, Volpi S and Aguilera G:
Anti-apoptotic actions of vasopressin in H32 neurons involve MAP
kinase transactivation and Bad phosphorylation. Exp Neurol.
211:529–538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Higashiyama M, Ishikawa S and Saito T,
Nakamura T, Kusaka I, Nagasaka S, Honda K and Saito T: Arginine
vasopressin inhibits apoptosis of rat glomerular mesangial cells
via V1a receptors. Life Sci. 68:1485–1493. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ikeda H, Suzuki Y, Suzuki M, Koike M,
Tamura J, Tong J, Nomura M and Itoh G: Apoptosis is a major mode of
cell death caused by ischaemia and ischaemia/reperfusion injury to
the rat intestinal epithelium. Gut. 42:530–537. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang G, Chen Z, Zhang F, Jing H, Xu W,
Ning S, Li Z, Liu K, Yao J and Tian X: Blockade of PKCβ protects
against remote organ injury induced by intestinal ischemia and
reperfusion via a p66shc-mediated mitochondrial apoptotic pathway.
Apoptosis. 19:1342–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ozacmak HS, Ozacmak VH, Barut F, Araslı M
and Ucan BH: Pretreatment with mineralocorticoid receptor blocker
reduces intestinal injury induced by ischemia and reperfusion:
Involvement of inhibition of inflammatory response, oxidative
stress, nuclear factor κB, and inducible nitric oxide synthase. J
Surg Res. 191:350–361. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang WM, Liu Z, Liu AJ, Wang YX, Wang HG,
An D, Heng B, Xie LH, Duan JL and Liu YQ: The zinc ion chelating
agent TPEN attenuates neuronal Death/apoptosis caused by
Hypoxia/ischemia via mediating the pathophysiological cascade
including excitotoxicity, oxidative Stress and inflammation. CNS
Neurosci Ther. 21:708–717. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu H, Wei X, Kong L, Liu X, Cheng L, Yan
S, Zhang X and Chen L: NOD2 is involved in the inflammatory
response after cerebral ischemia-reperfusion injury and triggers
NADPH oxidase 2-derived reactive oxygen species. Int J Biol Sci.
11:525–535. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang YJ, Zhang AQ, Zhao XX, Tian ZL and
Yao L: Nicorandil protects against ischaemia-reperfusion injury in
newborn rat kidney. Pharmacology. 92:245–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Zhou J, Zhang D, Song Y, She J and
Bai C: Bone marrow-derived mesenchymal stem cells enhance autophagy
via PI3K/AKT signalling to reduce the severity of
ischaemia/reperfusion-induced lung injury. J Cell Mol Med.
19:2341–2351. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrier L, Serradeil-Le Gal C, Schulte AM,
Vasina V, Gaultier E, Schroedel S, Ursino MG, Chaumaz G, Pascal M,
De Ponti F and Bueno L: Proinflammatory role of vasopressin through
V1b receptors in hapten-induced experimental colitis in rodents:
Implication in IBD. Am J Physiol Gastrointest Liver Physiol.
299:G1298–G1307. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boyd JH, Holmes CL, Wang Y, Roberts H and
Walley KR: Vasopressin decreases sepsis-induced pulmonary
inflammation through the V2R. Resuscitation. 79:325–331. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Maybauer MO, Maybauer DM, Enkhbaatar P,
Laporte R, Wiśniewska H, Traber LD, Lin C, Fan J, Hawkins HK, Cox
RA, et al: The selective vasopressin type 1a receptor agonist
selepressin (FE 202158) blocks vascular leak in ovine severe
sepsis*. Crit Care Med. 42:e525–e533. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nazari A, Sadr SS, Faghihi M, Azizi Y,
Hosseini MJ, Mobarra N, Tavakoli A and Imani A: Vasopressin
attenuates ischemia-reperfusion injury via reduction of oxidative
stress and inhibition of mitochondrial permeability transition pore
opening in rat hearts. Eur J Pharmacol. 760:96–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nakatani Y, Chin Y, Hara S and Kudo I:
Immediate prostaglandin E2 synthesis in rat 3Y1 fibroblasts
following vasopressin V1a receptor stimulation. Biochem Biophys Res
Commun. 354:676–680. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Miller RL, Sandoval PC, Pisitkun T,
Knepper MA and Hoffert JD: Vasopressin inhibits apoptosis in renal
collecting duct cells. Am J Physiol Renal Physiol. 304:F177–F188.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang W, Xing B, Yang L, Shi J and Zhou X:
Icaritin attenuates myocardial ischemia and reperfusion injury via
anti-inflammatory and anti-oxidative stress effects in rats. Am J
Chin Med. 43:1083–1097. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Y, Tian SY, Li YW, Zhang L, Yu JB,
Li J, Chen YY, Wang YX, Liang Y, Zhang XS, et al: Sevoflurane
preconditioning improving cerebral focal ischemia-reperfusion
damage in a rat model via PI3K/Akt signaling pathway. Gene.
569:60–65. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu X, Zhang C, Qian L, Zhang C, Wu K,
Yang C, Yan D, Wu X and Shi J: NF45 inhibits cardiomyocyte
apoptosis following myocardial ischemia-reperfusion injury. Pathol
Res Pract. 211:955–962. 2015. View Article : Google Scholar : PubMed/NCBI
|