Introduction
Osteoprotegerin is secreted from osteoblasts and has
an inhibitory effect on the differentiation and activation of
osteoclasts (1). Bone metabolism is
principally regulated by osteoblasts and osteoclasts, which are
responsible for the formation and resorption of bone, respectively
(2). Through the bone remodeling
activities of osteoblasts and osteoclasts, bone tissue in the
skeleton is continuously regenerated and renewed (3). Bone remodeling begins with the
resorption of bone by osteoclasts, followed by osteoblastic bone
formation (4). It has been
established that a decrease in bone mineral density results from an
imbalance in the bone remodeling process (3).
Dysfunction in bone remodeling leads to metabolic
bone disease associated with an increased risk of fracture,
including osteoporosis (5). Paget's
disease of bone is a common disorder characterized by focal areas
of increased and disorganized bone remodeling (6).
Osteoprotegerin and its cognate ligand receptor
activator of nuclear factor-κB (RANK) belong to the tumor necrosis
factor receptor family. Osteoprotegerin binds to RANK ligand as a
decoy receptor and prevents RANK ligand from binding to RANK,
resulting in the suppression of bone resorption through the
inhibition of osteoclast differentiation (1). Through this mechanism, the RANK/RANK
ligand/osteoprotegerin axis is considered to be an essential
regulatory system in the formation of osteoclasts (7).
Chlorogenic acid (CGA), which is a phenolic compound
and a main component of coffee, and (−)-epigallocatechin gallate
(EGCG), which is a polyphenolic compound and a primary constituent
of green tea, are considered to have beneficial properties for
human health, including anti-oxidative, anti-inflammatory and
anti-cancer properties (8–10). CGA has been documented to increase
mineralization in rat tibia and improve mechanical properties of
the femoral diaphysis (11). In
addition, CGA may suppress osteoclastic bone resorption by
downregulating the effects of RANK ligand (12). Similarly, EGCG may inhibit
osteoclastic bone resorption and supports osteoblastic bone
formation (13,14), and green tea consumption may be
associated with reduced age-related bone loss and fractures in the
elderly (13). However, the
underlying molecular mechanisms regarding the effects of CGA and
EGCG on bone metabolism are currently unknown.
Bone morphogenetic proteins (BMPs), which belong to
the transforming growth factor (TGF)-β superfamily, including TGF-β
and activin, promote bone formation by stimulating the
proliferation and differentiation of osteoblasts (15,16).
Intracellular signaling activities of BMPs generally occur through
a Smad (Smad1/5/8)-dependent pathway (15), although previous evidence suggests
that BMP signaling may also occur through a Smad-independent
pathway involving the mitogen-activated protein kinase (MAPK)
family (17,18). A previous study by the present
authors demonstrated that BMP-4 may stimulate the synthesis of
osteoprotegerin in osteoblast-like MC3T3-E1 cells, and that p38
MAPK may act as a positive regulator in osteoprotegerin synthesis
(19). In the present study, the
effects of CGA and EGCG on osteoprotegerin synthesis mediated by
BMP-4 in osteoblast-like MC3T3-E1 cells were investigated. It was
observed that EGCG but not CGA enhanced osteoprotegerin synthesis
stimulated by BMP-4 in osteoblasts.
Materials and methods
Materials
CGA and EGCG were purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). BMP-4 and a mouse osteoprotegerin
enzyme-linked immunosorbent assay (ELISA) kit (cat. no. MOP00) were
obtained from R&D Systems, Inc. (Minneapolis, MN, USA).
Rapamycin was obtained from Merck KGaA. Antibodies against
phosphorylated Smad1 (cat. no. 13820), p38 MAPK (cat. no. 9212),
phosphorylated (phospho)-p38 MAPK (cat. no. 4511), p70 S6 kinase
(cat. no. 9202) and phospho-p70 S6 kinase (cat. no. 9205S) were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Antibodies against GAPDH (cat. no. sc-25778) were purchased from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Secondary
antibodies, peroxidase-labeled goat anti-rabbit immunoglobulin G
(cat. no. 074-1506) were purchased from Seracare (Milford, MA,
USA). An enhanced chemiluminescence (ECL) western blotting
detection system was obtained from GE Healthcare Life Sciences
(Chalfont, UK). EGCG and rapamycin were individually diluted in
dimethyl sulfoxide (100 mM) and CGA was diluted in ethanol (100
mM). Both DMSO and ethanol were used at a maximum concentration of
0.1%, which did not affect the assay for osteoprotegerin or western
blot analysis.
Cell culture
Cloned osteoblast-like MC3T3-E1 cells derived from
newborn mouse calvaria (20) were
maintained as described previously (21). Briefly, cells were seeded into 35 mm
diameter dishes (5×104 cells/dish) for ELISA and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
experiments or 90 mm diameter dishes (2×105 cells/dish)
for Western blotting and cultured in α-minimum essential medium
(α-MEM; cat. no. M8042, Sigma-Aldrich; Merck KGaA) containing 10%
fetal bovine serum (FBS; cat. no. 12483-020; Gibco; Thermo Fisher
Scientific Inc. Waltham, MA, USA) at 37°C in a humidified
atmosphere of 5% CO2/95% air. After 5 days, the medium was
replenished with α-MEM containing 0.3% FBS at 37°C for 48 h. Cells
were then used in the following experiments.
Assay for osteoprotegerin
Cultured MC3T3-E1 cells were pretreated with 1, 3,
10, 30 or 50 µM of CGA or 1, 3 or 10 µM of EGCG at 37°C for 60 min,
then stimulated with 30 ng/ml BMP-4 or PBS supplemented with 0.01%
bovine serum albumin (Sigma-Aldrich; Merck KGaA) containing 0.1%
ethanol (as a vehicle) in 1 ml α-MEM containing 0.3% FBS at 37°C
for 0, 12, 24, 36 or 48 h. The conditioned medium was collected
following the incubation periods and the concentration of
osteoprotegerin was measured using the mouse osteoprotegerin ELISA
kit, according to the manufacturer's protocol.
RT-qPCR
Cultured MC3T3-E1 cells were pretreated with 10 µM
EGCG or vehicle at 37°C for 60 min, then stimulated with 70 ng/ml
BMP-4 or vehicle in 1 ml of α-MEM containing 0.3% FBS at 37°C for 6
h. Total RNA was isolated by TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) and was reverse
transcribed into complementary DNA using an Omniscript Reverse
Transcriptase kit (Qiagen Inc., Valencia, CA, USA) according to the
manufacturer's protocol. qPCR was performed using a LightCycler
Capillary system and Fast Start DNA Master SYBR-Green I kit (Roche
Diagnostics, Basel, Switzerland). The forward and reverse primers
for mouse osteoprotegerin mRNA were purchased from Takara
Biotechnology Co., Ltd. (Dalian, China). The sequences were as
follows: Forward 5′-CAATGGCTGGCTTGGTTTCATAG-3′ and reverse
5′-CTGAACCAGACATGACAGCTGGA-3′. The forward and reverse primers for
mouse GAPDH mRNA were synthesized based on the report of Simpson
et al (22) and obtained from
Sigma-Aldrich (Merck KGaA). The sequences were as follows: Forward
5′-AACGACCCCTTCATTGAC-3′ and reverse 5′-TCCACGACATACTCAGCAC-3′. The
reaction mixtures were incubated at 95°C for 10 min, followed by 40
cycles at 60°C for 5 sec and 72°C for 7 sec. The amplified products
were determined by a melting curve analysis (23) according to the system protocol. The
data were analyzed by second derivative maximum method using
LyghtCycler3 Data Analysis (Version 3.5.28; Roche Diagnostics).
Levels of osteoprotegerin mRNA were normalized to those of GAPDH
mRNA.
Western blot analysis
Cultured MC3T3-E1 cells were pretreated with 10, 20
or 30 µM of EGCG at 37°C for 60 min, then stimulated with 30 ng/ml
BMP-4 or vehicle, PBS supplemented with 0.01% bovine serum albumin
containing 0.1% ethanol, in 1 ml of α-MEM containing 0.3% FBS at
37°C for 45, 90 or 120 min according to our previous reports
(19,24–26).
Cells were then washed twice with phosphate-buffered saline and
lysed, homogenized and sonicated with 20 short 1 sec bursts using a
TOMY Ultrasonic Disruptor UD-211 (TOMY Digital Biology, Co., Ltd.,
Tokyo, Japan) in a lysis buffer containing 62.5 mM Tris/HCl (pH
6.8) 2% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol and 10%
glycerol. SDS-PAGE was performed according to the Laemmli method
(27) on 10% polyacrylamide gels.
The proteins were fractionated and transferred onto Immuno-Blot
polyvinyl difluoride (PVDF) membranes (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The membranes were blocked with 5% fat-free dry
milk in Tris-buffered saline-Tween-20 [TBS-T; 20 mM Tris-HCl (pH
7.6) 137 mM NaCl and 0.1% Tween-20) at room temperature for 2 h
prior to incubation with primary antibodies. Western blot analysis
was performed as described previously (28) using antibodies against phospho-Smad1,
GAPDH, phospho-p70 S6 kinase, p70 S6 kinase, phospho-p38 MAPK and
p38 MAPK as primary antibodies and peroxidase-labeled goat
anti-rabbit immunoglobulin G as secondary antibodies. The primary
and secondary antibodies were diluted to 1:1,000 with 5% fat-free
dry milk in TBS-T. Peroxidase activity on the PVDF membranes was
detected on an X-ray film using the ECL western blotting detection
system.
Measurements
The absorbance of enzyme immunosorbent assay samples
was measured at 450 nm with an EL 340 Bio Kinetic Reader (BioTek
Instruments, Inc., Winooski, VT, USA). Densitometric analysis of
western blotting was performed using a scanner and Image J 1.47
software (National Institutes of Health, Bethesda, MD, USA). Levels
of phosphorylated protein were calculated as the
background-subtracted signal intensity of each phosphorylation
signal normalized to the respective total protein signal, and
plotted as a fold increase relative to that of control cells
treated with vehicle reagent.
Statistical analysis
Data were analyzed using one-way analysis of
variance followed by a Bonferroni method for multiple comparisons
between pairs, using Microsoft Office Excel 2013 for Windows
(Microsoft Corporation, Redmond, WA, USA) and P<0.05 was
considered to indicate a statistically significant difference. All
data are presented as the mean ± standard error of the mean of
three replicate experiments from three independent cell
preparations.
Results
Differential effects of CGA or EGCG on
BMP-4-stimulated osteoprotegerin release in MC3T3-E1 cells
In a previous study by the present authors (19), it was demonstrated that BMP-4
stimulates osteoprotegerin synthesis in osteoblast-like MC3T3-E1
cells. The present study initially investigated the effect of CGA
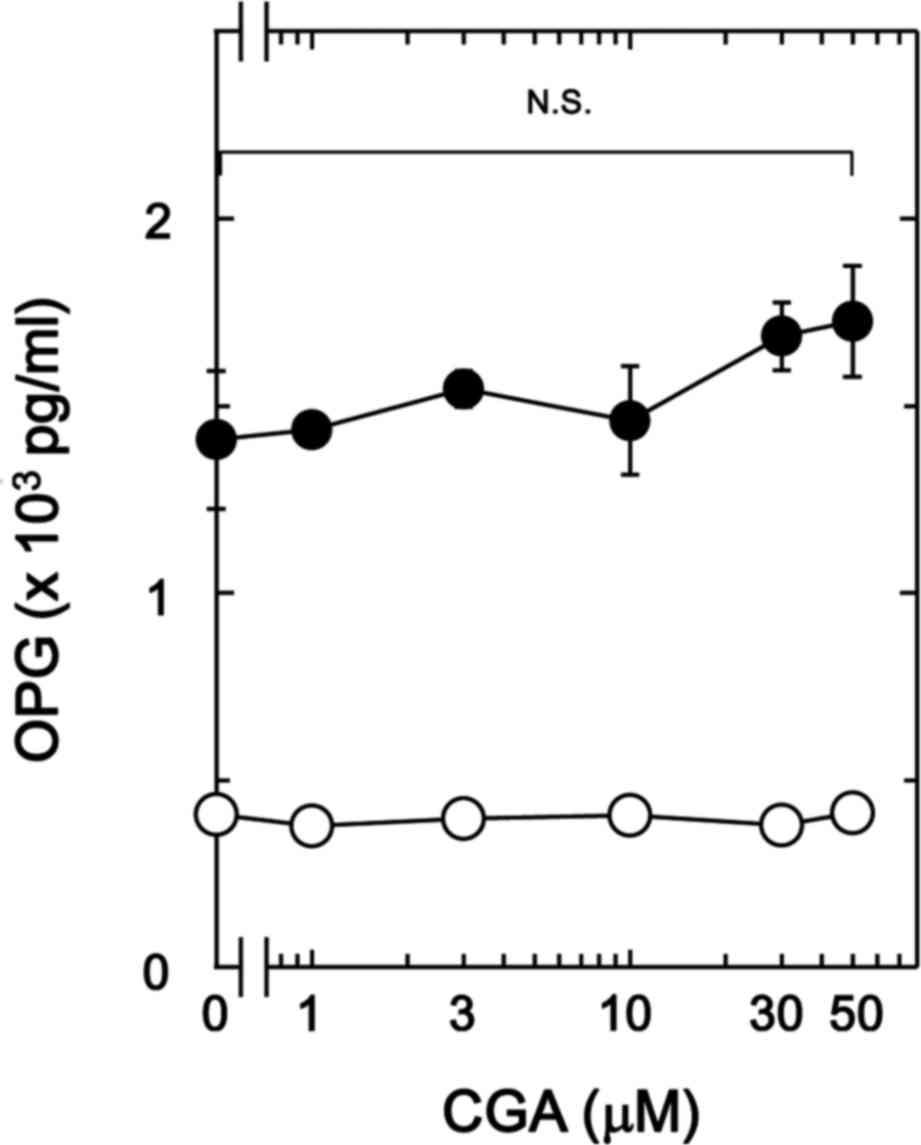
on BMP-4-stimulated osteoprotegerin release in MC3T3-E1 cells. It
was observed that CGA (≤50 nM) + BMP-4 had no significant effect on
BMP-4-stimulated osteoprotegerin release in comparison to the value
of vehicle + BMP-4 (Fig. 1).
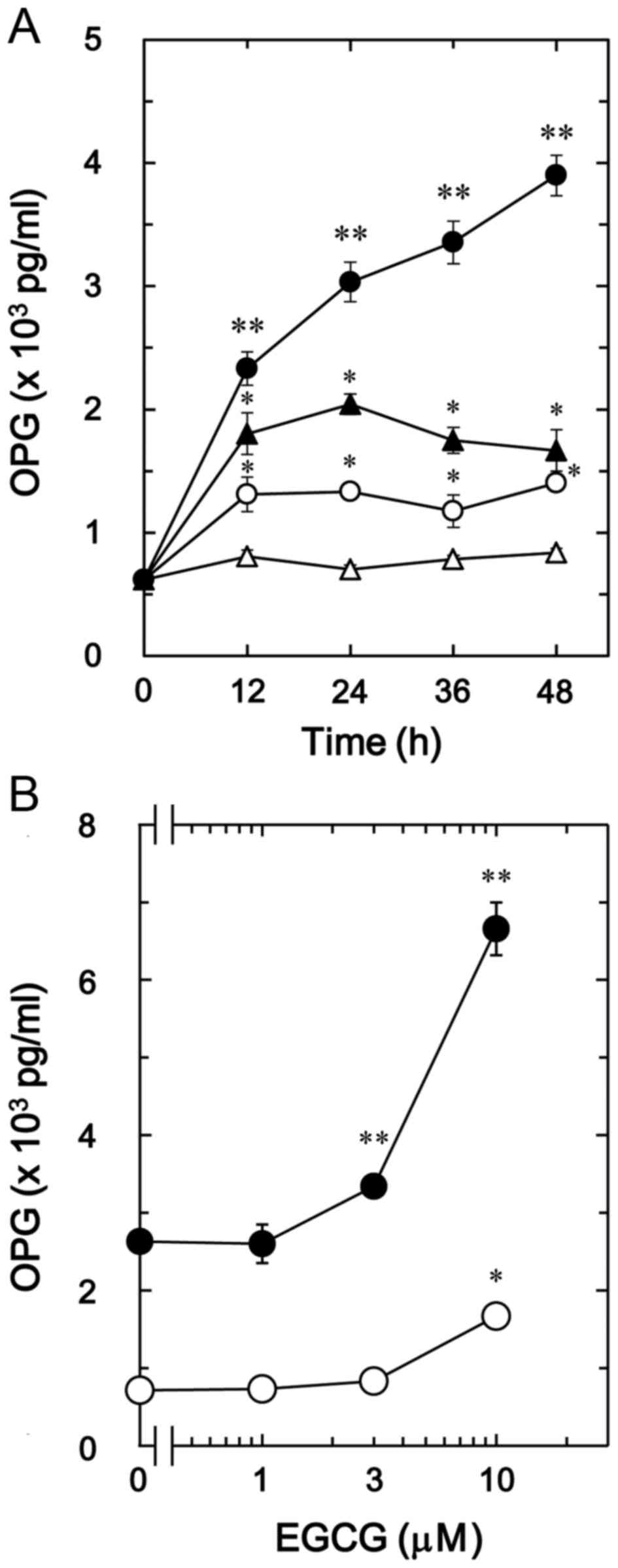
The effect of EGCG on BMP-4-stimulated
osteoprotegerin release was also investigated in MC3T3-E1 cells. In
contrast to CGA, it was observed that 30 µM of EGCG + BMP-4
(Fig. 2A) significantly enhanced
BMP-4-stimulated osteoprotegerin release in a time-dependent manner
compared with the value of vehicle + BMP-4 (P<0.005; Fig. 2A). In addition, the stimulatory
effects of EGCG + BMP-4 (Fig. 2B) on
osteoprotegerin release were dose-dependent between 1 and 10 µM in
comparison to the value of vehicle + BMP-4 (P<0.003; Fig. 2B).
EGCG stimulates BMP-4-induced
osteoprotegerin mRNA expression in MC3T3-E1 cells
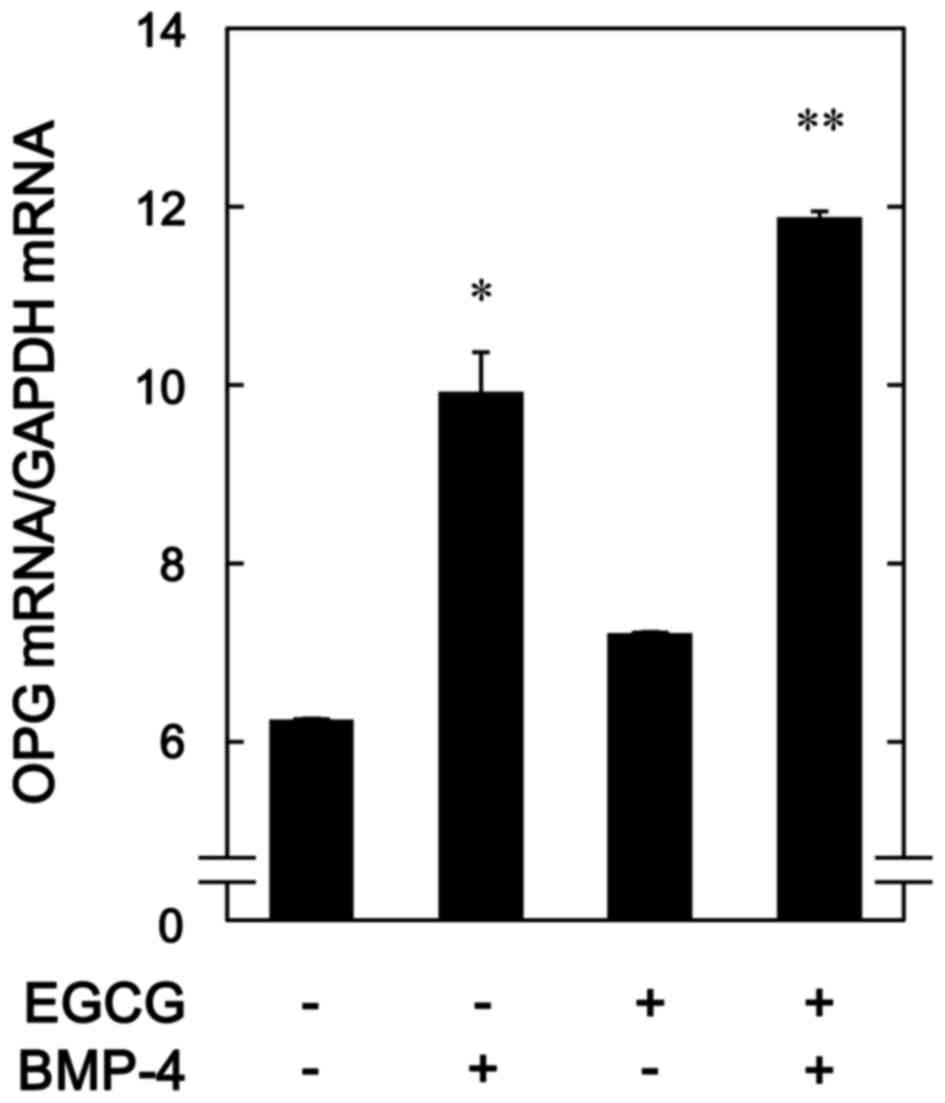
To determine whether the stimulatory effects of EGCG
on BMP-4-stimulated osteoprotegerin release was mediated by
transcriptional events in osteoblast-like MC3T3-E1 cells, the
effect of EGCG on BMP-4-induced expression of osteoprotegerin mRNA
was subsequently evaluated. It was observed that EGCG (10 µM) +
BMP-4 significantly enhanced BMP-4-induced osteoprotegerin mRNA
expression in comparison to the value of vehicle + BMP-4 (P=0.03;
Fig. 3).
EGCG has no effect on BMP-4-induced
phosphorylation of Smad1
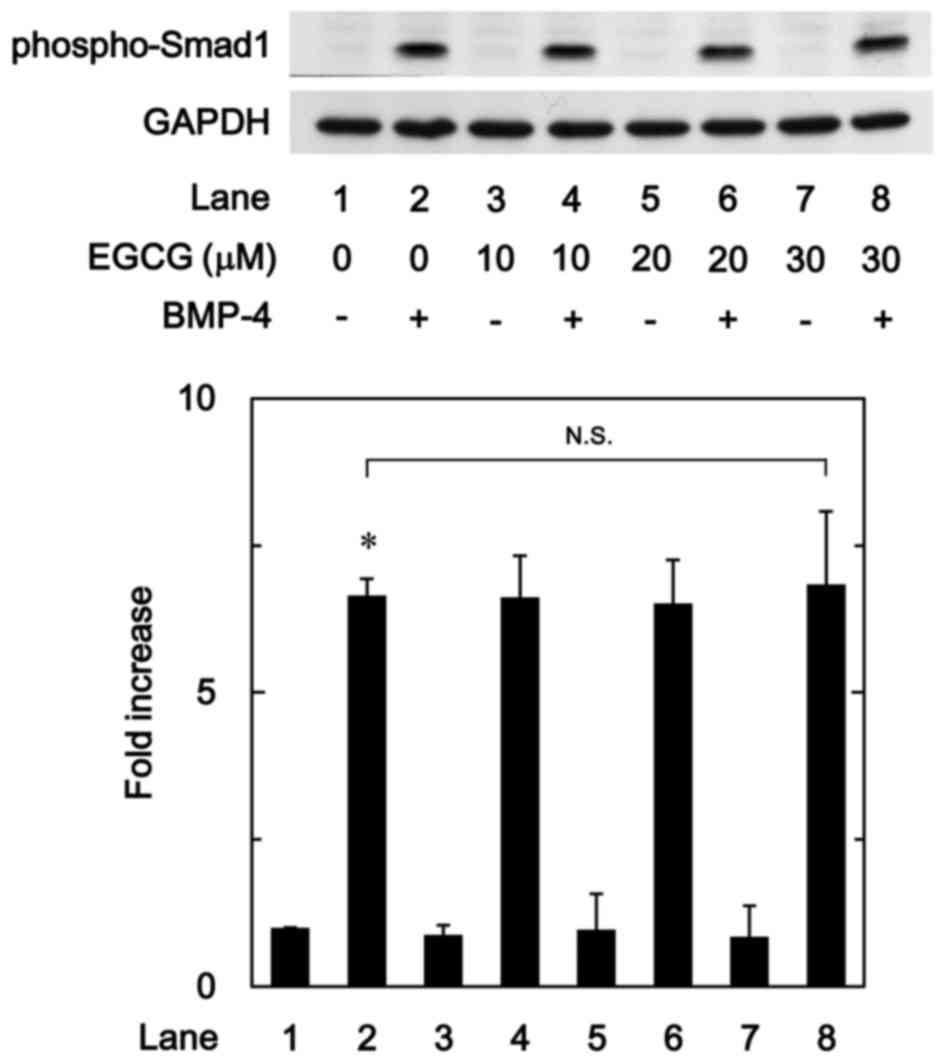
The Smad1/5/8 pathway is an intracellular signal
transduction system that principally mediates the effects of BMPs
(15). To determine whether the
stimulatory effects of EGCG on osteoprotegerin synthesis were
dependent on the activation of Smad1/5/8 in MC3T3-E1 cells, the
present study evaluated the effect of EGCG on BMP-4-induced
phosphorylation of Smad1. It was observed that vehicle alone or
EGCG + vehicle did not stimulate the phosphorylation of Smad1,
whereas vehicle + BMP-4 did. It was observed that EGCG (10–30 µM) +
BMP-4 had no effect on BMP-4-induced phosphorylation of Smad1 in
comparison to the value of vehicle + BMP-4 (Fig. 4).
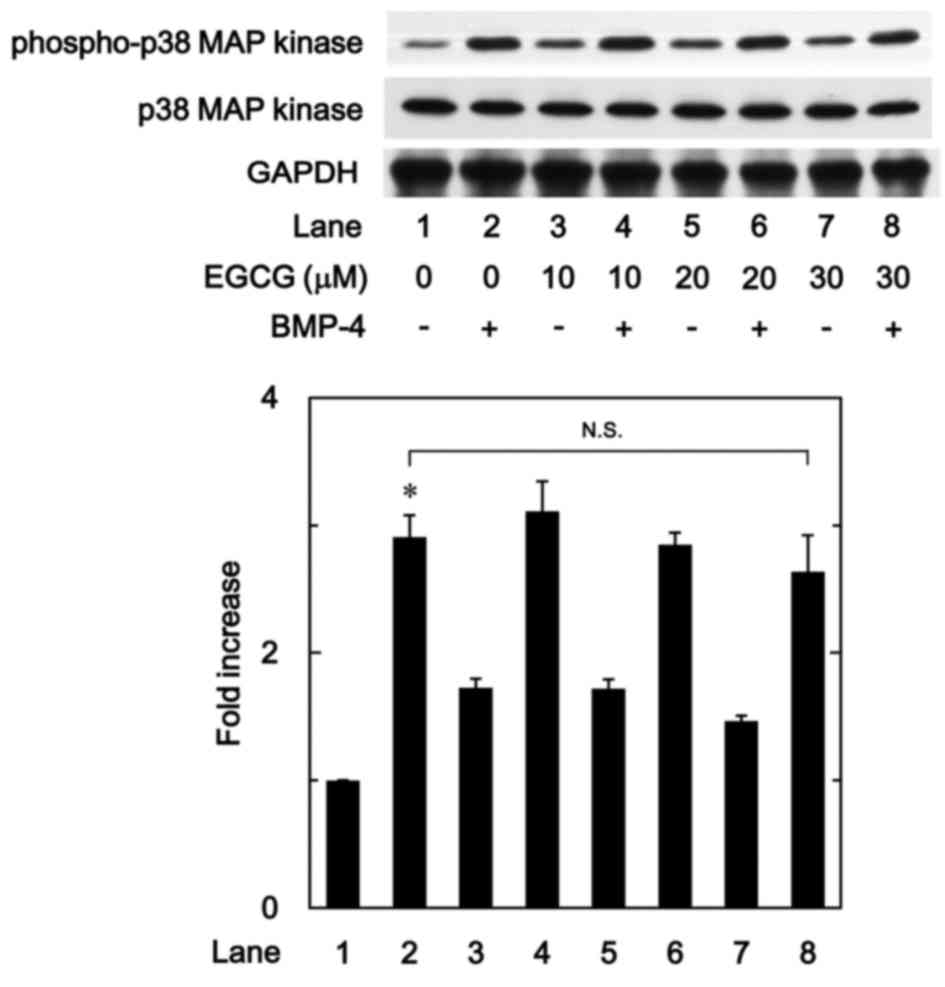
EGCG has little effect on
BMP-4-induced phosphorylation of p38 MAPK
In previous studies by our group (19,24),
BMP-4 induced the activation of p38 MAPK in osteoblast-like
MC3T3-E1 cells, and p38 MAPK was associated with the
BMP-4-stimulated osteoprotegerin synthesis in these cells.
Therefore, to determine whether the effect of EGCG on
BMP-4-stimulated osteoprotegerin synthesis was associated with
activation of p38 MAPK in MC3T3-E1 cells, the present study
evaluated the effect of EGCG on BMP-4-induced phosphorylation of
p38 MAPK. It was observed that EGCG (10–30 µM) + BMP-4 had no
significant effect on BMP-4-induced phosphorylation of p38 MAPK in
comparison to the value of vehicle + BMP-4 (Fig. 5).
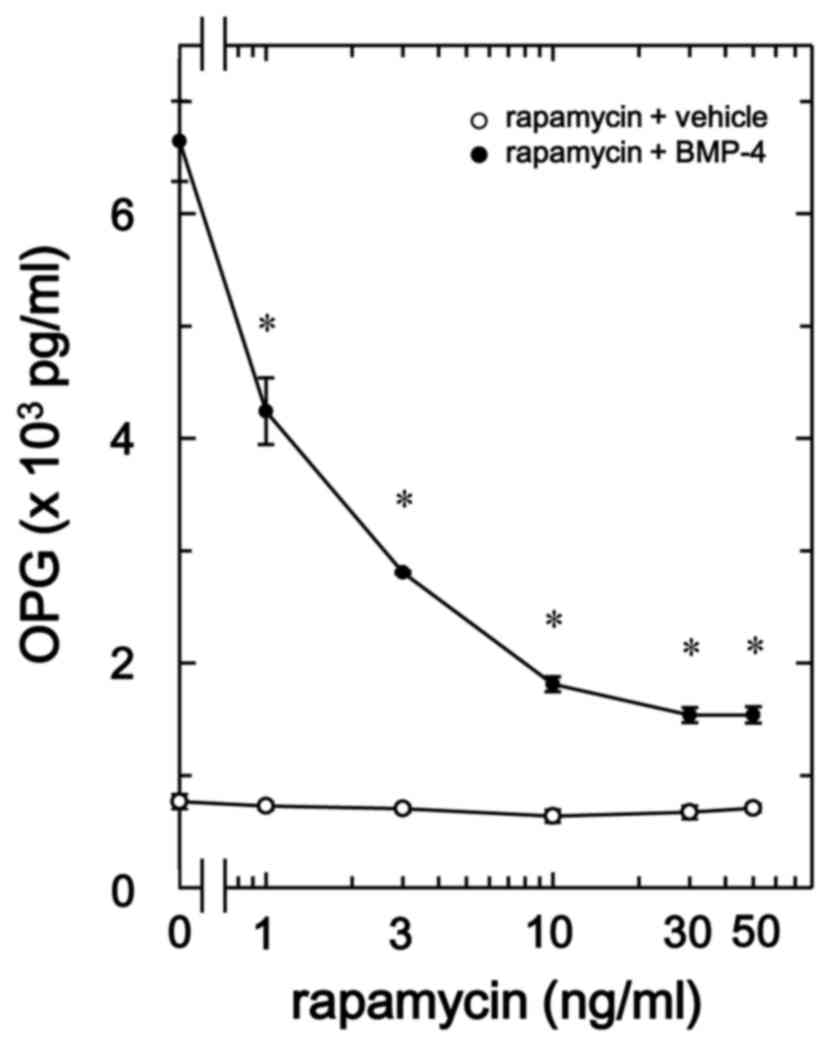
Rapamycin suppresses BMP-4-stimulated
osteoprotegerin release in MC3T3-E1 cells
BMP-4 has been demonstrated to induce the synthesis
of vascular endothelial growth factor through p70 S6 kinase in
osteoblast-like MC3T3-E1 cells (25). Therefore, the present study also
evaluated whether p70 S6 kinase serves a role in BMP-4-stimulated
osteoprotegerin synthesis in MC3T3-E1 cells. It was observed that
rapamycin, as an inhibitor of mammalian target of rapamycin (mTOR)
that activates p70 S6 kinase (29),
significantly suppressed BMP-4-stimulated osteoprotegerin release
in a dose-dependent manner between 1 and 50 ng/ml in comparison to
the value of vehicle + BMP-4 (P<0.007; Fig. 6).
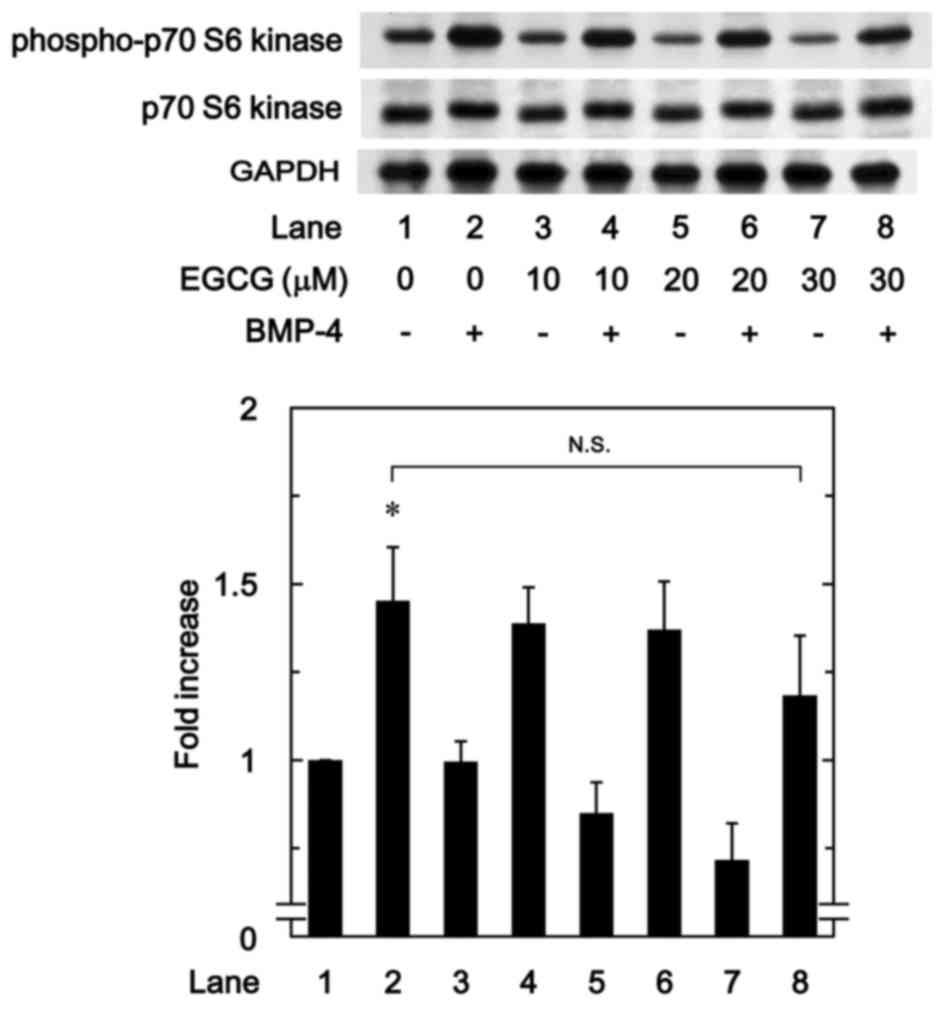
EGCG has little effect on
BMP-4-induced phosphorylation of p70 S6 kinase
To determine whether the effect of EGCG on
BMP-4-stimulated osteoprotegerin synthesis was due to the
activation of p70 S6 kinase in MC3T3-E1 cells, the present study
evaluated the effect of EGCG on BMP-4-induced phosphorylation of
p70 S6 kinase. However, it was observed that EGCG (10–30 µM) +
BMP-4 had no significant effect on BMP-4-induced phosphorylation of
p70 S6 kinase in comparison to the value of vehicle + BMP-4
(Fig. 7).
Discussion
In the present study, it was observed that EGCG,
which is a primary polyphenolic component of green tea,
significantly enhanced BMP-4-stimulated osteoprotegerin release in
osteoblast-like MC3T3-E1 cells. By contrast, CGA, a primary
polyphenolic component of coffee, had no significant effect on the
release of osteoprotegerin. It was also demonstrated that
BMP-4-induced expression of osteoprotegerin mRNA was significantly
enhanced by EGCG (10 µM) in MC3T3-E1 cells. These results suggest
that the stimulatory effects of EGCG on BMP-4-induced
osteoprotegerin release may be mediated at a transcriptional level.
Notably EGCG, but not CGA, may upregulate BMP-4-stimulated
synthesis of osteoprotegerin in osteoblast-like MC3T3-E1 cells.
It has been established that Smad proteins, as
intracellular signaling molecules, typically mediate the effects of
the TGF-β superfamily, including BMPs (15). In particular, the effects of BMPs are
exerted through Smad1/5/8, as a receptor-regulated Smad protein
(15). However, the present study
observed that EGCG had no significant effect on BMP-4-induced
phosphorylation of Smad1 in osteoblast-like MC3T3-E1 cells,
indicating that the stimulatory effect of EGCG on BMP-4-induced
osteoprotegrin synthesis does not occur through upregulation of the
Smad pathway. Recent studies have indicated that Smad-independent
pathways, including the MAPK superfamily, may mediate the effects
of BMPs in addition to Smad-dependent signaling (11,12). In
previous studies by the present authors (24,26), it
has been demonstrated that that BMP-4 promotes the activation of
p38 MAPK in osteoblast-like MC3T3-E1 cells, resulting in enhanced
synthesis of osteocalcin and VEGF. Therefore, the present study
evaluated the potential association between p38 MAPK and the effect
of EGCG on BMP-4-stimulated osteoprotegerin synthesis. However,
EGCG had no significant effect on BMP-induced phosphorylation of
p38 MAPK in MC3T3-E1 cells, indicating that the stimulatory effects
of EGCG on osteoprotegrin synthesis do not occur through p38 MAPK
signaling.
It has also been documented that BMP-4 may induce
the activation of p70 S6 kinase in osteoblast-like MC3T3-E1 cells,
and that activated p70 S6 kinase may limit BMP-4-stimulated
osteocalcin synthesis (30). Based
on these findings, the present study evaluated the potential
association of p70 S6 kinase with EGCG-enhanced osteoprotegerin
synthesis. Whereas EGCG had no significant effect on BMP-4-induced
phosphorylation of p70 S6 kinase, it was observed that
BMP-4-induced release of osteoprotegerin was suppressed by
rapamycin, as an inhibitor of mTOR and established activator of p70
S6 kinase (29). Collectively, these
data suggest that EGCG within osteoblasts may act at a point
downstream of Smad proteins, p38 MAPK, p70 S6 kinase, and/or other
signaling targets, where it is potentially associated with the
stimulation of osteoprotegerin synthesis. Future studies are
warranted to verify the molecular mechanisms underlying the effects
of EGCG on osteoprotegerin synthesis in osteoblasts.
Polyphenolic compounds present in beverages,
including green tea and coffee, are considered to have beneficial
properties for human health. In the present study, it was
demonstrated that EGCG, but not CGA, enhanced BMP-4-stimulated
osteoprotegerin synthesis in osteoblast-like MC3T3-E1 cells. EGCG
is a catechin that is predominantly present in green tea. Green tea
consumption may be associated with increased bone mass, improved
bone mineral density and a decreased risk of fracture (13). In addition, BMP-4 is associated with
bone formation and serves a key role in the process of fracture
healing (16). Conversely,
osteoprotegerin serves as a decoy receptor of the RANK ligand and
blocks RANK-RANK ligand interactions required for the
differentiation and activation of osteoclasts (1). Therefore, the stimulatory effects of
EGCG on BMP-4-stimulated osteoprotegerin synthesis may lead to
attenuation in bone resorption, resulting in an imbalance in bone
metabolism towards increased bone formation. In the present study,
EGCG enhanced BMP-4-stimulated osteoprotegerin synthesis at ≥3 µM.
In human volunteers, it has been documented that a single EGCG
dosage of 1,600 mg/day leads to a maximum plasma concentration of
11.08 µM (31). Therefore, the
concentration of EGCG used in the present in vitro study may
be achievable in vivo.
In conclusion, the present results suggest that
EGCG, but not CGA, may enhance BMP-4-stimulated osteoprotegerin
synthesis in osteoblasts. The potential modulatory effects of EGCG
osteoblast function and bone metabolism may be useful in the
prevention of fractures, particularly amongst the elderly. Future
studies are now warranted to determine the precise molecular
effects of EGCG on bone metabolism.
Acknowledgements
The authors wish to thank Mrs. Yumiko Kurokawa for
her technical assistance. The present study was supported by the
Ministry of Education (grant no. 19591042), the Ministry of Health,
Labor and Welfare (grant no. H25-Aging-General-004) and the
National Center for Geriatrics and Gerontology (grant no. 25-4,
26-12), Japan.
References
|
1
|
Simonet WS, Lacey DL, Dunstan CR, Kelley
M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, et
al: Osteoprotegerin: A novel secreted protein involved in the
regulation of bone density. Cell. 89:309–319. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zuo C, Huang Y, Bajis R, Sahih M, Li YP,
Dai K and Zhang X: Osteoblastgenesis regulation signals in bone
remodeling. Osteoporos Int. 23:1653–1663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hadjidakis DJ and Androulakis II: Bone
remodeling. Ann N Y Acad Sci. 1092:385–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rachner TD, Khosla S and Hofbauer LC:
Osteoporosis: Now and the future. Lancet. 377:1276–1287. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Seitz S, Priemel M, Zustin J, Beil FT,
Semler J, Minne H, Schinke T and Amling M: Paget's disease of bone:
Histologic analysis of 754 patients. J Bone Miner Res. 24:62–69.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tat S Kwan, Padrines M, Théoleyre S,
Heymann D and Fortun Y: IL-6, RANKL, TNF-alpha/IL-1: Interrelations
in bone resorption pathophysiology. Cytokine Growth Factor Rev.
15:49–60. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
George SE, Ramalakshmi K and Rao LJ Mohan:
A perception on health benefits of coffee. Crit Rev Food Sci Nutr.
48:464–486. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thielecke F and Boschmann M: The potential
role of green tea catechins in the prevention of the metabolic
syndrome-a review. Phytochemistry. 70:11–24. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shimizu M, Adachi S, Masuda M, Kozawa O
and Moriwaki H: Cancer chemoprevention with green tea catechins by
targeting receptor tyrosine kinases. Mol Nutr Food Res. 55:832–843.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Folwarczna J, Pytlik M, Zych M, Cegieła U,
Nowinska B, Kaczmarczyk-Sedlak I, Sliwinski L, Trzeciak H and
Trzeciak HI: Effects of caffeic and chlorogenic acids on the rat
skeletal system. Eur Rev Med Pharmacol Sci. 19:682–693.
2015.PubMed/NCBI
|
|
12
|
Kwak SC, Lee C, Kim JY, Oh HM, So HS, Lee
MS, Rho MC and Oh J: Chlorogenic acid inhibits osteoclast
differentiation and bone resorption by down-regulation of receptor
activator of nuclear factor kappa-B ligand-induced nuclear factor
of activated T cells c1 expression. Biol Pharm Bull. 36:1779–1786.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen CL, Yeh JK, Cao JJ and Wang JS: Green
tea and bone metabolism. Nutr Res. 29:437–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Singh R, Akhtar N and Haqqi TM: Green tea
polyphenol epigallocatechin-3-gallate: Inflammation and arthritis.
(corrected). Life Sci. 86:907–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyazono K, Maeda S and Imamura T: BMP
receptor signaling: Transcriptional targets, regulation of signals,
and signaling cross-talk. Cytokine Growth Factor Rev. 16:251–263.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krause C, De Gorter DJJ, Karperien M and
ten Dijke P: Signal transduction cascades controlling osteoblast
differentiationPrimer on the Metabolic Bone Diseases and Disorders
of Mineral Metabolism. Rosen CJ: 7th. John Wiley & Sons, Inc.;
Washington, DC: 2008, View Article : Google Scholar
|
|
17
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai J, Pardali E, Sánchez-Duffhues G and
ten Dijke P: BMP signaling in vascular disease. FEBS Lett.
586:1993–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuroyanagi G, Tokuda H, Yamamoto N,
Matsushima-Nishiwaki R, Mizutani J, Kozawa O and Otsuka T:
Resveratrol amplifies BMP-4-stimulated osteoprotegerin synthesis
via p38 MAPK in osteoblasts. Mol Med Rep. 12:3849–3854.
2015.PubMed/NCBI
|
|
20
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vivo differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2 in
osteoblast-like cells. Exp Cell Res. 198:130–134. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: A
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
23
|
Pryor RJ and Wittwer CT: Real-time
polymerase chain reaction and melting curve analysis. Methods Mol
Biol. 336:19–32. 2006.PubMed/NCBI
|
|
24
|
Kozawa O, Hatakeyama D and Uematsu T:
Divergent regulation by p44/p42 MAPK and p38 MAPK of bone
morphogenetic protein-4-stimulated osteocalcin synthesis in
osteoblasts. J Cell Biochem. 84:583–589. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kozawa O, Matsuno H and Uematsu T:
Involvement of p70 S6 kinase in bone morphogenetic protein
signaling: Vascular endothelial growth factor synthesis by bone
morphogenetic protein-4 in osteoblasts. J Cell Biochem. 81:430–436.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tokuda H, Hatakeyama D, Shibata T,
Akamatsu S, Oiso Y and Kozawa O: P38 MAPK regulates
BMP-4-stimulated VEGF synthesis via p70 S6 kinase in osteoblasts.
Am J Physiol Endocrinol Metab. 284:E1202–E1209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 glioma cells. J
Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Corradetti MN, Inoki K and Guan KL:
TSC2: Filling the GAP in the mTOR signaling pathway. Trend Biochem
Sci. 29:32–38. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Minamitani C, Tokuda H, Adachi S,
Matsushima-Nishiwaki R, Yamauchi J, Kato K, Natsume H, Mizutani J,
Kozawa O and Otsuka T: P70 S6 kinase limits tumor necrosis
factor-alpha-induced interleukin-6 synthesis in osteoblast-like
cells. Mol Cell Endocrinol. 315:195–200. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ullmann U, Haller J, Decourt JP, Girault
N, Girault J, Richard-Caudron AS, Pinceau B and Weber P: A single
ascending dose study of epigalocatechin gallate in healthy
volunteers. J Int Med Res. 31:88–101. 2003. View Article : Google Scholar : PubMed/NCBI
|