Introduction
Hepatitis B virus (HBV) infection is a considerable
health problem worldwide, with an estimated 2 billion of the global
population currently infected, of which 250 million are symptomatic
(1). Chronic HBV infection in humans
may lead to severe liver damage, such as cirrhosis and
hepatocellular carcinoma (2). For
treatment of chronic HBV, numerous antiviral nucleotide analog
drugs have been developed, such as adefovir (ADV), or tenofovir,
which are widely used to suppress viral replication as the
first-line defence in developing countries (3). ADV and tenofovir are two nucleoside
reverse transcriptase inhibitors; they possess few side-effects,
but a number of cases of nephrotoxicity have reportedly been caused
by these agents (4–6). A prospective study found that the
frequency of renal toxicity was 3–8% in HBV patients who were
treated with ADV therapy for 5 years (7). Thus, this increased number of patients
suffering from renal toxicity requires further investigation.
In the present study, four typical cases are
presented to investigate this complication. The study protocol was
approved by Medical Ethics Committee of Fuzhou Infectious Disease
Hospital (Fuzhou, China). All of data were used with the expressed
permission of the patients and were published anonymously. Written
informed consent was obtained from the patients prior to
publication.
Case study
Case 1
A 61-year-old male patient was referred to our
hospital after visiting several hospitals one year ago with pain in
both ankles, which gradually expanded to the knees and lumbar back
and was accompanied by recent multiple rib fractures. Since the
onset of these symptoms, the patient had experienced weight loss of
8 kg and a height decrease of 2 cm. He had a 46-year history of
HBV-related hepatitis and a 22-year history of diabetes. Notably,
24 years ago he underwent a splenectomy due to cirrhosis. Since
2008, he had been receiving ADV antiretroviral therapy until
admission (5 years in total).
The results of physical examination (data not shown)
were predominantly normal, with the exception of the rib cage
(which was tender on both the sides), indicating that he was
suffering from chronic HBV. In laboratory examination results,
HBsAg, HBeAb, HBcAb were positive, indicating that the patient was
suffering from chronic HBV (Table
I).
 | Table I.Biochemical examination of Case 1. |
Table I.
Biochemical examination of Case 1.
| Target in blood | Mount | ↑or↓ | Reference range | Target in urine | Mount | ↑or↓ | Reference range |
|---|
| TBil | 22 µmol/l | ↑ | 3–25 µmmol/l | α1-MG | 2.89 mg/dl | ↑ | 0-1.2 mg/dl |
| ALP | 175 U/l | ↑ | 45–125 U/l | β2-MG | 0.976 mg/dl | ↑ | 0-0.02 mg/dl |
| GLU | 7.8 mmol/l | ↑ | 3.9–6.1 mmol/l | P | 21 mmol/l |
|
|
| P | 0.6 mmol/l | ↓ | 0.9–1.62 mmol/l | Cl− | 81 mmol/l | ↓ | 170–250 mmol/24
h |
| K+ | 3.45 mmol/l | ↓ | 3.5–5.3 mmol/l | K+ | 35 mmol/l |
| 25–100 mmol/24 h |
|
|
|
|
| Na+ | 114 mmol/l | ↓ | 130–260 mmol/24
h |
|
|
|
|
| Ca2+ | 5.6 mmol/l |
| 2.5–7.5 mmol/24
h |
|
|
|
|
| GFR | 67.08 ml/min | ↓ | 80–120 ml/min |
|
|
|
|
| Cystatin C | 1.32 mg/l | ↑ | 0-1.03 mg/l |
|
|
|
|
| UN | 7.66 mmol/l | ↑ | 2.9–8.2 mmol/l |
The observations of the diagnostic examination are
recorded as follows:
I: Chest computed
tomography (CT): i) Presence of multiple lesions in both lungs,
suggesting inflammation; ii) multiple bilateral rib fractures; and
iii) reduced pancreas volume with multiple calcification and
hardened arteries.
II: Abdominal CT: i)
Liver cirrhosis; ii) multiple small cysts in the liver; iii)
multiple gallbladder stones; iv) absent spleen; and v) subcutaneous
nodules on the right anterior abdomen and spleen injury.
III: Thoracic and lumbar
spine magnetic resonance imaging (MRI): i) Thoracic vertebral
degeneration; ii) lumbar vertebral degeneration; and iii)
protrusion of L3-4, L4-5 intervertebral discs.
IV: MRI of the knees: i)
Bilateral contusion in distal femur and proximal tibia indicating a
posterior cruciate ligament injury; and ii) bilateral degenerative
changes in anterior horn of lateral meniscus and posterior horn of
medial meniscus, accompanied by bilateral knee joint effusion and
degeneration.
V: PET-CT: i) Multiple
lesions on the left lung and right upper lobe were diagnosed of
infectious diseases; ii) left maxillary sinusitis; iii) liver
cirrhosis, cysts in the right lobe of liver, splenectomy, and
portal hypertension changes (enlargement of portal vein and
abdominal subcutaneous varicose veins); iv) gall bladder stones,
cholecystitis, double kidney small stones and prostate
calcification; and v) obsolete fractures on the 3, 4, 6, 7, 9 right
ribs and the 6, 7, 8 left ribs; and vi) osteoporosis, bilateral
sternoclavicular joint degeneration and spinal hyperosteogeny.
VI: Pathological report
of renal biopsy: Slight diffusive mesangial proliferation was
accompanied by focal/segmental glomerulosclerosis (ischemic
sclerosis).
VII: Bone density: Mean
bone mineral density (BMD) of the lumbar spine was 0.862
g/cm2 with a T-score of −3.0. A dual femur BMD of 0.572
g/cm2 with a T-score of −3.7 indicated osteoporosis.
However, posterior superior iliac spine bone marrow cytological
analysis was normal.
The patient was diagnosed with the following
complications: i) HBV cirrhosis, compensatory stage; ii) acquired
Fanconi syndrome, ADV caused renal tubular lesion; iii)
hypophosphatemic osteomalacia; iv) post-splenectomy; and v) type 2
diabetes.
The treatment regimen was as follows: ADV therapy
was discontinued after diagnosis and was replaced with entecavir
(ETV) antiviral therapy. Meanwhile, the patient received sodium
glycerophosphate (2.16 g/day; Sino-Swed Pharmaceutical Co., Ltd.,
Beijing, China) via intravenous infusion, along with oral
administration of calcitriol capsules (0.25 µg/day; Roche Farma,
Madrid, Spain) and calcium D3 (1.5 g twice daily; Pfizer, Inc., New
York, NY, USA). Following this treatment regimen, body pain was
relieved and bone density was improved. The patient was discharged
and his treatment was amended to include concentrated divitamins
sodium syrup (10 ml, 3 times daily; Liwah Pharmaceutical Co. Ltd.,
Ningbo, China) for 12 months. Follow-up blood and urine tests were
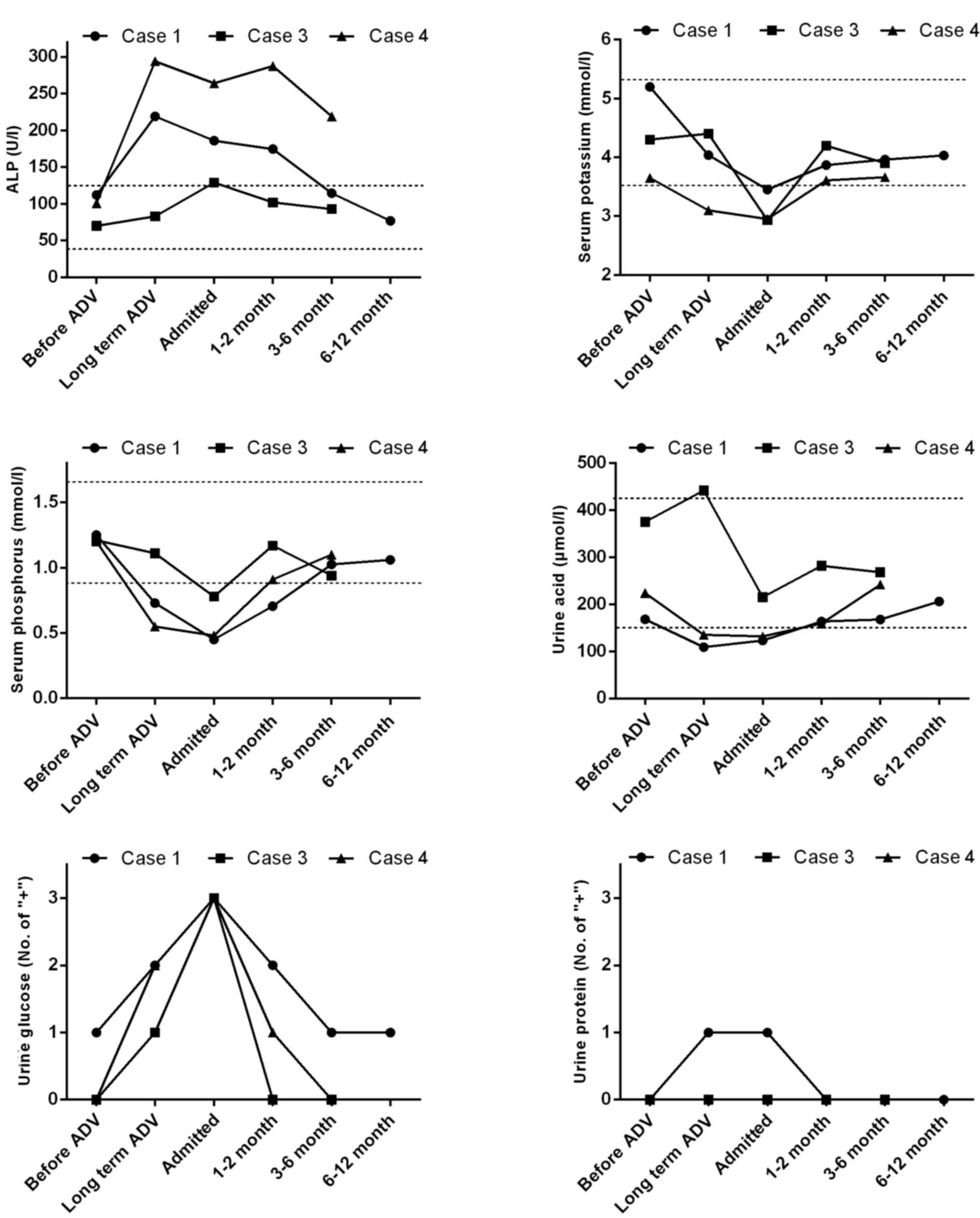
conducted at the indicated time points (Fig. 1), which showed that the alkaline
phosphatase (ALP), serum potassium, serum phosphorus, urine acid,
urine protein levels returned to normal after 3-month treatment.
Urine glucose levels did not return due to the patient's diabetes.
His hypophosphatemia was corrected and all body pain was diminished
with free movement possible. Following the successful treatment of
this patient, our hospital successively admitted another three
patients.
Case 2
A 55-year-old male patient visited our outpatient
clinic complaining of recurrent fatigue for 16 years with left heel
pain having developed in the past six months. A total of 16 years
previously, he noted a feeling of malaise and was found to have
abnormal liver function (details unknown) and was subsequently
diagnosed with HBsAg-positive ‘chronic hepatitis’ at a local
hospital. Since diagnosis, he had been admitted to local hospitals
17 times due to recurrent fatigue and abnormal liver function,
including fluctuating alanine aminotransferase and aspartate
aminotransferase levels (60–210 U/l and 45–86 U/l, respectively).
Diagnosis of HBV liver cirrhosis was confirmed by imaging results
and clinical diagnosis. Eight years before admittance to our
hospital, the patient was treated with antiviral therapy including
lamivudine (LMV), ETV and ADV. Two years prior to admission, the
patient's treatment protocol was amended to ETV+ADV antiviral
therapy due to recurrent symptoms and the detection of
2.08×106 copies/ml of HBV DNA during follow-up. Six
months before admittance, the patient began suffering from pain in
the left heel, which worsened after movement. Thus, the patient
visited our outpatient clinic for further treatment.
The patient's clinical history suggested that he was
HBsAg-positive for 22 years. Physical examination (data not shown)
demonstrated that his left heel was tender with mild lameness.
Laboratory examinations (Table II)
indicated that the patient suffered from a renal tubular lesion.
Bone density results indicated osteoporosis (lumbar spine T-score,
−2.9).
 | Table II.Physical and laboratory examination of
Case 2. |
Table II.
Physical and laboratory examination of
Case 2.
| Target in blood | Mount | ↑or↓ | Reference range |
|---|
| TBil | 23.2 µmol/l |
| 3–25 µmmol/l |
| Cr | 125.6 µmol/l | ↑ | 53–115 µmmol/l |
| Urine albumin | 31.0 mg/l | ↑ | <20 mg/l |
| Inorganic
phosphorus | 0.8 mmol/l | ↓ | 0.9–1.62 mmol/l |
| Potassium | 3.48 mmol/l | ↓ | 3.5–5.3 mmol/l |
| β2-MG | 9.00 mg/l | ↑ | 0-0.65 mg/l |
| NAG | 52.7 U/g.Cr | ↑ | 3–6 U/g.Cr |
| uRBP | 0.86 mg/l | ↑ | <0.7 mg/l |
| Lactate | 32.4 mg/dl | ↑ | <22 mg/dl |
| Cystatin C | 1.48 mg/l | ↑ | 0-1.03 mg/l |
The patient was diagnosed with: i) HBV liver
cirrhosis according to his disease history; and ii) acquired
Fanconi syndrome due to an ADV-induced renal tubular lesion.
Following diagnosis, ADV was discontinued while sodium
glycerophosphate was administered to improve hypophosphatemia and
calcitriol plus calcium D3 (suppliers as aforementioned) was
administered to improve osteoporosis. After a month-long treatment
regimen, serum phosphorus levels returned to normal and the patient
indicated that the left heel pain had been attenuated. To our
regret, this patient did not attend follow-ups at our hospital,
thus there is a lack of follow-up results.
Case 3
A 53-year-old male patient was admitted to our
outpatient clinic complaining of strengthless lower extremities and
difficulty walking for two years, which was particularly aggravated
in month prior to admission. Two years prior to admission, the
patient experienced lower extremity weakness with no evident
causes, but was not diagnosed or treated. For one month prior to
presentation at our hospital, he suffered from increased difficulty
walking, accompanied by limping and incapability in climbing
stairs.
Previous medical history (data not shown) revealed
that 10 years ago he suffered from HBV infection, liver lesions and
type 2 diabetes. Thereafter, this patient received treatment for
‘severe chronic HBV and type 2 diabetes at our hospital.
The therapeutic regimen administered to this patient
consisted of liver protection therapy and LMV antiviral therapy for
three months, which was discontinued after recovery. The patient
became resistant to LMV nine years ago, which was replaced with ADV
antiviral therapy until present (9 years in total).
Recent physical examination (data not shown)
indicated the patient had a dim complexion and bilateral lower
extremity muscle dystrophy with a muscle strength value of 4+.
Laboratory examinations (Table
III) indicated he was a chronically HBV-infected with
imbalanced electrolytes and liver and renal tubular lesion.
Meanwhile, accessory examination, including electromyography (EMG),
suggested neurogenic damage particularly in both lower extremities.
Bone density analysis indicated a left phalanx T-score of −3.63, a
Z-score of −3.09 and a ratio of 68% to peak bone density,
suggesting osteoporosis. Abdominal CT scan indicated: Normal
morphology, size, and parenchymal density of the liver; no
expansion of intrahepatic bile ducts with evenly distributed
intrahepatic vessels; normal size and shape of the gall bladder and
the spleen, and no intra-abdominal lymph nodes. On the basis of
these findings, the patient was diagnosed with: i) chronic HBV; ii)
diabetes type 2; and iii) ADV-induced Fanconi syndrome.
 | Table III.Biochemical examination of Case 3. |
Table III.
Biochemical examination of Case 3.
| Target in blood | Mount | ↑or↓ | Reference range | Target in urine | Mount | ↑or↓ | Reference range |
|---|
| Cr | 86 µmol/l |
| 53–115 µmmol/l | Urine glucose | 3+ |
| – |
| TBil | 12 µmol/l |
| 3–25 µmmol/l | β2-MG | 3179 mg/l | ↑ | <300 mg/l |
| ALP | 129 U/l | ↑ | 45–125 U/l |
|
|
|
|
| BUN | 6.8 mmol/l |
| 2.9–8.2 mmol/l |
|
|
|
|
| Cl− | 107 mmol/l |
| 96–108 mmol/l |
|
|
|
|
|
Ca2+ | 2.35 mmol/l |
| 2.08–2.6
mmol/l |
|
|
|
|
| P | 0.78 mmol/l | ↓ | 0.9–1.62
mmol/l |
|
|
|
|
| K+ | 2.93 mmol/l | ↓ | 3.5–5.3 mmol/l |
|
|
|
|
The treatment regimen followed was: ADV was
discontinued and replaced with ETV antiviral therapy in combination
with other supportive treatment, including compensation of
potassium with sustained-release tablets of potassium chloride.
Phosphate levels were elevated by administering glycerophosphate
sodium injection, and osteoporosis was ameliorated with calcitriol
and calcium tablets. The patient was discharged after his symptoms
improved. The primary monitoring indexes, including electrolytes
and urine glucose levels, returned to normal after one month of
treatment (Fig. 1).
Case 4
A 59-year-old female patient, who complained of
‘joint pain for two years followed by worsening of complications in
2 months’, was admitted to our hospital. She had a 20-year history
of liver cirrhosis and 12 years ago she underwent a splenectomy.
The patient began receiving ADV antiviral therapy in 2007 (6 years
prior to admission).
Physical examination (data not shown) of the patient
did not reveal any abnormal symptoms, and the laboratory
examination (Table IV) presented
imbalanced electrolytes, and liver and renal function abnormality.
Whole body PET-CT scan recorded the following: i) No abnormal
metabolic imaging development; ii) few obsolete lesions in the back
section of the left lung; iii) small stones in the left kidney; iv)
osteoporosis; and v) an old fracture of the right ankle. On the
basis of these observations, the patient was diagnosed with: i) HBV
liver cirrhosis (according to her disease history); ii) ADV-induced
Fanconi syndrome; iii) hypophosphatemic osteomalacia; and iv)
hypokalemia.
 | Table IV.Biochemical examination of Case
4. |
Table IV.
Biochemical examination of Case
4.
| Target in
blood | Mount | ↑or↓ | Reference
range | Target in
urine | Mount | ↑or↓ | Reference
range |
|---|
| ALP | 264 U/l | ↑ | 45–125 U/l | Urine glucose | 1+ |
| – |
| Cystatin C | 1.15 mg/l | ↑ | 0-1.03 mg/l | pH | 7.0 | ↑ | 5.5–6.5 |
| Cl− | 111 mmol/l | ↑ | 96–108 mmol/l | Cl− | 75.33 mmol | ↓ | 170–250 mmol/24
h |
|
Ca2+ | 2.07 mmol/l | ↓ | 2.08–2.6
mmol/l | K+ | 31.155 mmol |
| 25–100 mmol/24
h |
| P | 0.48 mmol/l | ↓ | 0.9–1.62
mmol/l |
|
|
|
|
| K+ | 2.95 mmol/l | ↓ | 3.5–5.3 mmol/l |
|
|
|
|
Following diagnosis, ADV was discontinued and was
replaced with ETV. After phosphorus, potassium and calcium
compensation, the patient's symptoms of joint pain were markedly
improved. The majority of the parameters were normal during
hospital review (Fig. 1), with the
exception of ALP.
Discussion
Fanconi syndrome is a disease characterized by
dysfunction of the proximal renal tubules resulting from various
pathogenic events. It was firstly reported by Lignae in 1924 and
further defined by Fanconi in 1936 (8). In this syndrome, there is excessive
excretion of glucose, bicarbonate, phosphate, uric acid, low
molecular weight urinary proteins, potassium, sodium, and calcium,
some amino acids, and also water from the urine due to abnormal
proximal tubule reabsorption and 1α-hydroxylase dysfunction. As a
consequence, patients present with clinical features that include
phosphate in the urine, renal glycosuria, renal aminoaciduria,
proteinuria, and hypophosphatemia, etc (9).
In the present study, common manifestations were
observed among all the four patients. Firstly, all the patients
were all aged above 50 years and were diagnosed as HBV cirrhosis
case with long-term use of ADV (2–9 years). Secondly, the patients
all suffered from systemic bone pain, osteoporosis, and walking
difficulties. Thirdly, laboratory examinations showed renal tubular
reabsorption dysfunction, imbalanced electrolyte acid-base ratio,
and elevated cystatin C levels, and bone density analysis indicated
osteoporosis. Finally, pathological analysis of kidney specimen
from Case 1 suggested mild diffusive renal mesangial proliferation
accompanied with focal/segmental glomerulosclerosis, mild tubular
atrophy plus interstitial fibrosis, and atherosclerosis. All the
patients presented with symptoms of severe hypophosphatemia, severe
diffused systemic pain and osteoporosis, which could be easily
misdiagnosed as osteomalacia caused by other metabolic bone
diseases leading to excessive loss of urinary phosphate.
ADV-induced Fanconi syndrome was the main reason that contributed
to increased urinary excretion of phosphorus in all the four cases
described in the present study.
Case 1, who was a middle-age man with no family
history of genetic disorders or hypoglycemia, exhibited normal
serum ceruloplasmin levels. Therefore, this may rule out the
possibility of some congenital or inherited genetic disorders,
including Pompe's syndrome and amyostatic syndrome. It is more
likely for him to have acquired Fanconi syndrome after 5-year
therapy of ADV (dose, 10 mg/day), which was administered to treat
HBV cirrhosis. Bone marrow biopsy was conducted twice; however, no
abnormalities were detected during the examination. In addition,
levels of serum globulin and immunoglobulin were normal in plasma
of the patient, which may exclude the diagnosis of multiple myeloma
and light chain nephropathy. Pathological analysis of renal biopsy
showed mild diffusive mesangial proliferation with focal segmental
glomerulosclerosis (ischemic sclerosis), mild tubular atrophy plus
interstitial fibrosis and atherosclerosis. In recent years, it has
been demonstrated that long term use of nucleoside analogues
(including ADV and tenofovir) can cause proximal tubule dysfunction
namely Fanconi syndrome (3,10). The mechanism that is most likely is
that nucleoside analogues may inhibit DNA synthesis in renal
tubular cell mitochondria resulting in loss of mitochondrial DNA in
proximal tubule. In addition, due to their large molecular weights
(501 and 636 kDa, respectively, according to the Drugbank database;
www.drugbank.ca), ADV and tenofovir may cause
drug accumulation in the renal tubules, which would disturb the
function of transporter proteins, ultimately leading to dysfunction
of the proximal tubule (11,12). In recent years, we have observed such
patients frequently in clinical practice, in patients who have
undergone >2 years of ADV therapy (range, 2–9 years) (13); however, the relationship of renal
toxicity with drug dose, patient age, and basal kidney diseases
requires further research. We hypothesize that the risk factors of
kidney damage include age (>50 years), degree of renal
impairment before treatment (with or without diabetic nephropathy
and hypertensive nephropathy), and drug history (combined with
other renal toxic drugs or not). Since two cases (cases 1 and 3)
had a history of diabetes, the kidneys of these patients were more
susceptible to damage due to the administration of diabetic
nephropathy drugs.
Taking into account that the main complaints of the
patients were muscle soreness, difficulty walking or spontaneous
fractures, many doctors' neglected the detection and diagnosis of
Fanconi syndrome induced by drugs and misdiagnosed the patients
with multiple myeloma or metastatic tumor of bone from other
diseases. Some patients were admitted to rheumatology, orthopedics,
nephrology or other departments in different hospitals and still
did not receive prompt diagnosis and effective treatment,
demonstrating a limited understanding of the disease.
Typical clinical manifestations of hypophosphatemia,
such as fatigue, proximal myopathy, dysphagia, intestinal
obstruction and systolic dysfunction are not noteworthy and
indicative of Fanconi syndrome, unless the phosphorus levels were
below 1 mg/dl (14). The central
nervous system may also be involved, causing irritability, delirium
or even coma, whereas hemolysis and granulocyte dysfunction may
occur rarely (15,16). Chronic hypophosphatemia leads to a
decline in bone mineral density, contributing to rickets in
children and osteomalacia in adults. In adults, skeletal
deformities are relatively rare, although diffusive bone pain may
occur all over the body, resulting in weight-bearing ambulation and
abnormal gait (17).
The mainstay of treatment of drug-induced Fanconi
syndrome is to discontinue the drugs that are suspected to have led
to development (including ADV or tenofovir), and switch to an
alternative antiviral strategy. Typically, the function of the
proximal tubules can be recovered after drug cessation. Meanwhile,
electrolyte disturbances and metabolic acidosis due to abnormal
proximal tubule reabsorption and excessive loss of bicarbonate,
phosphate, potassium, sodium and other substances requires
correction. In the present case series, all four patients were
diagnosed in a timely manner with prompt discontinuation of ADV in
combination with supportive treatment, including calcium
compensation and electrolyte imbalance correction. The therapeutic
effects were obvious with smooth healing of bone fractures, pain
relief and an improvement in walking difficulty noted.
In conclusion, the chances of misdiagnosing or
failing to diagnose drug-induced secondary Fanconi syndrome are
high. For example, in the present case series 2/4 patients (cases 1
and 3) were diagnosed with Fanconi syndrome after having continuous
walking difficulties for 2 years. This increases unnecessary agony
and stress for the patients. In clinical practice, Fanconi syndrome
should be considered in patients with walking difficulty onset in
adulthood accompanied with a history of Fanconi syndrome-inducible
medication. Glomerular and tubular function indicators, such as the
glomerular filtration rate, serum phosphorus concentration,
proteinuria and glycosuria, should be closely monitored when
Fanconi syndrome-inducible medication must be administered. Once
Fanconi syndrome is diagnosed, the suspected agent should be
immediately discontinued with prompt symptomatic treatment.
Acknowledgements
This work was supported by the National Science and
Technology project for the prevention and control of AIDS, viral
hepatitis and other major infectious diseases (grant no.
2008ZX10002-005), the National Natural Science Foundation of China
(grant no. 81602102), the Natural Science Foundation of Fujian
Province (grant nos. 2015J05174 and 2016J01592), the Project of
Nanjing Military Region (grant no. 15MS136), the Scientific
Research Project of Health and Family Planning Commission of Fujian
province (grant nos. 2014-2-43 and 2015-1-94) and the Scientific
Foundation of Fuzhou Health Department (grant no.
2014-S-139-3).
Glossary
Abbreviations
Abbreviations:
|
ADV
|
adefovir
|
|
ETV
|
entecavir
|
|
LMV
|
lamivudine
|
|
MRI
|
magnetic resonance imaging
|
|
PET-CT
|
positron emission tomography computed
tomography
|
|
ALP
|
alkaline phosphatase
|
References
|
1
|
World Health Organization, . Hepatitis B
Fact Sheet. 204:http://who.int/mediacentre/factsheets/fs204/en/January
23–2013
|
|
2
|
Zhu H, Wu J and Shen X: Genome-wide
association study: New genetic insights into HBV/HCV-related
hepatocellular carcinoma genomes. Scand J Gastroenterol. 1–7.
2016.
|
|
3
|
Uteng M, Mahl A, Beckmann N, Piaia A,
Ledieu D, Dubost V, Tritto E, Wolf A, Moulin P, Li L, Chibout SD,
et al: Comparative renal safety assessment of the hepatitis B
drugs, adefovir, tenofovir, telbivudine and entecavir in rats.
Toxicol Sci. 2016.PubMed/NCBI
|
|
4
|
Verhelst D, Monge M, Meynard JL, Fouqueray
B, Mougenot B, Girard PM, Ronco P and Rossert J: Fanconi syndrome
and renal failure induced by tenofovir: A first case report. Am J
Kidney Dis. 40:1331–1333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vigano M, Lampertico P and Colombo M: Drug
safety evaluation of adefovir in HBV infection. Expert Opin Drug
Saf. 10:809–818. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hadziyannis SJ, Tassopoulos NC, Heathcote
EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z,
Ma J, et al: Long-term therapy with adefovir dipivoxil for
HBeAg-negative chronic hepatitis B for up to 5 years.
Gastroenterology. 131:1743–1751. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fontana RJ: Side effects of long-term oral
antiviral therapy for hepatitis B. Hepatology. 49 Suppl
5:S185–S195. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mathew G and Knaus SJ: Acquired Fanconi's
syndrome associated with tenofovir therapy. J Gen Intern Med.
21:C3–C5. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Izzedine H, Launay-Vacher V, Isnard-Bagnis
C and Deray G: Drug-induced Fanconi's syndrome. Am J Kidney Dis.
41:292–309. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang XB, Zhu XC, Huang XY, Ye WJ and Wang
LX: Fanconi syndrome due to prolonged use of low-dose adefovir. J
Res Med Sci. 20:416–419. 2015.PubMed/NCBI
|
|
11
|
George N, Basu G, Mohapatra A, Zachariah
U, Abraham P, Korula A, Varughese S, Jacob CK and Tamilarasi V:
Adefovir nephrotoxicity in a renal allograft recipient. Indian J
Nephrol. 25:180–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fontana RJ: Side effects of long-term oral
antiviral therapy for hepatitis B. Hepatology. 49:S185–195. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin Y, Pan F, Wang Y, Chen Z, Lin C, Yao
L, Zhang X, Zhou R and Pan C: Adefovir dipivoxil-induced Fanconi
syndrome and its predictive factors: A study of 28 cases. Oncol
Lett. 13:307–314. 2017.PubMed/NCBI
|
|
14
|
Weisinger JR and Bellorín-Font E:
Magnesium and phosphorus. Lancet. 352:391–396. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takeda E: Calcium pros and cons
significance and risk of phosphorus supplementation. The necessity
of phosphorus supplementation for hypophosphatemia. Clin Calcium.
21:167–170. 2011.(Article in Japanese).
|
|
16
|
Håglin L: Using phosphate supplementation
to reverse hypophosphatemia and phosphate depletion in neurological
disease and disturbance. Nutr Neurosci. 19:213–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hamnvik OP, Becker CB, Levy BD and
Loscalzo J: Clinical problem-solving. Wasting away. N Engl J Med.
370:959–966. 2014. View Article : Google Scholar : PubMed/NCBI
|