Introduction
Chronic heart failure (CHF) is the result of the
majority of cardiovascular diseases, and is a major cause of death.
With the growth of the aging population, increased incidence of
hypertension and coronary heart disease, and declining mortality
from acute coronary heart disease, the prevalence of CHF is
increasing. Previous studies have shown that the myocardial cell
surface expresses 4 β-adrenergic receptors (βAR), the majority of
which are β1AR and β2AR. In human ventricle,
β1AR constitutes 70–80% of total βAR, while
β2AR constitutes the remaining 20–30%. The effects of
βAR activation are mainly mediated through β1AR and
β2AR. In the heart, stimulation of βAR is the most
potent means of increasing cardiac contractility and relaxation in
response to stress or a ‘fight-or-flight’ situation. However,
sustained βA stimulation promotes pathological cardiac remodeling
such as myocyte hypertrophy, apoptosis and necrosis, thus
contributing to the pathogenesis of CHF. Lack of cardiac
contractility mediated by βAR was the most significant
contradiction, and some suggested that increasing the expression of
cardiac βAR in patients with HF by transgenic technology could
reverse the lack of cardiac contractility. At present, research in
transgenic mice (1,2), rat models of HF (3) and other small animals confirmed that
increased expression of cardiac β2AR significantly
improved contractile function and demonstrated potential
development of β2AR gene therapy for HF. However, the
effect of different expression levels of the β2AR gene
in cardiocytes of large mammals with HF for improving contractile
function is less reported in the literature. In this study, an HF
model in canines was established by rapid pacing. Changes in the
expression of β2AR protein during HF, and the function
of cardiocytes after transfection with Adv-β2AR were
observed.
Materials and methods
Materials
Adult canines (male, 10–15 kg) were kindly supplied
by the Medical College of Xuzhou (Xuzhou, China).
SDS-polyacrylamide gel electrophoresis (PAGE) gel kit was from
BiYunTian Biotechnology Research Institute (Nantong, China).
Molecular weight Marker, anti-mouse IgG and anti-rabbit IgG were
from Sigma (St. Louis, MO, USA). Anti-β-actin was from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Anti-β2AR
(H-20):sc-569 was from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA). NBT/BCIP was from Promega Corp. (Madison, WI, USA).
Protease inhibitor mixture was from Merck Millipore (Billerica, MA,
USA). Single cell dynamic edge detection system was from IonOptix
Corp. (Westwood, MA, USA). Full wavelength microplate reader was
from Bio-Rad (Hercules, CA, USA). Gel electrophoresis system and
semi-dry transfer system were from Bio-Rad (Berkeley, CA, USA).
Decolorization shaker was obtained from Taicang Instrument
(Taicang, China).
Establishment of HF model
Adult canines were weighed after intraperitoneal
anesthesia by pentobarbital sodium (3%, 1 ml/kg). Next, canines
underwent tracheal intubation and were connected to a ventilator
(tidal volume was 200–300 ml, frequency was 12 times/min,
exhale/inhale ratio was 1/1.5). A transverse incision at the 5th
intercostal of the left chest was made to expose the heart,
followed by a longitudinal pericardial incision. The anode of the
pacing lead was placed on the surface of the right ventricle, the
pericardium was sutured, and the cathode of the pacing lead was
planted on the surface of the left pericardium. The chest was then
closed. The anode and cathode of the pacing lead pierced the skin
of the back and was connected with a pacemaker. We started the
pacemaker after one week to maintain rapid pacing at 240 times/min,
and measured cardiac function after four weeks of pacing by
echocardiography. The animal experiments were approved by the
Animal Ethics Committee of Xuzhou Medical University.
Determination of cardiac function in
canines
Cardiac function was measured under anesthesia
(method ibid.) by echocardiography in the control group (no pacing
after surgery) at 1 and 5 weeks after surgery and in the HF group
before pacing (1 week after surgery) and after pacing for four
weeks (5 weeks after surgery). We took the section of the
parasternal long axis, and used M-ultrasound to measure in the
guided two-dimensional ultrasound. Measurement data averaged three
consecutive cardiac cycles. Observational indices included:
Internal diameter of left atrium (LA), internal diameter of left
ventricular (LV) end-diastolic, internal diameter of LV
end-systolic, interventricular septal thickness end-diastolic, LV
ejection fraction (EF) and LV fractional shortening were obtained
by the computer system of the ultrasonic detector.
Isolation and culture of
cardiocytes
Each canine was anesthetized (method ibid.) and the
chest was opened under mechanical ventilation. The heart was
removed and quickly placed in frozen Tyrode's solution. The left
anterior branch of the coronary artery of the heart was connected
to a constant temperature perfusion device, and was successively
perfused with Tyrode's solution, calcium-free solution and enzyme
solution. Digested myocardium was cut-off and placed in a dish
containing enzyme solution, cut into thin slices, oscillated,
filtered, and centrifuged. The supernatant was removed and
incubated twice. Cells were cultured in M199 medium.
Transfection of cardiocytes
Cultured cardiocytes were treated with E1 defective
recombinant adenovirus as a carrier of the human
β2AR-enhanced green fluorescent protein (EGFP) or EGFP
gene. The multiplicity of infection was 100.
Western blotting
Cardiocytes were collected after 48 h of adenovirus
transfection, washed twice with M199 medium, centrifuged, and the
supernatant was removed. Cell pellet was mixed with buffer
containing protease inhibitor cocktail. Cells were lysed by
sonication and cryopreserved at −80°C. Bovine serum albumin was
used as standard to determine protein content.
The following operations were carried out at 4°C.
Samples were mixed with equal volumes of 4X Laemmli sample buffer
and boiled in a water bath for 5 min for protein denaturation.
Equal amounts of protein (100 µg) were separated by SDS-PAGE, and
electrically transferred to nitrocellulose (NC) membranes by
semi-dry method. After NC membranes were incubated at room
temperature for 3 h in blocking solution, anti-β2AR
antibody (1:200) was added and incubated for 4 h at room
temperature or overnight at 4°C. Membranes were washed with TBST (5
min × 3), and incubated in alkaline phosphatase (AP) labeled
secondary antibody working solution for 2 h at room temperature.
Membranes were washed again in TBST (5 min×3) and washed in water.
NBT/BCIP kit was used to color fresh AP reagent, and washed in
running water to terminate the reaction. Color bands in the
scanning film were analyzed with Image-Pro Plus software (version
10.0; Media Cybernetics, Silver Springs, MD, USA); SigmaStat
(version 3.5; Jandel Scientific, San Rafael, CA) and SigmaPlot
(version 11.0; Systat Software, San Jose, CA, USA). The optical
density of the bands were marked by multiples of the control group
on the same membrane.
Determination of intracellular cAMP
levels
Cardiocytes were collected 48 h after transfection
with adenovirus. Cells were lysed by freezing and thawing,
supernatant was collected and intracellular cAMP was assayed by
ELISA. Some cells were treated with isoproterenol (ISO) and allowed
to react for 2 h. The final concentration of ISO was
10−6 mol/l.
Determination of contractile function
of cardiocytes
The length-time curve of cardiocytes were recorded
through a single cell dynamic edge detection system. Cells were
perfused with Krebs-Henseleit solution containing 119 mM NaCl, 4.7
mM KCl, 0.94 mM MgSO4·7H2O, 1.2 mM
KH2PO4, 25 mM NaHCO3, 11.5 mM
Glucose·H2O and 1 mM CaCl2, pH 7.4, and were
electrically stimulated at 0.5 Hz. Cells with stable contraction
amplitude changes after intervention were selected to record the
results. The percentage of cell contraction amplitude, time to peak
contraction (TTP), time to 50% relaxation (R50) and time to R90
were obtained by IonWizard analysis software (IonOptix Corp.). The
concentration of ISO that caused 50% maximum contraction was
indicated as pD2.
Statistical analysis
Data are presented as mean ± standard deviation.
Comparisons were made by Student's t-test or paired t-test when
appropriate. Analysis of pD2 was through nonlinear regression
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical manifestations
Canines in the HF group showed loss of appetite and
gradually decreased activity after two weeks of rapid pacing. After
4 weeks, they experienced shortness of breath and significantly
decreased diet. Canines in the control group did not show these
changes over the same period.
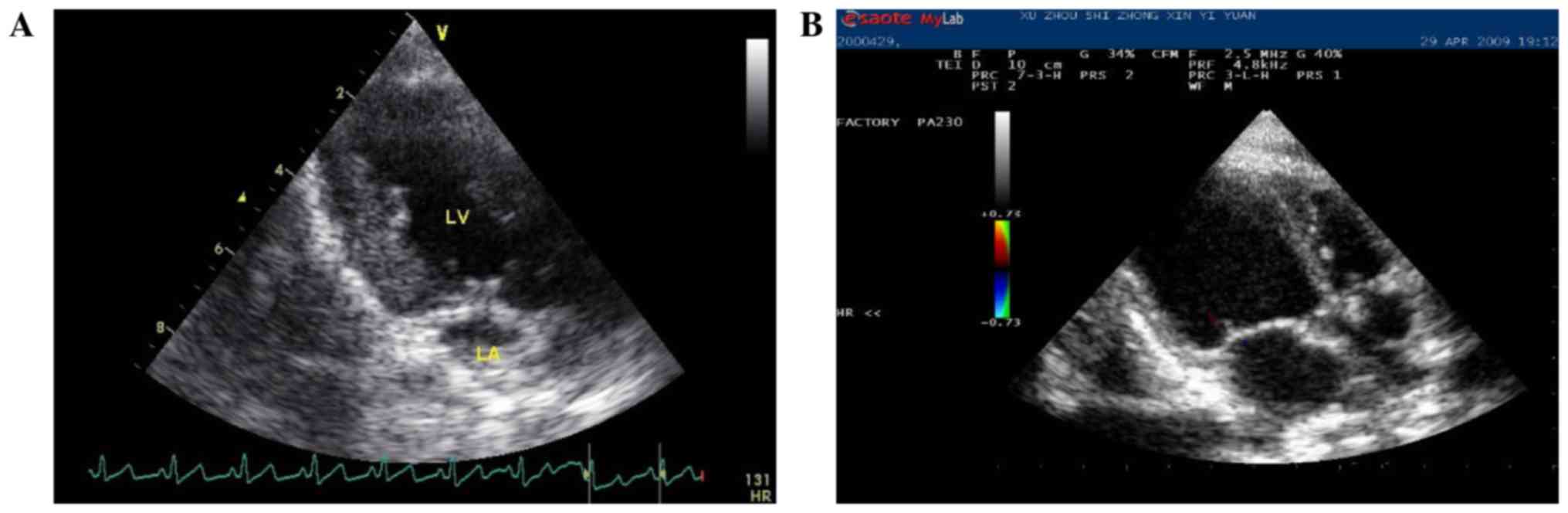
The change of echocardiography index
before and after 4 weeks of rapid pacing
Canines in the HF group showed mitral regurgitation
of different degrees and the related indexes showed significantly
increased internal diameter of LA and left LV. In addition,
myocardial thickness and EF decreased significantly (Table I and Fig.
1).
 | Table I.The result of the determination of
the heart fuction of canines (n=10, mean ± standard deviation). |
Table I.
The result of the determination of
the heart fuction of canines (n=10, mean ± standard deviation).
|
| Control group | Heart failure |
|---|
|
|
|
|
|---|
|
Characteristics | 1 week after
surgery | 5 weeks after
surgery | Before pacing | Pacing for 4
weeks |
|---|
| LA, mm | 16.1±1.2 | 15.5±0.9 | 15.7±1.2 |
21.3±1.0a |
| IVS, mm |
6.9±0.5 |
6.8±0.3 |
6.8±0.4 |
4.5±0.4a |
| LVEDD, mm | 29.4±2.3 | 30.3±2.3 | 27.7±1.9 |
38.9±2.2a |
| LVESD, mm | 18.8±2.1 | 20.3±2.1 | 17.8±1.5 |
32.9±2.1a |
| EF, % | 67.5±4.4 | 63.0±4.2 | 67.7±6.5 |
32.7±6.9a |
| FS, % | 36.2±3.4 | 33.1±3.1 | 36.2±4.9 |
15.2±3.7a |
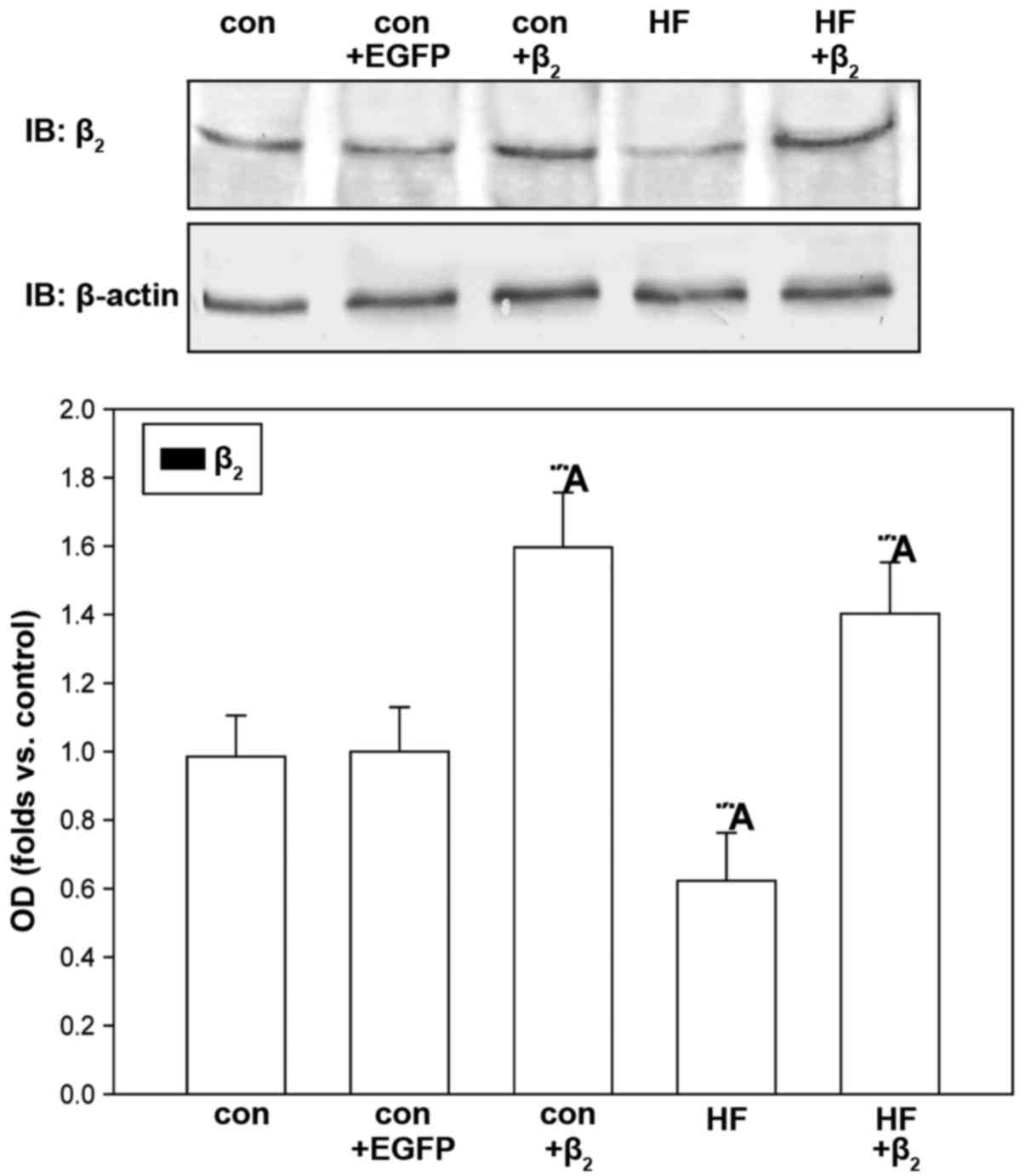
Identification of β2AR
protein expression in cardiocytes cultured for 48 h
Western blot analysis showed that, compared with
untransfected cells, β2AR protein expression of
cardiocytes increased significantly in both the control group and
HF group after transfection with β2AR-EGFP for 48 h.
Compared with the control group, β2AR protein expression
of cardiocytes in HF group did not change significantly.
β2AR protein expression of cardiocytes in the control
group did not change significantly either after transfection with
the EGFP gene for 48 h (Fig. 2).
The effect of β2AR gene
overexpression on intracellular cAMP concentration of
cardiocytes
ELISA analysis showed that, compared with the
control group, intracellular cAMP concentration in the HF group
decreased significantly in the basic state (concentration of
calcium was 1 mmol/l) and ISO stimulated state (concentration of
ISO was 10−6 mol/l) (Fig.
3A). Cardiocytes of the control group were then transfected
with β2AR-EGFP or EGFP for 48 h. Compared with
untransfected cells, intracellular cAMP concentration of the
control group increased significantly in the basic and ISO
stimulated states after transfection with β2AR-EGFP,
while intracellular cAMP concentration of the control group did not
change significantly in either basic or ISO stimulated states after
transfection with EGFP (Fig.
3B).
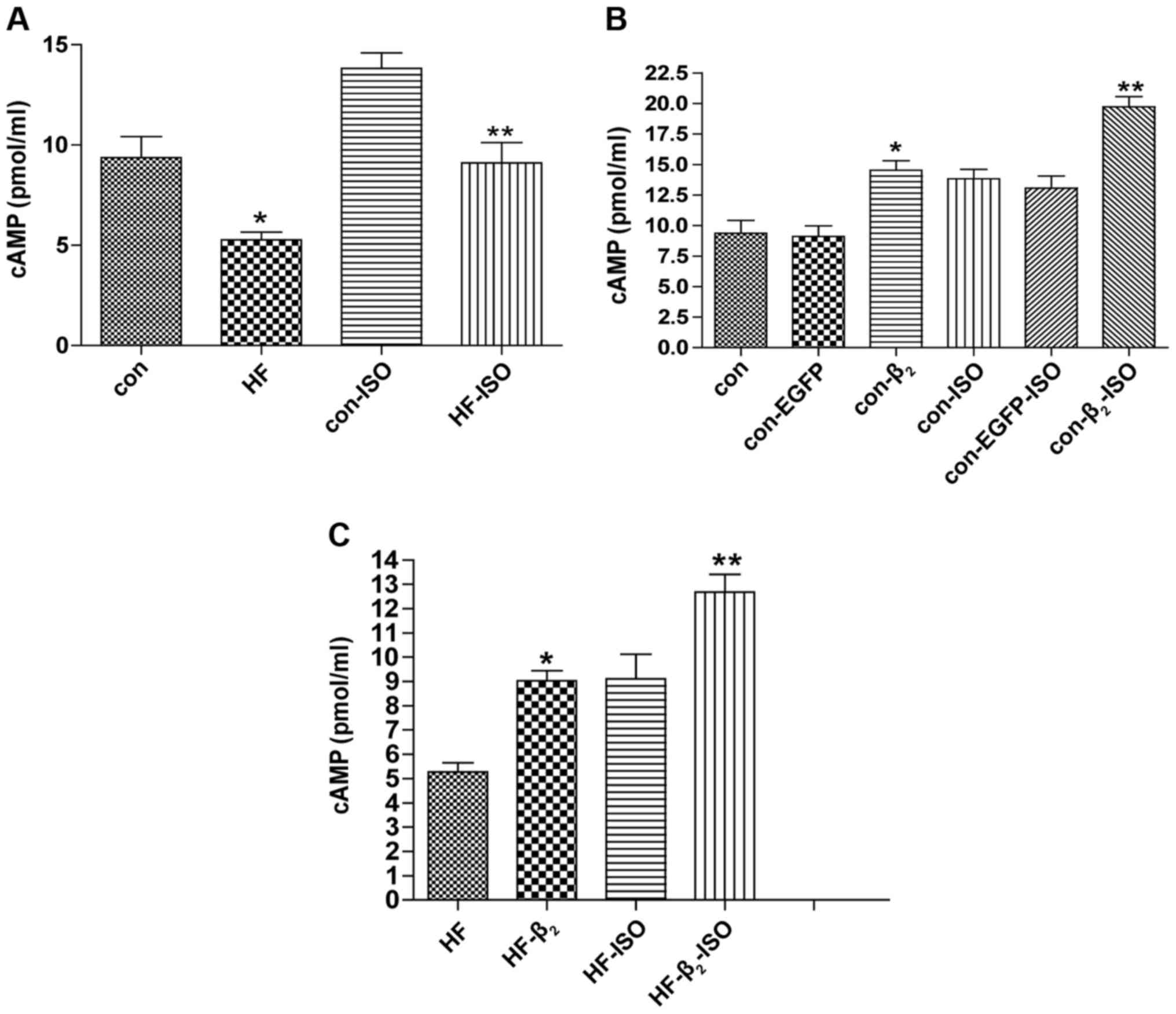 | Figure 3.Changes of intracellular cAMP
concentration after transfection with β2AR-EGFP. (A and
B) Compared with con, *P<0.05, n=6; compared with con-ISO,
**P<0.01, n=6. (C) Compared with HF, *P<0.05, n=6; compared
with HF-ISO, **P<0.01, n=6. β2AR,
β2-adrenergic receptor; EGFP, enhanced green fluorescent
protein; con, control; HF, heart failure; ISO, isoproterenol. |
Cardiocytes of the HF group were also
transfected with the β2AR-EGFP gene for 48 h
Compared with untransfected cells, intracellular
cAMP concentration of the HF group increased significantly in basic
and ISO stimulated states after transfection with
β2AR-EGFP (Fig. 3C).
The effect of β2AR gene overexpression
on contractile function of cardiocytes
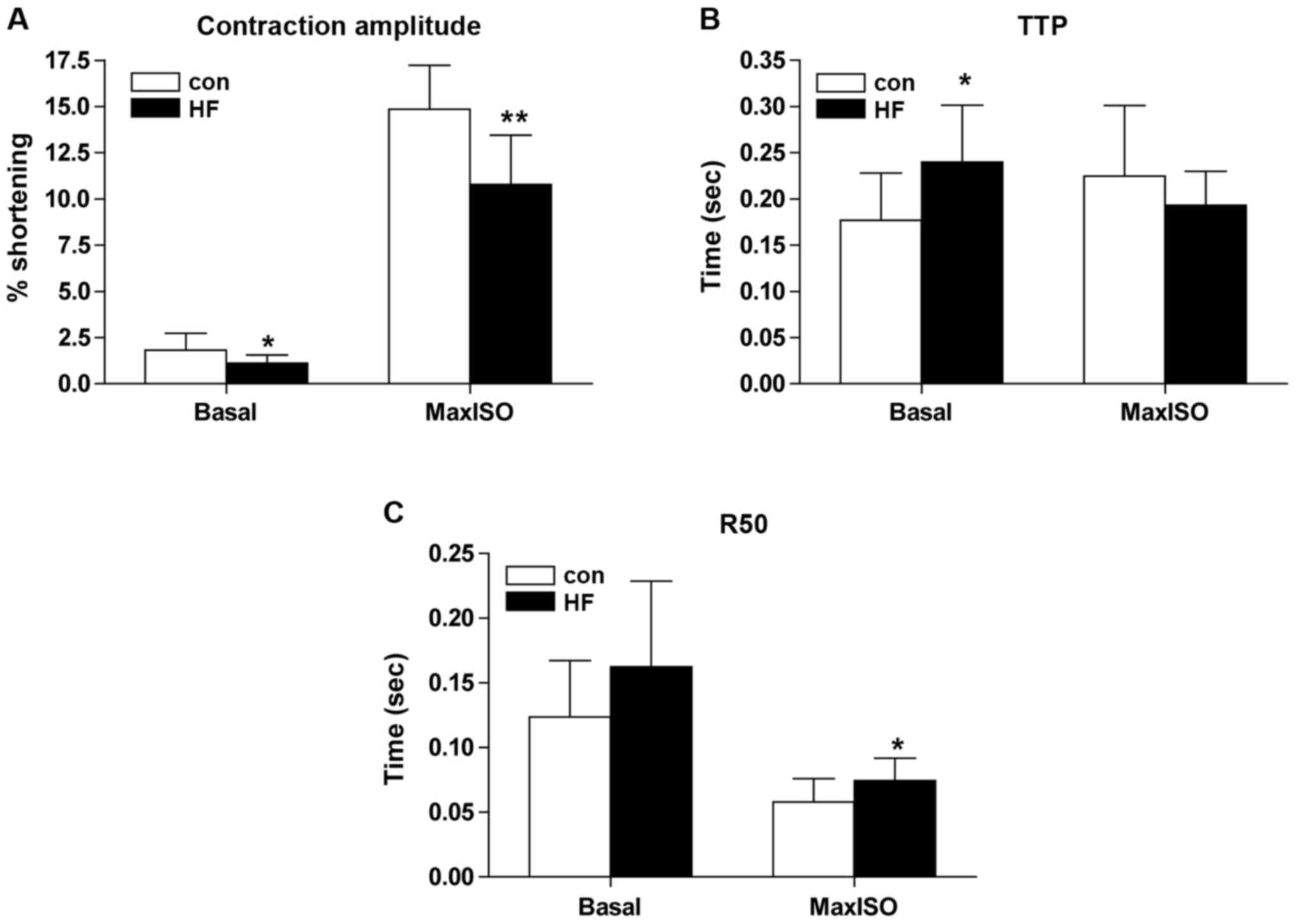
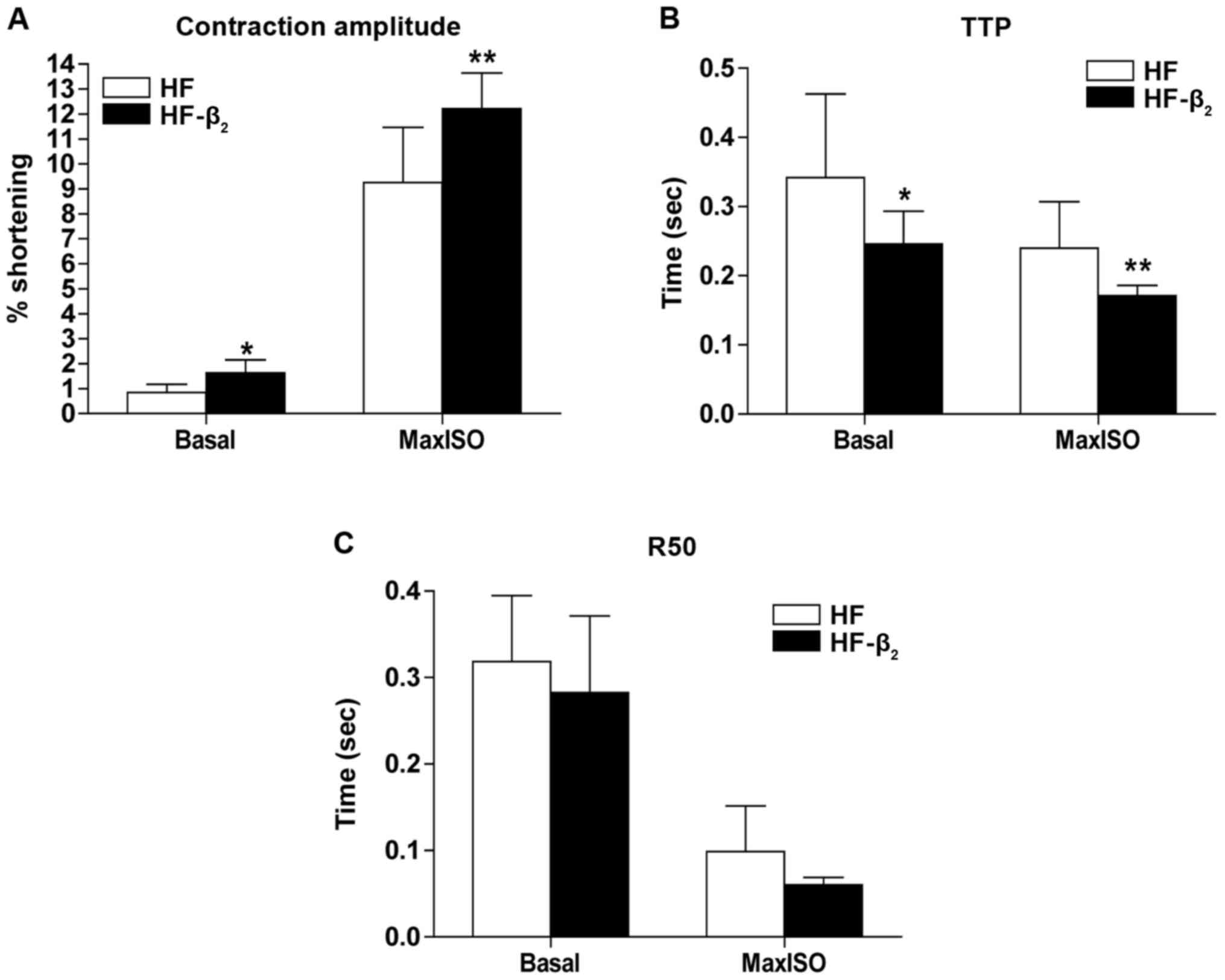
Results of contractile function of cardiocytes
separated immediately showed that compared with the control group
(n=15), the percentage of cell contraction amplitude decreased
significantly (Fig. 4A), TTP
increased significantly (Fig. 4B) in
the basic state (concentration of calcium was 1 mmol/l), the
percentage of cell contraction amplitude decreased (Fig. 4A), and R50 increased significantly
(Fig. 4C) in the state of maximal
contraction stimulated by ISO of cardiocytes in the HF group.
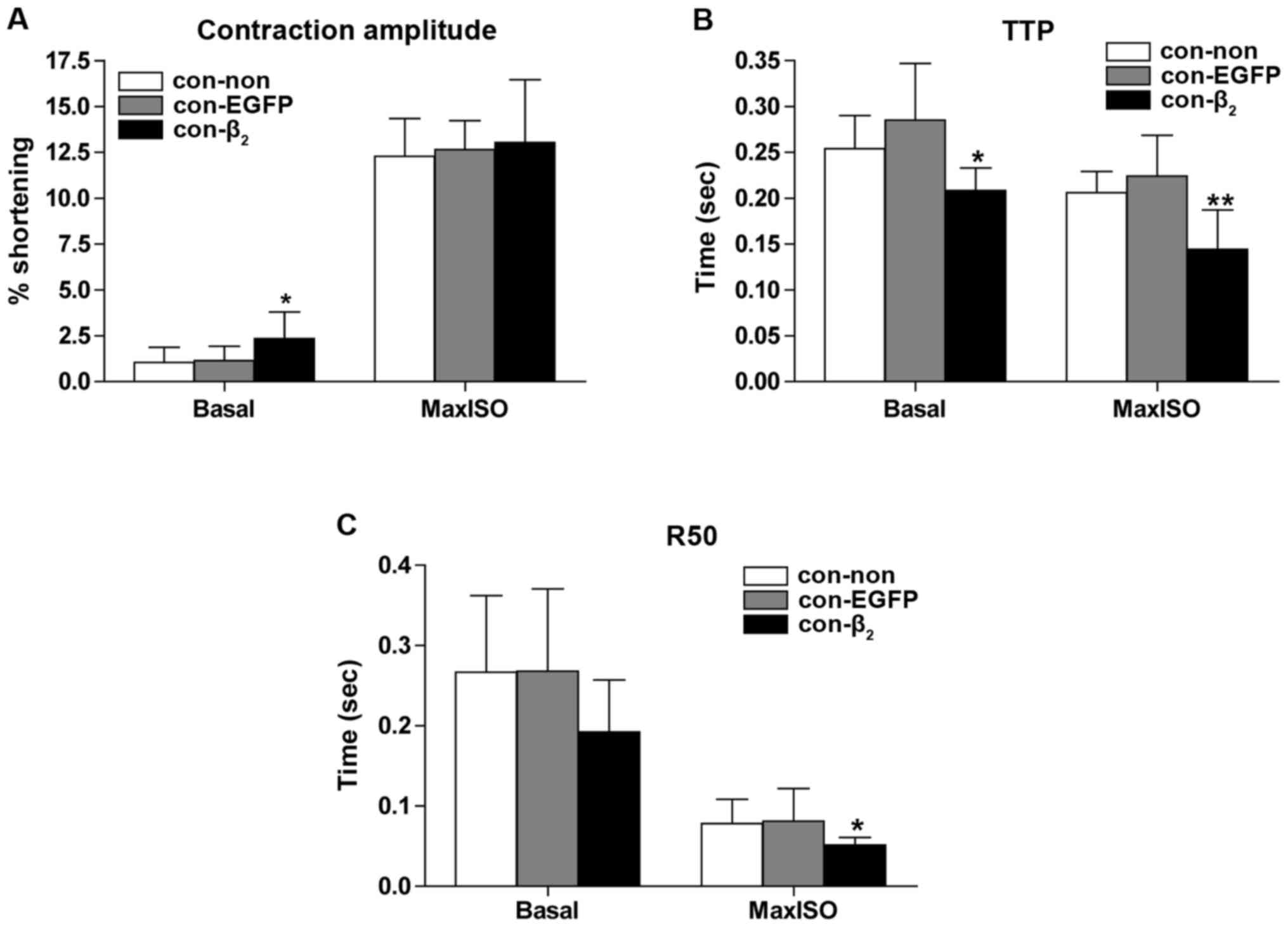
Control cardiocytes were next divided into three
groups: untransfected (n=11), transfected with β2AR-EGFP
(n=8), and transfected with EGFP (n=9). Contractile function was
determined after culture for 48 h. Compared with untransfected, the
percentage of cell contraction amplitude increased (Fig. 5A), TTP decreased (Fig. 5B) significantly in basic state, and
TTP and R50 decreased (Fig. 5B and
C) significantly in the state of maximal contraction of
cardiocytes transfected with β2AR-EGFP. Contractile
function of cardiocytes in the control group did not change
significantly in either basic or maximal contraction states after
transfection with EGFP.
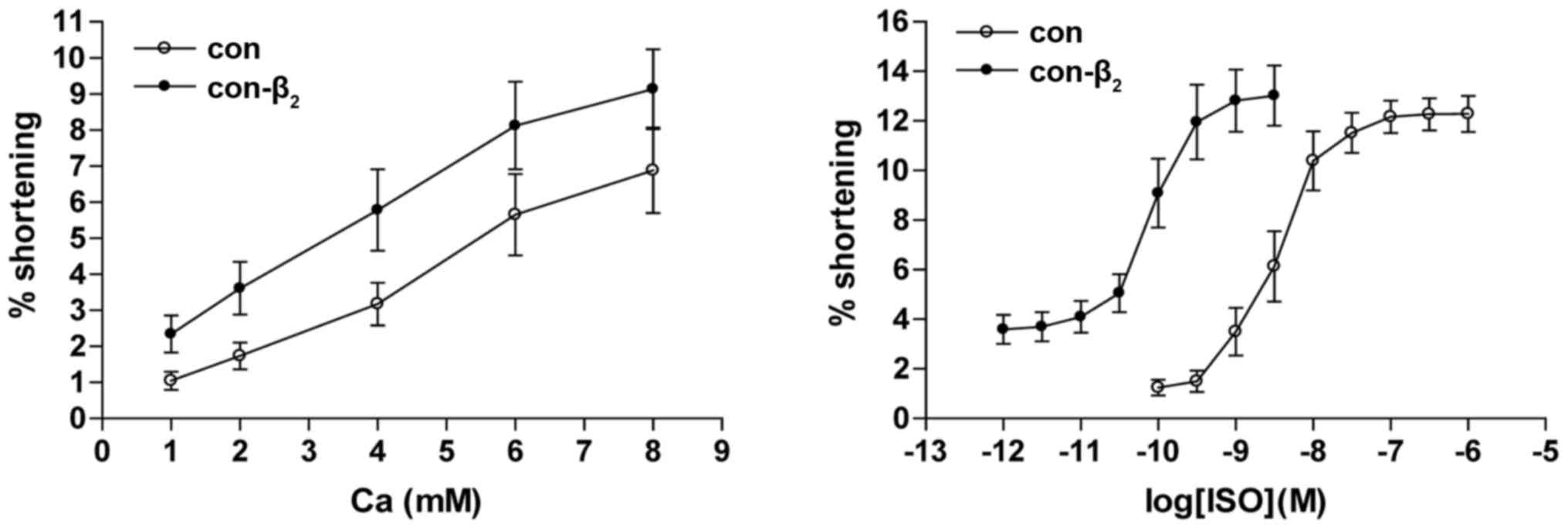
The values of the Ca2+ concentration vs
percentage of contraction amplitude curve of cardiocytes
transfected with β2AR-EGFP gene was higher than in
untransfected cells, which indicated that the percentage of
contraction amplitude of cardiocytes transfected with
β2AR-EGFP was higher than in untransfected cells with
the same Ca2+ concentration in the control group. The
values of the semi-logarithmic cumulative concentration of ISO
versus the percentage of contraction amplitude curve of cardiocytes
transfected with β2AR-EGFP were smaller in untransfected
cells (Fig. 6). Compared with
untransfected cells, pD2 of cardiocytes transfected with
β2AR-EGFP decreased significantly (3.000±0.063 vs.
0.126±0.039 nM, P<0.01).
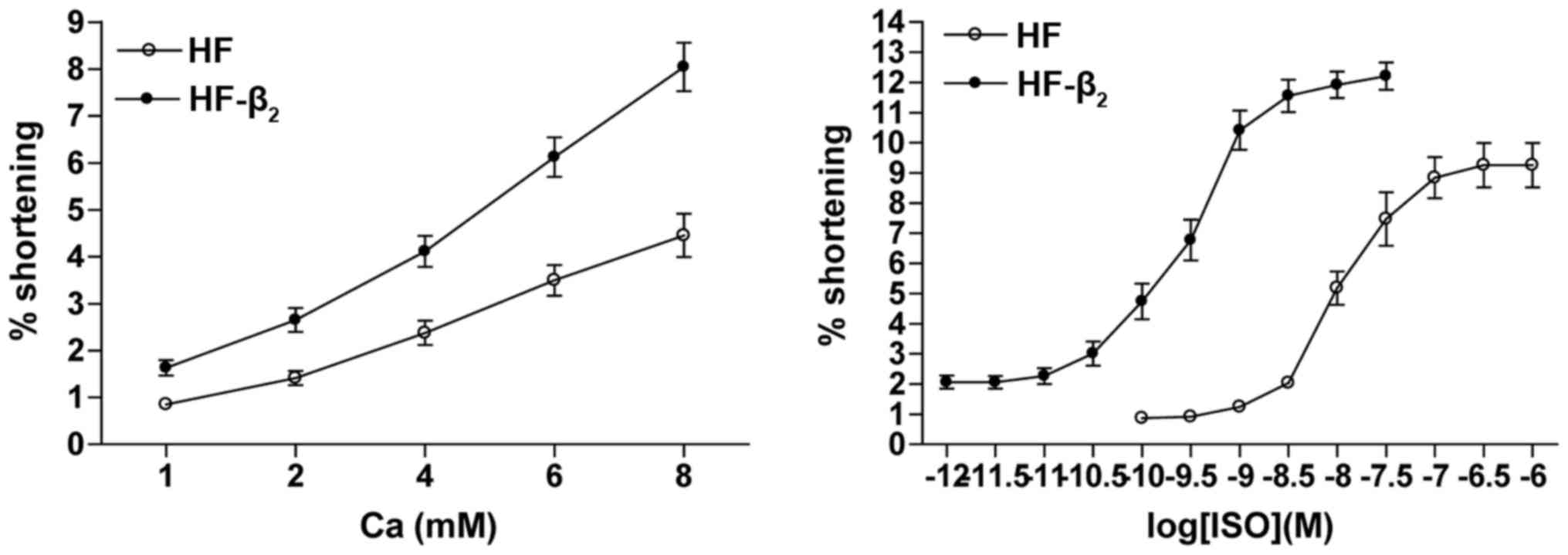
Cardiocytes in the HF group were also divided into
groups: Untransfected (n=11) and transfected with
β2AR-EGFP (n=10). Contractile function was determined
after culture for 48 h. Compared with untransfected cells, the
percentage of cell contraction amplitude increased (Fig. 7A) and TTP decreased (Fig. 7B) significantly in both basic and
maximal contraction states of cardiocytes transfected with
β2AR-EGFP, while R50 of cardiocytes in the HF group did
not change significantly in either the basic or maximal contraction
state after transfection with β2AR-EGFP (Fig. 7C).
The values of the curve of Ca2+
concentration versus the percentage of contraction amplitude of
cardiocytes transfected with β2AR-EGFP was higher than
in untransfected cells, indicating that the percentage of
contraction amplitude of cardiocytes transfected with
β2AR-EGFP was higher than in untransfected cells with
the same Ca2+ concentration in the HF group. The values
of the plot of semi-logarithmic cumulative concentration of ISO
versus the percentage of contraction amplitude curve of cardiocytes
transfected with β2AR-EGFP were lower than the
untransfected cells in the HF group, compared with untransfected
(Fig. 8). The pD2 of cardiocytes
transfected with β2AR-EGFP decreased significantly
(6.310±0.351 vs. 0.159±0.016 nM, P<0.01; Fig. 8).
Discussion
In mammals, including humans, the predominant βAR
subtypes expressed in the heart are β1AR and
β2AR. The traditional view of β1AR signal
transduction is that stimulation of the receptor activates the
Gs-AC-cAMP cascade, leading to PKA-dependent
phosphorylation of a set of regulatory proteins. Studies over the
past decade have shown that stimulation of β2AR
activates both the Gs-AC-cAMP and
Giα-Giβγ-PI3K-Akt cascades (4). Therefore, βAR subtypes fulfill
different, even opposite, physiological and pathophysiological
roles via activating subtype-specific signaling pathways in the
heart.
A series of studies showed that cardiac-specific
overexpression of β1AR and β2AR had very
different effects on the process of myocardial remodeling and the
prognosis of HF. Studies in mice with modest (5–40-fold)
cardiac-specific overexpression of human β1AR revealed
dilated cardiomyopathy, severe cardiac remodeling, and premature
death (5), while cardiac-specific
overexpression of human β2AR (100–200-fold) in mice
markedly enhanced cardiac contractility, even in the absence of
β-agonist, without obvious pathological consequence at the age of
12 months (2). Studies in rats with
HF also indicated that moderate over-expression of the
β2AR gene in cardiocytes could improve contractility
(3).
The canine model of CHF was induced by rapid right
ventricular pacing and is similar to human dilated cardiomyopathy
in aspects of clinical manifestations, hemodynamics, activation of
neurohumoral factors and ventricular remodeling. The results showed
that clinical manifestations and echocardiographic performance
after four weeks of rapid pacing in canines were consistent with
CHF. The process of HF in the canine model was observed dynamically
by echocardiography and had the advantages of being non-invasive,
convenient, and reproducible.
The expression of β2AR protein,
intracellular cAMP and contractile function of cardiocytes in
canines were measured in this study. The expression of
β2AR protein in the HF cardiocytes did not change, but
contraction function and intracellular cAMP concentration
decreased. This may be related to changes of βAR regulation in the
heart during HF: i) CHF caused by diverse etiologies is
characterized by elevated levels of circulating catecholamines and
hyperadrenergic drive, as well as concurrent selective
desensitization of β1AR, leading to a markedly blunted
βAR-mediated contractile response. ii) Sustained β1AR
stimulation caused by hyperadrenergic drive leading to changes of
β1AR signal transduction from the
β1AR-Gs-AC-cAMP-PKA cascade to
β1AR-Gs-Ca2+-CaMK II (6), thus promoting cardiac hypertrophy and
cardiocyte apoptosis (7). iii)
Sustained β2AR stimulation caused by hyperadrenergic
drive leading to phosphorylation of β2AR, mediated by
PKA, inducing changes of β2AR signal transduction from
β2AR-Gs-AC-cAMP-PKA cascade to
β2AR-Giα-Giβγ-PI3K-Akt cascade
(8). The
β2AR-Gi pathway could have anti-apoptotic
effects (9,10), but cannot mediate positive inotropic
effects, and the β2AR-Gi pathway could
compartmentalize the Gs-AC-cAMP-PKA cascade and then
confine and negate the β2AR-Gs-mediated
positive inotropic effect, spatially and functionally (11–13).
β2AR protein expression and intracellular
cAMP concentration of cardiocytes in the control group increased
significantly after transfection with β2AR-EGFP, while
the percentage of cell contraction amplitude in the maximal
contraction state did not change significantly after transfection
with β2AR-EGFP. This may be related to the following
factors: i) β1AR accounts for the majority of cardiac surface βAR
in mammalian species, including humans. Contraction of cardiocytes
induced by ISO is primarily mediated by the
β1AR-Gs pathway under normal physiological
conditions. ii) β2AR coupled with the Gs, Gi
and β2AR-Gi pathways could compartmentalize the
β2AR-Gs pathway. β2AR-Gi and
β2AR-Gs coupling increased when
β2AR was overexpressed, so that the percentage of cell
contraction amplitude of cardiocytes in the control group in the
maximal contraction state did not change significantly after
transfection with β2AR-EGFP.
β2AR protein expression, intracellular
cAMP concentration and the percentage of cell contraction amplitude
of cardiocytes in the HF group increased significantly after
transfection with β2AR-EGFP. There was a significant
difference in the percentage of cell contraction amplitude of
cardiocytes in the control group in the maximal contraction state,
but it did not change significantly after transfection with
β2AR-EGFP. This may be because of concurrent selective
desensitization of β1AR in CHF. The advantages of
β2AR become more apparent under conditions of
β2AR overexpression. With increased β2AR
expression, increased β2AR-Gs coupling can
repair damaged contractile function. In addition, increased
β2AR-Gi coupling can inhibit cardiac
hypertrophy, apoptosis and has other cell protective effects,
inhibiting the harmful effects induced by excessive activation of
the β2AR-Gs pathway. β2AR protein
expression, intracellular cAMP concentration and contractile
function of cardiocytes in the control group did not change
significantly after transfection with EGFP. This demonstrated that
EGFP co-transfected with β2AR did not have non-specific
effects.
At present, β2AR overexpression in
cardiocytes of HF has been considered as a potential means of gene
therapy for CHF (14). This study
demonstrated that transgenic β2AR overexpression in
cardiocytes effectively repaired their damaged contraction
function, and may be related to changes of signal transduction of
βAR during CHF. How safe and effective moderately overexpressed
β2AR in vivo is, and the long-term effects of
β2AR overexpression on survival require further
exploration.
Acknowledgements
This study was supported by the National Nature
Science Foundation of China (grant no. 30572073); the Life Health
Science and Technology Projects funded by the Jiangsu Special Funds
(grant no. BL2012019); the Jiangsu Key Talents of Medical Science
(grant no. RC2007024); the Xuzhou Science and Technology
Development Project (grant no. XF10C029); and the Xuzhou Science
and Technology Development Project (grant no. XM12B062).
References
|
1
|
Dorn GW II, Tepe NM, Lorenz JN, Koch WJ
and Liggett SB: Low- and high-level transgenic expression of
β2-adrenergic receptors differentially affect cardiac hypertrophy
and function in Gαq-overexpressing mice. Proc Natl Acad Sci USA.
96:pp. 6400–6405. 1999; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milano CA, Allen LF, Rockman HA, Dolber
PC, McMinn TR, Chien KR, Johnson TD, Bond RA and Lefkowitz RJ:
Enhanced myocardial function in transgenic mice overexpressing the
β2-adrenergic receptor. Science. 264:582–586. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gong HB, Lu Q, Wang L and Wang J:
Relationship between expression of β2-AR protein on cadiocyte and
the cardiac function of heart failure rat. Xiandai Shengwu Yixue
Jinzhan. 9:1024–1027. 2009.
|
|
4
|
Xiao RP, Ji X and Lakatta EG: Functional
coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G
protein in cardiac myocytes. Mol Pharmacol. 47:322–329.
1995.PubMed/NCBI
|
|
5
|
Engelhardt S, Hein L, Wiesmann F and Lohse
MJ: Progressive hypertrophy and heart failure in β1-adrenergic
receptor transgenic mice. Proc Natl Acad Sci USA. 96:pp. 7059–7064.
1999; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang W, Zhu W, Wang S, Yang D, Crow MT,
Xiao RP and Cheng H: Sustained β1-adrenergic stimulation modulates
cardiac contractility by Ca2+/calmodulin kinase signaling pathway.
Circ Res. 95:798–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhu WZ, Wang SQ, Chakir K, Yang D, Zhang
T, Brown JH, Devic E, Kobilka BK, Cheng H and Xiao RP: Linkage of
β1-adrenergic stimulation to apoptotic heart cell death through
protein kinase A-independent activation of Ca2+/calmodulin kinase
II. J Clin Invest. 111:617–625. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Daaka Y, Luttrell LM and Lefkowitz RJ:
Switching of the coupling of the beta2-adrenergic receptor to
different G proteins by protein kinase A. Nature. 390:88–91. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu WZ, Zheng M, Koch WJ, Lefkowitz RJ,
Kobilka BK and Xiao RP: Dual modulation of cell survival and cell
death by β2-adrenergic signaling in adult mouse cardiac myocytes.
Proc Natl Acad Sci USA. 98:pp. 1607–1612. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Communal C, Singh K, Sawyer DB and Colucci
WS: Opposing effects of β1- and β2-adrenergic receptors on cardiac
myocyte apoptosis: Role of a pertussis toxin-sensitive G protein.
Circulation. 100:2210–2212. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiao RP, Avdonin P, Zhou YY, Cheng H,
Akhter SA, Eschenhagen T, Lefkowitz RJ, Koch WJ and Lakatta EG:
Coupling of β2-adrenoceptor to Gi proteins and its physiological
relevance in murine cardiac myocytes. Circ Res. 84:43–52. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kuschel M, Zhou YY, Cheng H, Zhang SJ,
Chen Y, Lakatta EG and Xiao RP: Gi protein-mediated functional
compartmentalization of cardiac β2-adrenergic signaling. J Biol
Chem. 274:22048–22052. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jo SH, Leblais V, Wang PH, Crow MT and
Xiao RP: Phosphatidylinositol 3-kinase functionally
compartmentalizes the concurrent Gs signaling during β2-adrenergic
stimulation. Circ Res. 91:46–53. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vinge LE, Raake PW and Koch WJ: Gene
therapy in heart failure. Circ Res. 102:1458–1470. 2008. View Article : Google Scholar : PubMed/NCBI
|