Introduction
Aspergillus spp. are a ubiquitous fungus in the
environment, which are easily identified in soil, water and various
types of decomposing organic matter (1). Inhalation of Aspergillus spores causes
pulmonary aspergillosis, which is the most common form of
aspergillus filamentous fungal infection, and is often diagnosed in
patients with immune deficiencies or systemic diseases (2). Aspergillosis is currently divided into
the following categories (3): i)
Invasive pulmonary aspergillosis (IPA), a severe disease and major
cause of mortality in severely immunocompromised patients; ii)
chronic necrotizing aspergillosis (CNA), presents as a locally
invasive disease and is observed primarily in patients who are
mildly immunocompromised or have chronic lung disease; and iii)
allergic bronchopulmonary aspergillosis (ABPA), a non-invasive
hypersensitivity pulmonary disease caused by Aspergillus.
Aspergilloma is a fungus ball that develops in a pre-existing
cavity in lung parenchyma, which may be easily detected by X-ray
and computed tomography (CT) scans (3). In addition, a novel category of
aspergillosis with semi-invasive form (SIA) has been described
(4).
Endobronchial aspergilloma (EBA) is a rare disease,
described as a massive non-invasive overgrowth of the aspergillus
species in bronchial lumen, causing space-occupying lesions
(5,6). It is often incidentally diagnosed
during bronchoscopy to evaluate the cause of hemoptysis (5,6), and may
be considered as another form of pulmonary aspergillosis. At
present, there are very few reports regarding the characteristics
of EBA. The present study aimed to investigate the clinical and
radiological characteristics in addition to the bronchoscopic
appearance in patients with EBA through a representative case from
Shanghai Pulmonary Hospital affiliated to Tongji University
(Shanghai, China) and a review of the current literature.
Case report
Study design
The present study reports a case of EBA with no
prior treatment that was diagnosed at Shanghai Pulmonary Hospital
affiliated to Tongji University, informed consent was also obtained
from the patient. A retrospective analysis of the clinical and
radiological characteristics and bronchoscopic results was also
completed by combining this case with 16 other previously reported
EBA cases (5–10). All patients we subjected to a chest
CT, bronchoscopy and histological examination. The tissue used for
histological examination was obtained via bronchoscopy biopsy of
the mass in the trachea, avoiding necrotic tissue or secondary
infection areas, with a volume of at least 0.5×0.5×0.2 cm and had a
fresh non-necrotic color. The tissue was fixed using 10% neutral
formaldehyde for 24 h at room temperature, routinely selected,
embedded, sliced in continuous sections with a thickness of 4 µm
and hematoxylin-eosin (HE) stained. The test results and basic
clinical data were collected. Primary evidence to confirm EBA were
as follows: i) Presence of bronchoscopy-visible intraluminal mass
and necrotic tissue; ii) characteristic appearance of Aspergillus
species under microscopic examination, such as septate fungal
hyphae bearing acute branching angles; iii) positive result of
Aspergillus culture; and iv) following the elimination of other
possibilities, such as tumors, foreign bodies, parasites or mucus
by evaluating the patient's personal history, performing
bronchoscopy and pathology examinations.
Representative case
A 48-year-old male was admitted to Shanghai
Pulmonary Hospital affiliated to Tongji University due to a
recurrent fever, cough, expectoration and bloody sputum lasting 3
months. The patient had a 20-year history of smoking, and a 4-year
history of diabetes and was undergoing diet therapy. On admission,
he presented with normal vital signs. Physical examination of the
chest revealed no evident crackles or wheezing. Complete blood
count results were normal. Routine blood chemistry and tumor
markers were all within normal limits. Mycodextranase and latex
agglutination tests from the blood were negative. Human
immunodeficiency virus antibody test was negative. Chemical
luminescence of syphilis test was negative.
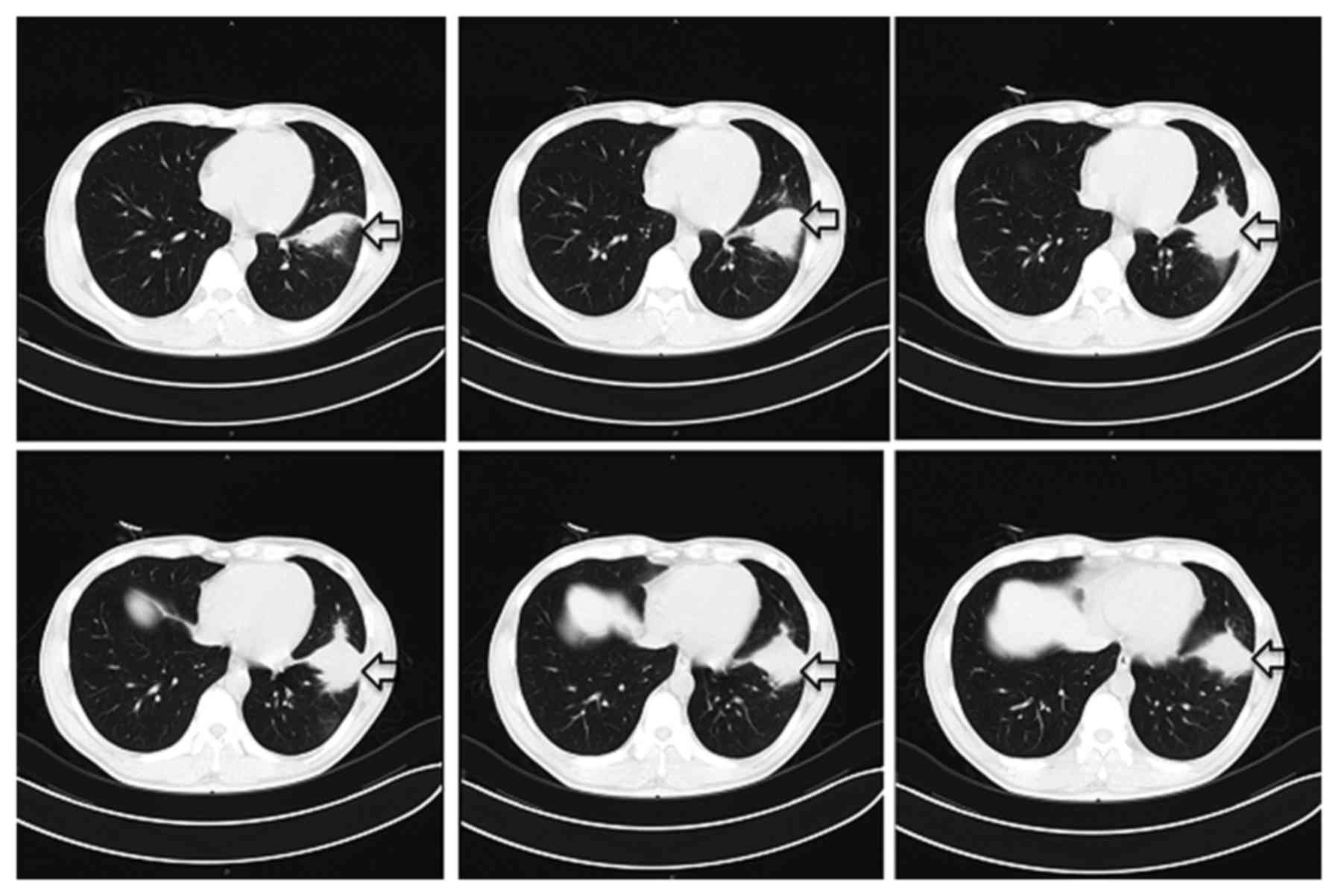
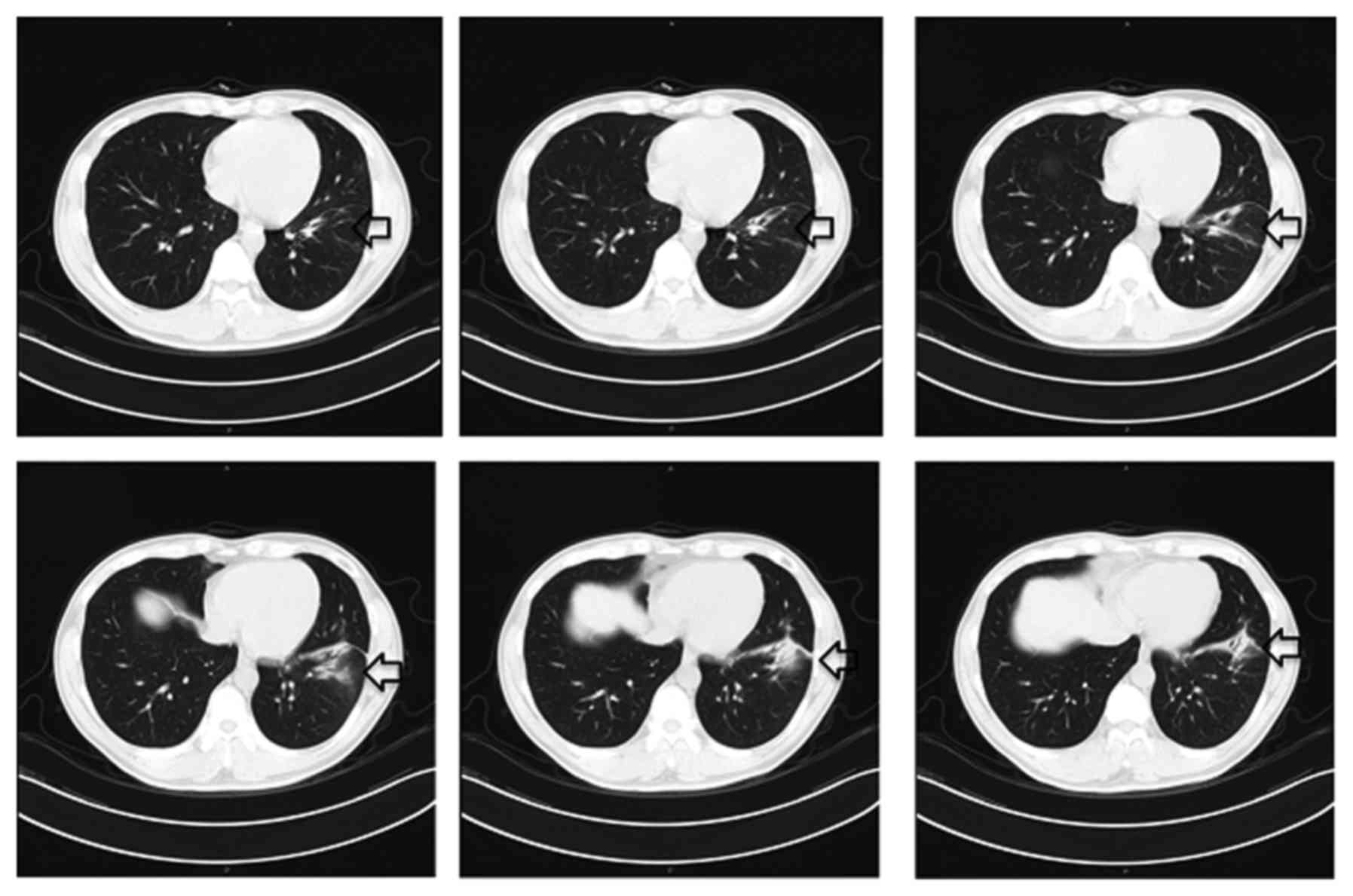
An intial CT scan of the chest revealed a spiculated
mass with exudation in the left lower lobe (Fig. 1). 18F- deoxyglucose (FDG)
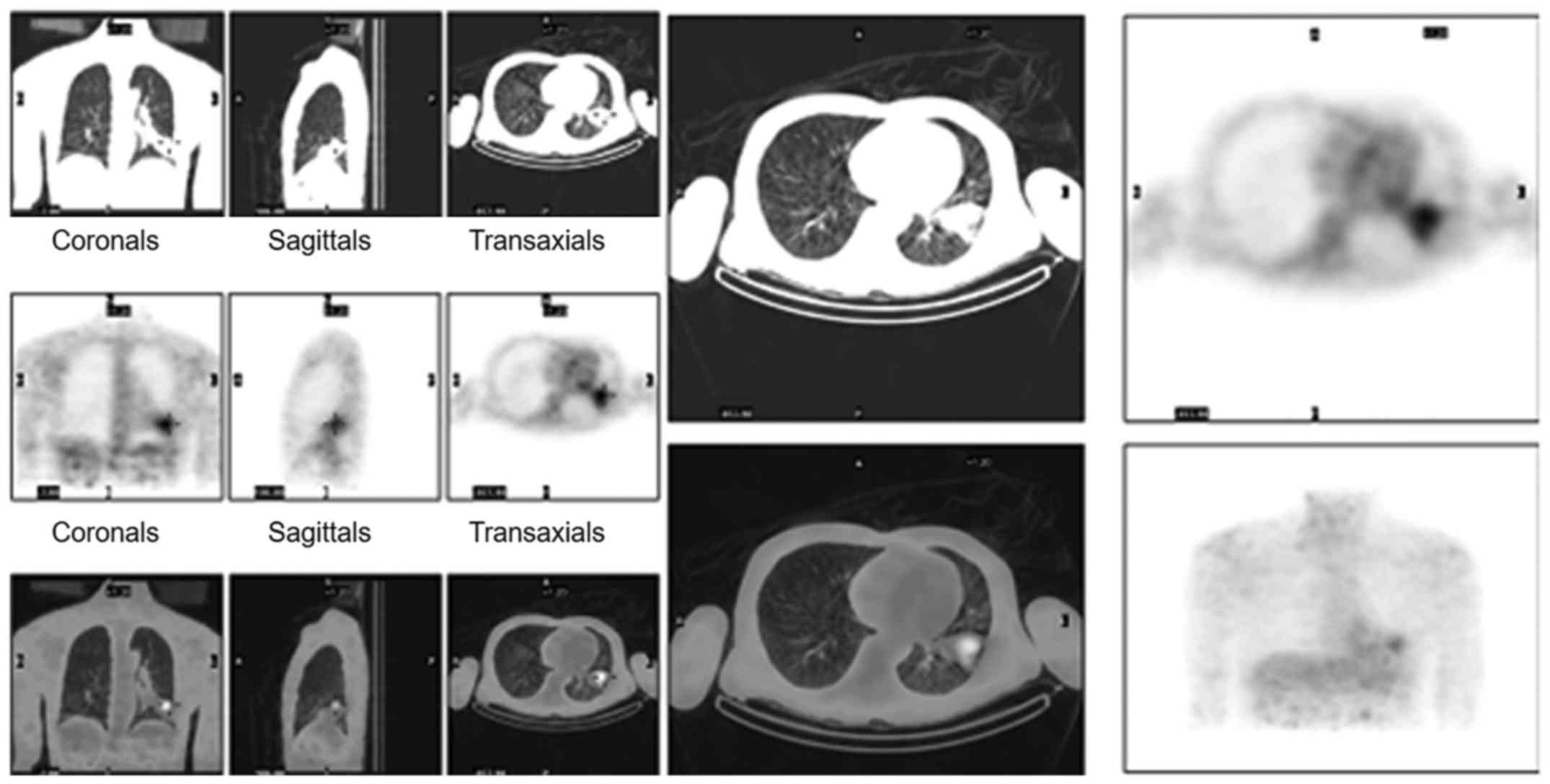
dual-head coincidence single-photon emission computerized
tomography (DHTC) indicated that the maximum tumor to non-tumor
ratio was 11.63 and therefore, the possibility of lung cancer was
not eliminated (Fig. 2). Initial
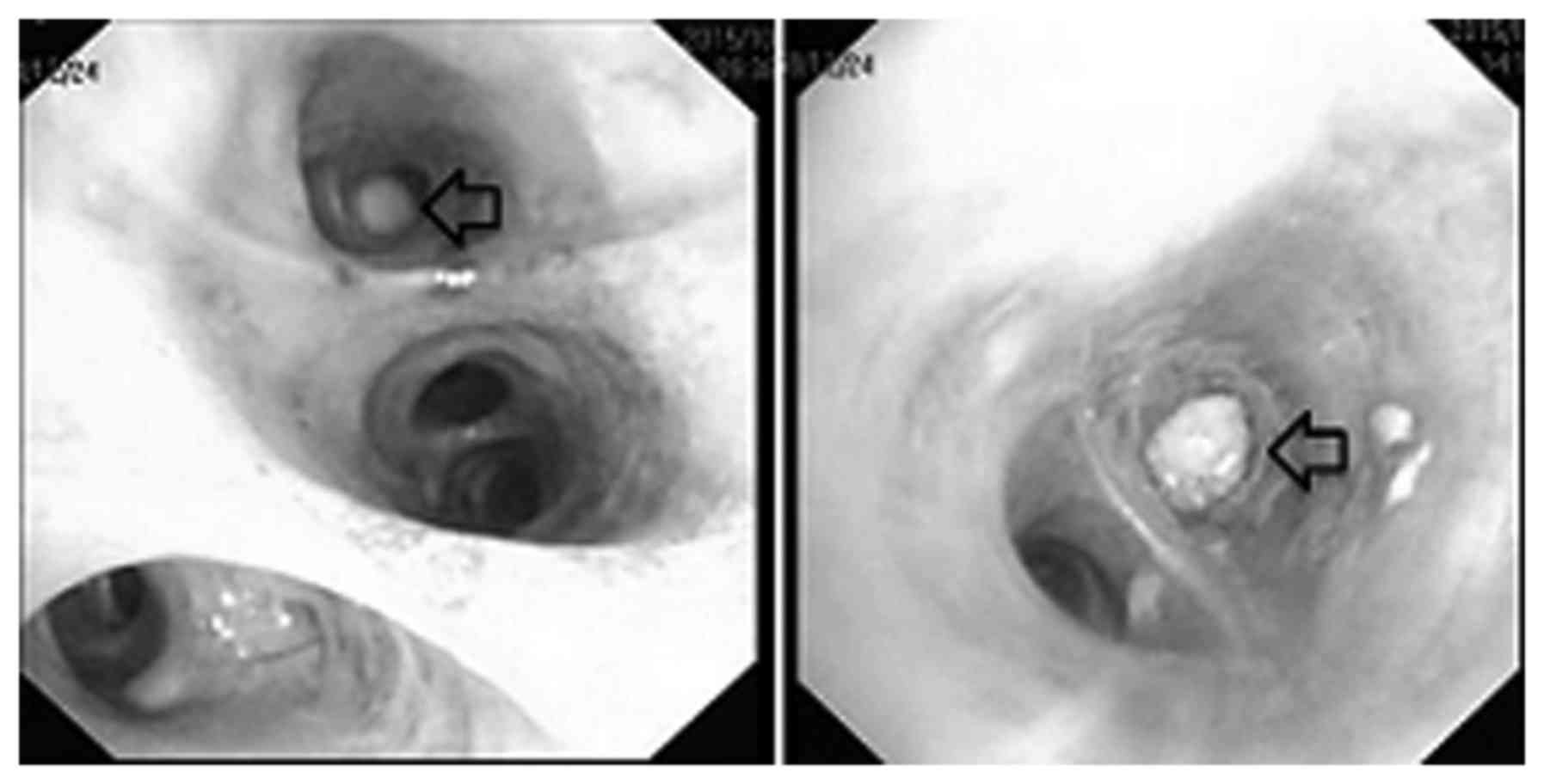
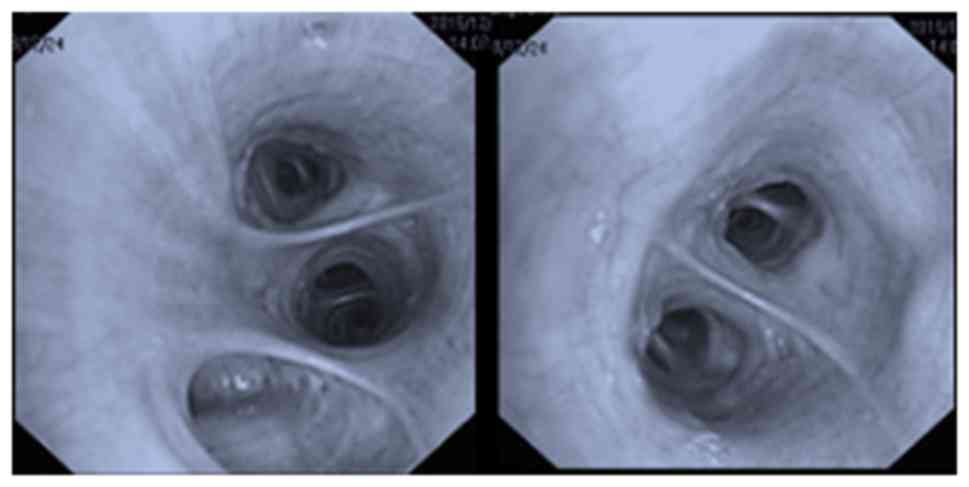
fiberoptic bronchoscopy (FOB) examination (11) revealed a white necrotic mass in the
medial anterior basal segment of left lower lobe, which completely
obstructed the subsegmental bronchus (Fig. 3). Removal of the mass was attempted
but was not successful as it was impacted in the narrow lumen.
Notably, histopathological examination performed using HE staining
of the bronchoscopic biopsied specimen was indicated to be
negative. FOB was performed a second time 2 days later and a
purulent material partially obstructing the lumen was identified at
the same site. The lumen was finally unblocked following repetitive
clearing of the necrotic material and sputum aspiration. The
clearing work performed with a BF-1T260 bendable bronchoscopy
(Olympus, Ishikawa, Japan) and biopsy forceps (Aohua, Shanghai,
China), to cut and remove the tissue, 10 min a time for a total of
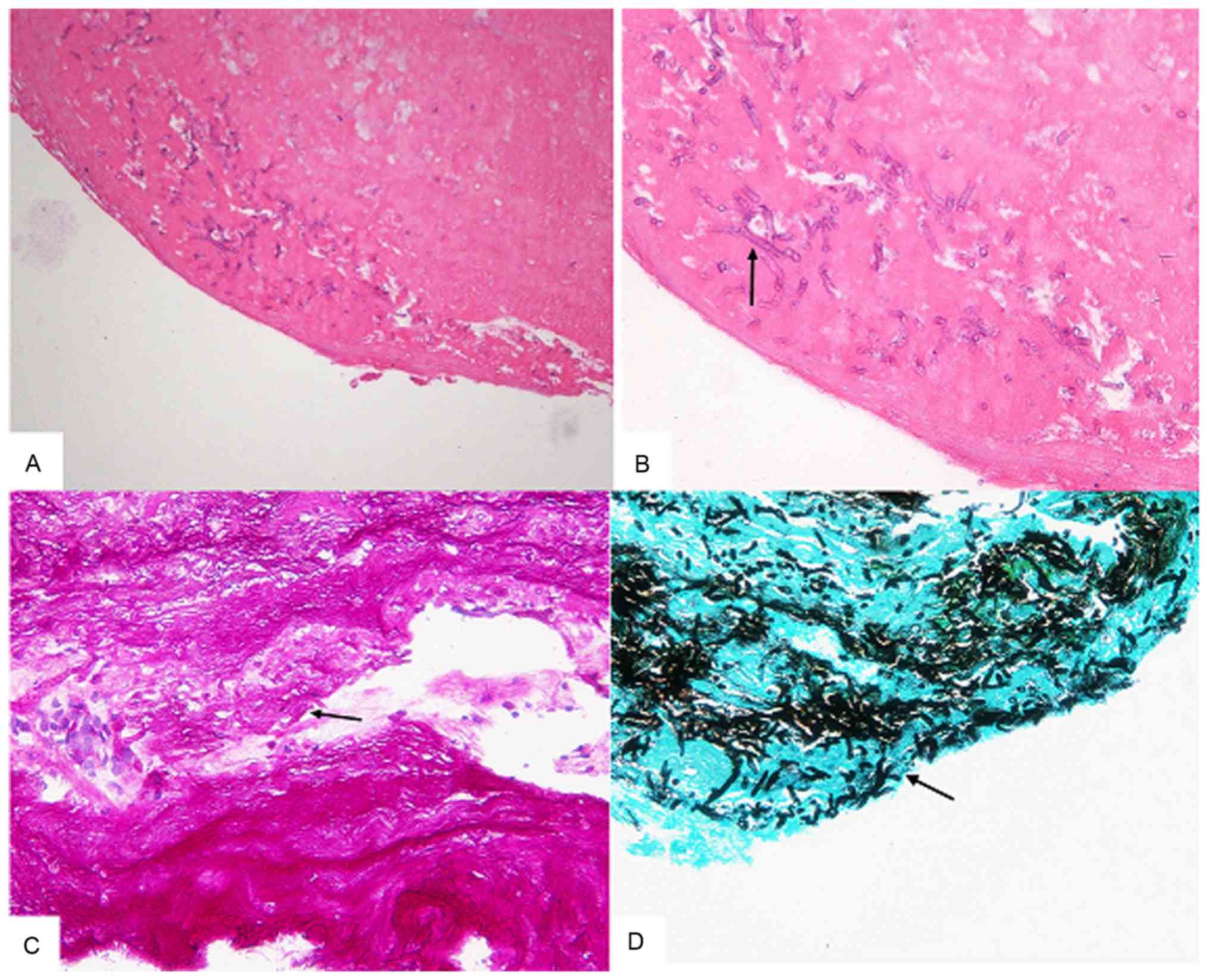
3 times. Histopathological examination of the bronchoscopic
biopsied specimen indicated the presence of necrotic tissue and
Aspergillus. Periodic acid-Schiff staining was performed and
samples were exposed to 0.5% periodic acid for 5 min, Schiffs'
reagent for 15 min and then washed twice using 0.5% sodium
metabisulfite for 1 min each time before bright green staining was
performed for 10 sec at room temperature. Furthermore, hexamine
silver staining was conducted and samples were stained using silver
solution for 1.5 h at 65°C first, then exposed to 0.25% chloride
for 2 min, 3% sodium thiosulfate for 1 min and 0.1% bright green
staining for 1 min at room temperature. Periodic acid-Schiff and
hexamine silver staining results were positive (Fig. 4). The patient was then treated with
oral voriconazole (Pfizer, New York, United States) 200 mg once a
day for 1 month. The lumen was reassessed with the bronchoscope and
was observed to be completely unobstructed (Fig. 5). A repeat chest CT demonstrated that
the left lung lesion was largely absorbed (Fig. 6). In addition, the patient's symptoms
were noticeably relieved, no fever was observed, bloody sputum was
no longer observed 3 days post interventional therapy. Complete
blood count and routine blood chemistry markers were maintained at
normal levels.
Literature comparison
By combining the present case with similar cases
identified in the literature, a retrospective analysis of the
clinical and radiological characteristics in addition to the
bronchoscopic appearance was performed. The key word ‘endobronchial
aspergilloma’ was searched in the PubMed NCBI database (ncbi.nlm.nih.gov/pubmed?holding=icnsibslib), by
excluding cases without histopathological confirmation and simple
Aspergilloma. In total, 17 patients meeting the EBA diagnosis
criteria were included (including the case reported in the current
study). The number of males (13/17; 76.5%) was notably higher,
compared with women (4/17; 23.5%), and ages ranged from 33 to 82
years old (median age, 59). Of the 17 patients, 9 (52.9%) were
smokers. Diagnoses of previous lung diseases included pulmonary
tuberculosis (n=8; 47.6%), lung cancer (n=4; 23.5%), pulmonary
resection (early stage lung cancer; n=1; 5.9%) and foreign body of
the right main bronchus (n=1; 5.9%). Notably, 2 patients had
previously been diagnosed with endobronchial lung cancer covered by
aspergillosis (patients 13 and 14). A total of 8 patients had
non-pulmonary comorbidities, including dilated cardiomyopathy
(n=1), primary thrombocytosis (n=1), intracerebral hemorrhage
(n=1), chronic heart failure (n=2), subarachnoid hemorrhage (n=1),
hypertension (n=1), rheumatoid arthritis (n=1), hepatichemangima
(n=1) and diabetes (n=4). None of the patients exhibited evidence
of severe immune deficiency. Primary clinical symptoms were
hemoptysis (9/17; 53%), cough (7/17; 41.2%) and dyspnea (3/17;
17.6%). Microbiology examination was performed in the bronchial
lavage fluid of 12 patients, among whom only 2 exhibited
aspergilloma, and 5 patients were treated with antifungal drugs
(unknown) but the treatment response was not clear, with the
exception for the present patient. Clinical characteristics of the
17 patients with EBA are summarized in Table I.
 | Table I.Clinical characteristics of 17
patients with endobronchial aspergilloma. |
Table I.
Clinical characteristics of 17
patients with endobronchial aspergilloma.
| No. | Sex | Age | Symptom | Underlying
disease | History of pulmonary
TB | Smoking status | Microbiologic
examination of BW | Treatment | (Refs.) |
|---|
| 1 | M | 75 | Dyspnea, cough | DCMP | – | Ex | Aspergillus spp. No
MTB isolated | Itraconazole via IV
and oral for 4 weeks | (6) |
| 2 | F | 53 | Cough, sputum,
dyspnea | HT | – | NS | Not completed | – | (6) |
| 3 | M | 70 | Cough, sputum | NSCLC | 2 months | CS | Not completed | Oral itraconazole for
3 weeks | (6) |
| 4 | M | 70 | Hemoptysis | – | 25 years | CS | No MTB isolated | – | (6) |
| 5 | M | 51 | Hemoptysis | – | 30 years |
| No MTB isolated | – | (6) |
| 6 | M | 46 | Hemoptysis,
Dyspnea | – | 6 years | CS | No MTB isolated | – | (6) |
| 7 | M | 57 | Cough, sputum | ET,
hepatichemangima | 30 years | NS | No MTB
isolated | – | (6) |
| 8 | M | 36 | Hemoptysis | – | 7 years | Ex | No MTB
isolated | – | (6) |
| 9 | M | 76 | Cough, sputum | SDH, ICH | 10 years | Ex | No Aspergillus spp.
isolated No MTB isolated | – | (6) |
| 10 | M | 50 | Hemoptysis,
cough | NSCLC | – | CS | Not completed | – | (6) |
| 11 | M | 64 | No | CHF, RA | 6 months | – | Not completed | Voriconazole for a
number of months | (5) |
| 12 | M | 59 | Hemoptysis | None | None | NS | Not completed | – | (8) |
| 13 | F | 56 | Shortness of
breath, hemoptysis | NSCLC, pneumonia
and bronchitis | – | – | No fungal organisms
or acid-fast bacilli were identified in sputum cultures. | Voriconazole, not
clear | (7) |
| 14 | F | 82 | Shortness of
breath | NSCLC, COPD, CHF,
Diabetes, gastroesophageal reflux disease | None | CS A fumigatus | Sputum culture grew
not clear | Itraconazole, | (7) |
| 15 | M | 48 | Fever, cough,
expectoration and blood-tinged sputum | Diabetes | None | Ex | No Aspergillus spp.
isolated No MTB isolated | None | The present
study |
| 16 | F | 75 | Fever and
chills | Diabetes | None | NS | No Aspergillus spp.
isolated No MTB isolated | None | (9) |
| 17 | M | 33 | Blood-tinged
sputum, chest pains | Pneumothorax
surgery | None | NS | No Aspergillus spp.
isolated No MTB isolated | None | (10) |
X-ray examination was performed for 13 patients, and
all but 1 exhibited abnormal manifestations. The abnormal region
was most often in the upper lobe (9/13; 69.2%), than in the left
(8/13; 61.5%). Primary manifestations were pulmonary fibrotic
changes along with lung volume loss, similar to old pulmonary
tuberculosis. Mass or nodular shadows were also detected as
presentations.
CT scanning indicated that Aspergillus lesions were
more likely to be identified in the left lung (13/17; 76.5%) than
in the right (4/17; 23.5%). Lesions were most common in the left
upper lobe (6/17; 35.3%) and left lower lobe (6/17; 35.3%) on the
CT scan. The types of Aspergillus identified by chest CT included:
Space-occupying lesions (n=10; 58.8%), aspergilloma (n=3; 17.6%),
pneumonic consolidation (n=2; 11.8%), and ground glass opacity
(n=1; 5.9%). CT results indicated a mass with a multi-lobulated
contour in 3 patients (patients 1, 10 and 15), and an endobronchial
lesion, such as a foreign body or broncholithiasis, was suspected
in 4 patients (patients 2, 8, 16 and 17). Multiple calcified
nodules and fibrotic changes as sequelae of previous pulmonary
tuberculosis were noted in 6 patients (patients 4–9).
Bronchoscopy examination identified a mass in the
bronchial lumen of all 17 patients and 15 patients presented with
endobronchial obstruction by necrotic material. The abnormal
regions under bronchoscopy were consistent with the CT locations.
Table II summarizes the
radiological characteristics and the bronchoscopic appearance in 17
patients with EBA.
 | Table II.Radiographic and bronchoscopy
findings in 17 patients with endobronchial aspergilloma. |
Table II.
Radiographic and bronchoscopy
findings in 17 patients with endobronchial aspergilloma.
| No. | Chest X-ray | Chest CT | Bronchoscopy |
|---|
| 1 | Round shaped mass
lesion, LUL | Well-marginated and
multi-lobulating mass in LUL, non-enhanced low density mass. | Protruding whitish
mass in LUL apicoposterior segment |
| 2 | No definite
lesion | Small sized
high-density lesion suggesting foreign body or broncholith in
RBI. | Foreign body
adjacent granulation tissue covered with necrotic tissue in
RBI. |
| 3 | RUL lobectomy
state | RUL lobectomy
state. No parenchymal lesion. | Whitish and
necrotic tissue in anastomosis site of RUL. |
| 4 | Sequelae of TB,
LUL | Sequelae of
pulmonary TB in LUL with endobronchial lesion in LUL apical
segment, focal GGO in LUL. | Small whitish mass
in LUL upper division. |
| 5 | Sequelae of TB,
both upper lobes | Soft tissue mass,
low density and non-enhanced, with focal calcification causing
obstruction of LUL apical segment. | Whitish mass-like
lesion in LUL. |
| 6 | Sequelae of TB of
both lungs | Sequelae of
pulmonary TB in both upper lobes, cavitary lesion in RUL and RML,
fungus ball in LUL. Ground glass opacity in LLL superior
segment. | Large
yellowish-necrotic mass obstructing LUL apicoposteriorsegment. |
| 7 | Sequelae of TB,
LUL | Pneumonic
consolidation in lingular division of LUL. | Mucus like
yellowish mass in lingular division of LUL |
| 8 | Sequelae of TB and
Fungus ball formation, LUL | Severe
emphysematous change in the two lungs. Fungus ball in LUL and
aspirated blood in the two lower lung fields. | Whitish to yellow
necrotic mass in LUL. |
| 9 | Sequelae of TB in
RUL and pneumonic consolidation in LLL | Severe fibrotic
change and volume loss in right lung and pneumonic consolidation in
LLL. | Mass with yellowish
and dark brownish exudates in LUL apicoposterior segment. |
| 10 | Mass like shadow in
left hilum | Low-density and
lobulating mass in LLL causing obstruction of LLL superior
segment. | Protruding mass
with yellow necrotic material in LLL superior segment. |
| 11 | – | Cavitary lesion
with a suggestive image of a fungus ball, in the lingular division
of the LUL. | A large yellowish
hard mass with almost complete obstruction of the lower segment of
the lingular division of the LUL, |
| 12 | Nodular opacity in
left hilar field | Chest revealed a
2.4×1.8 cm spiculated mass and surround small nodular opacities in
superior segment of left lower lobe. | Irregular
mass-like, brownish material, which completely obstructed the
subsegmental bron chus and a foreign body in superior segmental
bronchus of left lower lobe. |
| 13 | – | Left pulmonary
infrahilar soft tissue mass with an endobronchial component
extending into the left lower lobe. | Friable, white mass
almost completely obstructing patients left lower lobar
bronchus. |
| 14 | – | Right main
bronchial mass was identified, causing collapse of the right upper
lobe. Furthermore, a number of smaller nodules were observed in the
lung parenchyma bilaterally. | Numerous fragments
of carcinoid in part covered by fibrin, necrotic debris, and fungi,
the latter consistent with aspergillus. |
| 16 | Diffuse
consolidation in the left lower lobe | A 1.0×0.5-cm
calcified endobronchial lesion with post-obstructive pneumopathy in
the left lower bronchus. | Mass blocking the
left basal segment was removed by grasping forceps. The mass was
attached firmly to the bronchus and slight bleeding occurred at the
removal site. The mass had an irregular yellow and black surface
and broke apart easily. |
| 17 | Ill-defined nodular
density; at the left infrahilar area | Further evaluation
of the nodular density, a 1.5×2.5-cm relatively well-defined, oval
shaped mass at the left lower lobe near the origin of the
laterobasal segmental bronchus. | Irregularly shaped
yellowish mass of ~1 cm completely obstructing the laterobasal
segmental bronchial orifice at the left lower lobe, the mass was
movable. |
Discussion
Aspergilloma often occurs in lung parenchyma or the
lung cavity (12). EBA is a rare
space-occupying disease of the endobronchial fungi, and it may not
belong to the current classification of Aspergillus spp. (IPA, CNA,
ABPA and SIA). It is a substantive or cavitary lesion caused by an
overgrowth of aspergilloma with a non-invasive form in
endobronchial areas through inhalation and fumigation, which is
always limited to the main bronchus (13). EBA may present either as the sole
manifestation of pulmonary aspergillosis or with other forms of
pulmonary aspergillosis. CNA and ABPA may be associated with the
EBA or the visible aspergilloma inside the lung cavity,
demonstrated by bronchoscopy. Among 10 cases of EBA reported by Ma
et al (6), 2 patients had
lung parenchymal lesions with EBA in the lung cavity.
It is not easy to directly diagnose EBA by imaging
or other noninvasive tests. In the present study, all patients were
diagnosed incidentally when having a bronchoscopy and this was
confirmed by histological examination and culture. Only 3 patients
were suspected to have Aspergillus on the basis of CT results.
Hemoptysis (9/17; 53%) and cough (7/17; 41.2%) were
the most common symptoms of EBA, and hemoptysis specifically, may
be a result of the Aspergillus colonization, which makes the
diagnosis via FOB important (6). The
current study suggested the detection rate of the microbiology
examination using bronchial lavage fluid was low (16.7%);
therefore, histopathological examination was required. None of the
17 cases were reported to have exhibited complications or adverse
effect following biopsy.
Impaired immune function and certain comorbidities,
such as active tuberculosis or diabetes, are important factors for
fungal infection. Although there were no reports regarding the
patients' immune status in the current study, it was revealed that
of the 17 patients, 12 (70.6%) had previously been diagnosed with
lung diseases, including pulmonary tuberculosis and lung cancer,
and 8 patients (47.6%) had non-pulmonary comorbidities, such as
dilated cardiomyopathy, essential thrombocytosis, cerebrovascular
accident, hypertension and diabetes.
In patients with normal immune function, the
formation of aspergilloma was considered to require a nest
structural change that induces the airflow stasis, which helps
Aspergillus in the airflow to colonize (6). In the present study, 8 patients with
old pulmonary tuberculosis were included, and they exhibited
destructive and fibrotic changes of the parenchyma, which
potentially resulted in airway stenosis and obstruction. In
addition, the majority of those who had no history of tuberculosis
presented with a narrow or completely blocked lumen caused by lung
cancer, lung resection, foreign bodies or granulation tissue, which
was confirmed through imaging and bronchoscopy. Therefore, this
change of the bronchial lumen may be considered to be an important
factor to potentially induce airflow stasis and Aspergillosis
colonization in bronchioles, in addition to the formation of
aspergilloma. Lesions were also identified in the left lung more
often than in the right, and it is suggested that this is because
the left lung is anatomically closer to the heart and aorta. In
addition, the left main bronchus is longer and narrower than the
right side, thus it is easier to induce airflow stasis in the
bronchioles of the left lung, causing EBA (13).
Notably, in the present study, 4 patients had lung
cancer, of which 1 underwent surgery, 1 was affected by aspergillus
(6), and the other 2 reported by
Nilsson et al (7) had
endobronchial tumor covered by aspergilloma. In Nilsson's report,
the fungal hyphae were identified via histopathology and the
patients were subsequently treated with anti-fungal drugs
(unknown). However, in both the reported cases, the mass kept
growing and finally the tumor was identified through further
biopsy. There was no clear evidence to link Aspergillus and cancer
development in the literature (7)
and it is rarely reported that fungal infection may co-exist with
solid types of cancer in immunocompetent patients (14,15).
Malignant tumor may be a factor for local fungal infection
including EBA.
The optimal treatment of EBA has not yet been
established. The short-term follow-up in the current study
confirmed that through interventional treatment using bronchoscopy
and systemic antifungal therapy, the prognosis of EBA is excellent.
Nevertheless, Jung et al (8)
argued that for clinically asymptomatic patients, there is no
requirement for treatment, and in cases of mild hemoptysis such as
in the present patient's case, medical therapy with bed rest,
humidified oxygen, cough suppressants and postural drainage is
sufficient (8). To the best of our
knowledge, there is no consistent evidence that aspergilloma
responds to systemic administration of antifungal agents (12). In certain reports, surgical treatment
was a good alternative (16–18), but the pulmonary function and
tolerance of patients should be considered. It is suggested that
selecting appropriate treatments according to different conditions
of patients is reasonable, and the combination of direct
bronchoscopic intervention with antifungal therapy may be
advantageous.
In conclusion, the dominant symptom for EBA was
identified to be hemoptysis in the present analysis. Chest CT
demonstrated that aspergillosis lesions were more frequently
identified in the left lung compared with the right. It was also
revealed that endobronchial aspergilloma often occurs in
individuals with comorbidities that may potentially impair immune
function and underlying lung diseases that cause lumen structural
change or bronchial obstruction. Previous studies have provided
sufficient evidence that endobronchial aspergilloma may be clearly
diagnosed by bronchoscopy biopsy, but the potential for the
co-exististing of tumors requires consideration (5–10).
Finally, based on clinical experience, the combination of
bronchosopic intervention and anti-fungal therapy may be an
effective treatment for patients with EBA.
Acknowledgements
The abstract of this manuscript was presented at the
ERS International Congress 3rd-7th September 2016 in London, UK and
published as abstract no. PA3715 in the European Respiratory
Journal 48 (suppl 60): 2016.
Glossary
Abbreviations
Abbreviations:
|
EBA
|
endobronchial aspergilloma
|
|
CT
|
computer tomography
|
|
IPA
|
invasive pulmonary aspergillosis
|
|
CAN
|
chronic necrotizing aspergillosis
|
|
ABPA
|
allergic bronchopulmonary
aspergillosis
|
|
SIA
|
semi-invasive aspergillosis
|
|
FDG
|
18F- deoxyglucose
|
|
FOB
|
fiberoptic bronchoscopy
|
|
DCMP
|
dilated cardiomyopathy
|
|
HT
|
hypertension
|
|
NSCLC
|
non-small cell lung cancer
|
|
ET
|
essential thrombocytosis
|
|
SDH
|
subarachnoid hemorrhage
|
|
ICH
|
intracranial hemorrhage
|
|
TB
|
tuberculosis
|
|
BW
|
bronchial washing
|
|
MTB
|
Mycobacterium tuberculosis
|
|
IV
|
intravenous
|
|
GGO
|
ground glass opacity
|
|
LLL
|
left lower lobe
|
|
LUL
|
left upper lobe
|
|
RML
|
right middle lobe
|
|
RUL
|
right upper lobe
|
|
RBI
|
right bronchus intermedius
|
References
|
1
|
Silva EF, Mde P Barbosa, Oliveira MA,
Martins RR and e Silva J Fontinele: Chronic necrotizing pulmonary
aspergillosis. J Bras Pneumol. 35:95–98. 2009.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hospenthal DR, Kwon-Chung KJ and Bennett
JE: Concentrations of airborne aspergillus compared to the
incidence of invasive aspergillosis: Lack of correlation. Med
Mycol. 36:165–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zmeili OS and Soubani AO: Pulmonary
aspergillosis: A clinical update. QJM. 100:317–334. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chabi ML, Goracci A, Roche N, Paugam A,
Lupo A and Revel MP: Pulmonary aspergillosis. Diagn Interv Imaging.
96:435–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Araújo D, Figueiredo M and Monteiro P:
Endobronchial aspergilloma: An unusual presentation of pulmonary
aspergillosis. Rev Port Pneumol (2006). 22:61–62. 2016.PubMed/NCBI
|
|
6
|
Ma JE, Yun EY, Kim YE, Lee GD, Cho YJ,
Jeong YY, Jeon KN, Jang IS, Kim HC, Lee JD and Hwang YS:
Endobronchial aspergilloma: Report of 10 cases and literature
review. Yonsei Med J. 52:787–792. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nilsson JR, Restrepo CS and Jagirdar J:
Two cases of endobronchial carcinoid masked by superimposed
aspergillosis: A review of the literature of primary lung cancers
associated with aspergillus. Ann Diagn Pathol. 17:131–136. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jung SW, Kim MW, Cho SK, Kim HU, Lee DC,
Yoon BK, Jeong JP and Ko YC: A case of Endobronchial aspergilloma
associated with foreign body in immunocompetent patient without
underlying lung disease. Tuberc Respir Dis (Seoul). 74:231–234.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yeo CD, Baeg MK and Kim JW: A case of
endobronchial aspergilloma presenting as a broncholith. Am J Med
Sci. 343:501–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim JS, Rhee Y, Kang SM, Ko WK, Kim YS,
Lee JG, Park JM, Kim SK, Kim SK, Lee WY and Chang J: A case of
endobronchial aspergilloma. Yonsei Med J. 41:422–425. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
British Thoracic Society Bronchoscopy
Guidelines Committee, a Subcommittee of the Standards of Crae
Committee of the British Thoracic Society, . British thoracic
society guidelines on diagnostic flexible bronchoscopy. Thorax. 56
Suppl I:i1–i21. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patterson KC and Strek ME: Diagnosis and
treatment of pulmonary aspergillosis syndromes. Chest.
146:1358–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soubani AO and Chandrasekar PH: The
clinical spectrum of pulmonary aspergillosis. Chest. 121:1988–1999.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tashiro T, Izumikawa K, Tashiro M,
Takazono T, Morinaga Y, Yamamoto K, Imamura Y, Miyazaki T, Seki M,
Kakeya H, et al: Diagnostic significance of aspergillus species
isolated from respiratory samples in an adult pneumology ward. Med
Mycol. 49:581–587. 2011.PubMed/NCBI
|
|
15
|
Ohmagari N, Raad II, Hachem R and
Kontoyiannis DP: Invasive aspergillosis in patients with solid
tumors. Cancer. 101:2300–2302. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stevens DA, Kan VL, Judson MA, Morrison
VA, Dummer S, Denning DW, Bennett JE, Walsh TJ, Patterson TF and
Pankey GA: Practice guidelines for diseases caused by Aspergillus.
Infectious diseases society of america. Clin Infect Dis.
30:696–709. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sagan D and Goździuk K: Surgery for
pulmonary aspergilloma in immunocompetent patients: No benefit from
adjuvant antifungal pharmacotherapy. Ann Thorac Surg. 89:1603–1610.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee JG, Lee CY, Park IK, Kim DJ, Chang J,
Kim SK and Chung KY: Pulmonary aspergilloma: Analysis of prognosis
in relation to symptoms and treatment. J Thorac Cardiovasc Surg.
138:820–825. 2009. View Article : Google Scholar : PubMed/NCBI
|