Introduction
With the aging population, the occurrence of
osteoporotic vertebral compression fracture (OVCF) caused by
osteoporosis has been increasing yearly (1). Currently, both traditional PVP and
traditional percutaneous kyphoplasty (PKP) can improve clinical
symptoms quickly in the treatment of OVCF with good clinical
effects (2), and PKP is superior to
traditional PVP in correcting kyphosis, recovering the affected
vertebra height and reducing the leakage of bone cement, however,
PVP is generally used clinically due to the low price, definite
curative effect and convenient operation and mastery (3). In the treatment of peripheral wall
damage-type OVCF, traditional PVP can cause a leakage of bone
cement and other complications (4).
The improved percutaneous vertebroplasty (improved PVP) is a type
of new technique combined with the advantage and principle of PKP
on the basis of traditional PVP. When the bone cement goes into the
doughing stage after the drawing stage, it is pushed in using push
rod according to the characteristics of permeability and
non-permeation of bone cement in the doughing stage in order to
realize uniform distribution and expanded support, thus achieving
the therapeutic effect of vertebral plasty (5). The comparative study on the
biomechanics of improved PVP and traditional PKP can greatly reduce
the subjective factors and provide a scientific and stable clinical
basis, however, there are few reports in China and other countries
due to a lack of effective experimental models. Our study detected
a change in two mechanical indexes of vertebral models, compressive
strength and stiffness, before and after the improved PVP and
traditional PKP, and compared the bone cement filling rate between
both through vertebral peripheral wall damage-type OVCF models of a
new calf (12–14 weeks) treated by decalcifying agent (Shandon
TBD-1) with the vertebral compression fracture caused by mechanical
device and peripheral wall damage realized by drop weight method
(6).
Materials and methods
Specimens thoracolumbar spine
A total of 15 thoracolumbar vertebra specimens
(T9-L4) were taken from 4 new calves (12–14 weeks). Conventional
X-ray examination was used to rule out deformity, benign and
malignant tumor and fracture, and to remove the surrounding soft
tissue immediately and free vertebral body in the intervertebral
space. The dual-energy X-ray BMD tester was used to determine the
vertebral BMD of each free specimen and the specimen was wrapped by
a double-layer plastic wrap and stored in the refrigerator at
−20°C. The specimen was unfrozen for 24 h in the refrigerator at
4°C before each experiment.
Experimental equipment
Dual-energy X-ray BMD tester (Norland Corp., Fort
Atkinson, WI, USA), Micro CT (Scanco Medical AG, Brüttisellen,
Switzerland), mechanical test equipment (Department of Mechanics,
Anhui University of Technology, Hefei, China), PVP and PKP
instrument (Shandong Weigao Group Medical Polymer Co., Ltd.,
Weihai, China), bone cement (Shandong Weigao Group Medical Polymer
Co., Ltd.), decalcifying agent (Shandon TBD-1) (Thermo Fisher
Scientific, Waltham, MA, USA) were all obtained commercially.
Preparation of osteoporosis model
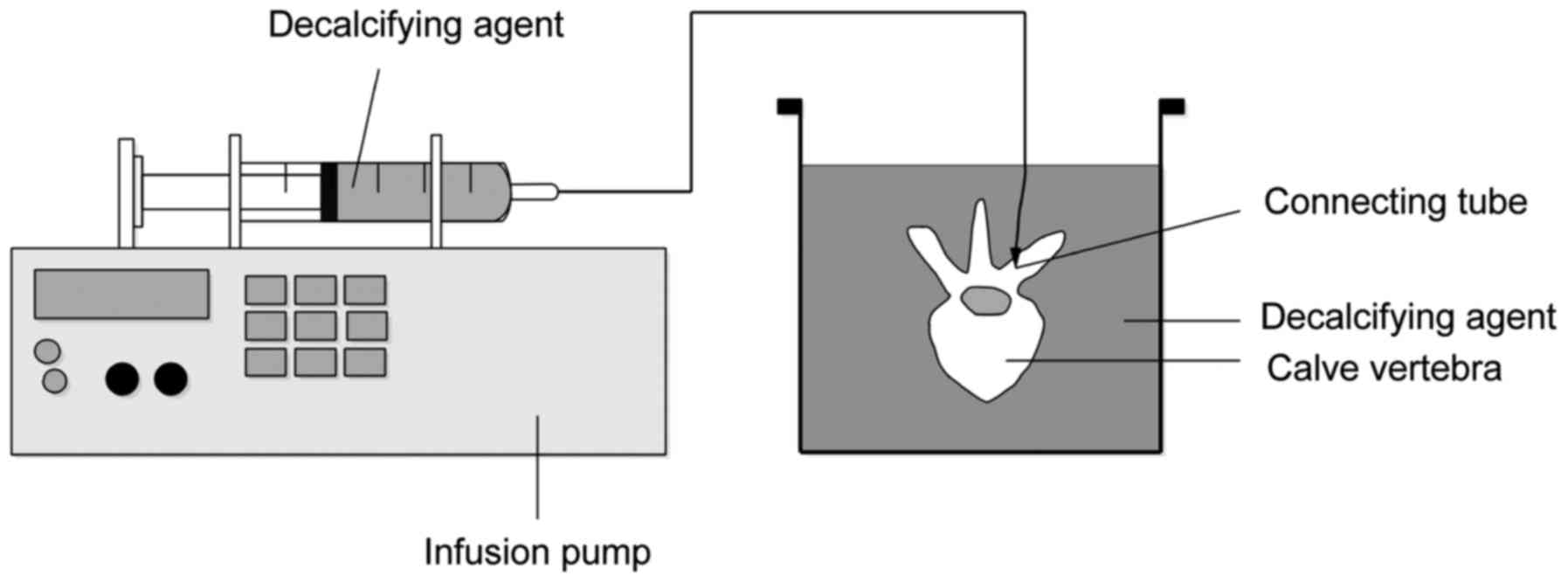
According to literature, we drilled the poles in the
intersection of superior articular process periphery and the
transverse process on both sides of the vertebral body,
respectively (diameter × depth =6.5 × 40 mm). We injected 50 ml of
decalcifying agent (Shandon TBD-1) into the nail hole on one side
of the vertebral body and placed it in a glass bowl filled with
decalcifying agent. Then, we used 6 cm-long catheter to connect the
infusion pump, and injected 480 ml of the decalcifying agent
(Shandon TBD-1), into the nail hole within 12 h at an average rate
of 40 ml/h. The same method was applied to the nail hole on the
other side of the vertebral body and each specimen was rinsed by
distilled water and dried after decalcification for 24 h. The BMD
of specimens before and after decalcification was detected, and the
osteoporotic vertebral model was established successfully on the
condition that BMD after decalcification was lower than that before
decalcification (P<0.05, Fig. 1
for model diagram).
Preparation of vertebral peripheral
wall damage-type OVCF
We used the vernier caliper to measure the front and
back, left and right height of each vertebral model. We used the
dental base acrylic resin powder to embed the upper and lower ends
of the vertebral body. We adopted the mechanical test equipment to
compress at 5 mm in posterior margin of the anterior vertebral
cortex at a rate of 10 mm/min until the vertebral anterior edge was
compressed by 25%, thereby achieving the vertebral anterior edge
compression fracture; we recorded and drew the
strength-displacement curve. According to the knee and slope of the
curve, the initial strength and stiffness of each vertebral
specimen were obtained. We also used the drop weight method to
determine the peripheral wall damage, and measured the front and
back, left and right height of each vertebral model, as well as the
vertebral volume, again using the vernier caliper. We determined
the anterior height of the fractured vertebral body, and used the
vertebral CT scan to judge whether there was vertebral peripheral
wall damage in order to further determine whether the vertebral
peripheral wall damage-type OVCF model of calf was established
successfully. This study was approved by the Ethics Committee of
the People's Hospital of Maanshan. Signed written informed consents
were obtained from all participants before the study.
Vertebral plasty
A total of 15 vertebral models were randomly divided
into three groups: the improved PVP group (Group A), traditional
PKP group (Group B) and the control group (Group C). The control
group was the blank group of peripheral wall damage-type OVCF
model, namely the group without processing. There was no
significant difference in the vertebral volume, BMD and initial
stiffness and strength of the vertebral body among the three
groups. The operation process was generally the same as that in
literature (3), and the key to the
improved PVP was that the bone cement in the doughing stage was
pushed in using a push rod when the bone cement came into the
doughing stage after drawing stage. The vertebral anterior height
of both groups was measured.
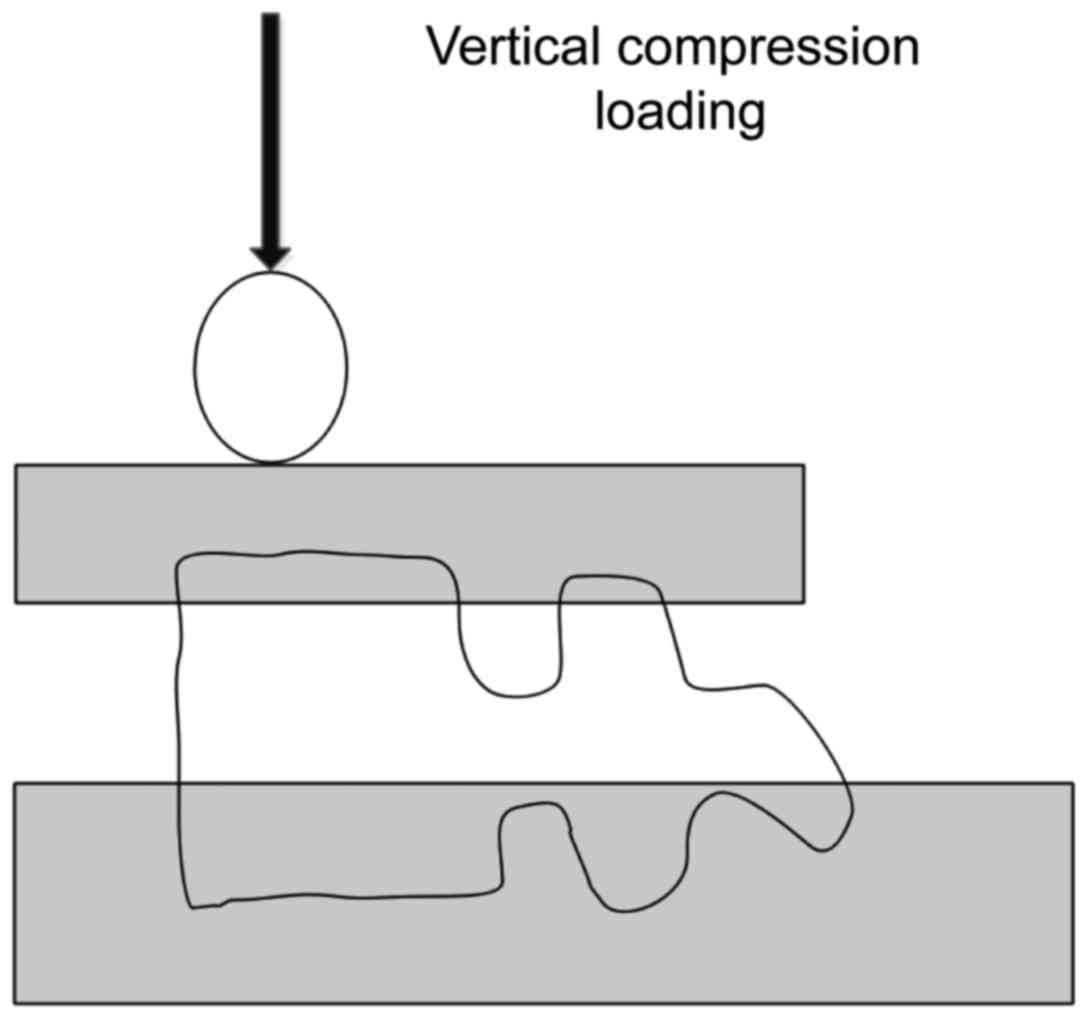
Biomechanical experiment
After the injection of bone cement, all vertebral
bodies were stored in plastic bags and placed in the refrigerator
at 4°C for 24 h; they were retrieved for the experiment. The dental
base acrylic resin powder was used to fix the single vertebral
body, and then the anterior edge of bone cement-enhanced vertebral
body was compressed at the rate of 10 mm/min until it was
compressed by 25% in order to measure the stiffness and strength
(Fig. 2).
Imaging observation
Micro CT was conducted for specimens in three groups
in order to ensure the formation of vertebral peripheral wall
damage-type OVCF model, and Micro CT was conducted again after the
vertebral plasty was used to observe the bone cement filling
rate.
Data collection
The following data were collected from specimens: ⅰ)
BMD of each vertebral body before and after decalcification; ⅱ) the
front and back, left and right height of the vertebral body before
and after the model building; ⅲ) the anterior height of the
fractured vertebral body after model building and operations in
Groups A and B; ⅳ) initial intensity and initial stiffness after
model building; strength and stiffness of the vertebral body after
operations in Groups A and B and ⅴ) bone cement filling rate.
Statistical processing
SPSS l3.0 software (SPSS, Inc., Chicago, IL, USA)
was used, and the paired t-test was used in the comparison of BMD,
strength and stiffness, and BMD, initial strength and initial
stiffness before decalcification in each group. The ANOVA test was
used in the intergroup comparison and P<0.05 suggested that the
difference was statistically significant.
Results
Basic condition of vertebral body
During the whole test process, the vertebral body of
the calf did not suffer from comminuted fracture, and all of the 15
vertebral models that were selected were anterior wall damage with
a similar damage degree. BMD after decalcification in Groups A, B
and C was significantly lower than that before decalcification
(P<0.05), and there was no significant difference in BMD before
and after decalcification among the three groups (P<0.05). After
the establishment of vertebral models and grouping, there were no
statistically significant differences in the anterior height and
vertebral body volume among the three groups (P>0.05). The bone
cement in both improved PVP and traditional PKP exceeded the middle
line of vertebral body, and the difference was not statistically
significant in the bone cement filling amount between Groups A and
B (P>0.05, Table I).
 | Table I.Comparison of basic condition of
vertebral models in three groups (x±s). |
Table I.
Comparison of basic condition of
vertebral models in three groups (x±s).
| Groups | Sample size | Vertebral body volume
V2 (ml) | Anterior height H2
(cm) | BMD G1
(g/cm2) | BMD G2
(g/cm2) | Bone cement amount
(ml) |
|---|
| Group A | 5 | 20.03+5.45 | 2.14±0.28 | 1.425±0.072 | 1.074±0.065 | 3.75±0.55 |
| Group B | 5 | 19.76±7.03 | 2.20±0.18 | 1.482±0.056 | 1.059±0.075 | 3.65±0.75 |
| Group C | 5 | 19.87±6.65 | 2.15±0.25 | 1.502±0.063 | 1.058±0.081 | – |
| P0 | – | 0.847 | 0.414 | 0.885 | 0.929 | 0.478 |
Comparison of strength and stiffness
values in Groups A and B before and after operation
The postoperative strength in Groups A and B after
the operation was 1.036±300 and 1.045±200, respectively, which was
significantly higher than the initial strength in both groups
(P<0.05). The postoperative stiffness in Groups A and B after
the operation was 395±250 and 470±270 N/mm, respectively, which was
slightly lower than initial stiffness, however, the differences
were not statistically significant (P>0.05). In the comparison
of postoperative strength and stiffness in Groups A and B, the
postoperative strength in Group A was lower than that in Group B;
differences were not statistically significant (P>0.05). There
was no significant difference in postoperative stiffness between
Groups A and B (P>0.05, Table
II).
 | Table II.Comparison of statistical results of
each index in groups A and B before and after operation. |
Table II.
Comparison of statistical results of
each index in groups A and B before and after operation.
| Group | Initial strength | Postoperative
strength | Initial
stiffness | Postoperative
stiffness | P2 | P3 |
|---|
| Group A | 700±220 | 1.036±300 | 450±230 | 395±250 | 0.021 | 0.574 |
| Group B | 710±230 | 1.045±200 | 487±220 | 470±270 | 0.036 | 0.793 |
| P1 | 0.721 | 0.826 | 0.647 | 0.604 |
|
|
Discussion
Overview of minimally invasive therapy
of OVCF
With regards to the surgical treatment of OVCF,
vertebral plasty, as an emerging minimally invasive spinal
technique, can rapidly ease pain, promote restoration of affected
vertebral height, and is characterized by a simple operation, short
learning curve and high safety, which can shorten the patient's
bed-rest time, reduce related complications, and significantly
improve quality of life (7). In
terms of the clinical effects, traditional PVP and PKP, as two
types of traditional vertebral plasty, can significantly alleviate
a patients' pain without significant difference (8). However, the PKP is superior to
traditional PVP in correcting kyphosis and recovering the vertebral
height since the difference of PKP lies in that the special
inflatable balloon that is injected into the vertebral body through
the working channel before the injection of bone cement. The high
pressure injector is connected to expand the balloon in order to
form a larger cavity, though the core of the two types of surgical
methods is to inject bone cement into the vertebral body.
Therefore, the former is uniform dispersion, while the latter is
the non-uniform dispersion. In addition, the injection amount of
bone cement in PKP is more than that in traditional PVP, which
greatly enhances the stiffness and strength of the vertebral body,
and therefore, the PKP is superior to traditional PVP in correcting
the kyphosis and recovering vertebral height (9). In addition, the balloon expansion
reduces the incidence of bone cement leakage and other surgical
complications, thus, most scholars tend to choose the traditional
PKP for clinical treatment of peripheral wall damage-type OVCF and
traditional PVP can cause leakage of bone cement and other
complications (4). Of course,
traditional PVP is characterized by a simple operation, definite
curative effect and low cost, and therefore, it is widely used in
treatment of general-type OVCF.
Mechanism of treatment of OVCF with
improved PVP
The improved PVP is a type of new technique that is
combined with the advantage and principle of PKP on the basis of
traditional PVP. With the help of the full understanding of the
performance of bone cement, when the bone cement comes into the
doughing stage after the drawing stage, it is pushed in using a
push rod according to the characteristics of permeability and
non-permeation of bone cement in doughing stage, which fully
combines the characteristics of uniform distribution of bone cement
in traditional PVP (10). This
avoids the disadvantages of traditional PVP of bone cement leakage
with the similar effects of balloon dilatation support to PKP
(11). An improved PVP can determine
the treatment effects of vertebral plasty, and reduce the risk of
complications, such as permeation.
Possibility, selection and method of
establishing effective experimental vertebral animal model
quickly
There is a paucity of research on the clinical
effects of improved PVP and traditional PKP on the peripheral wall
damage-type OVCF, and there are fewer reports in China and other
countries due to a lack of effective biomechanics in the
experimental model (12). Therefore,
the establishment of animal model of peripheral wall damage-type
OVCF is the key to this study. According to literature, the
diagnostic standard of osteoporosis in BMD is 2.5 times lower than
the mean peak of BMD of normal bones (13), and the mechanical characteristics
that constitute the bones are generally considered as a combination
of collagen fibers and calcium phosphate, among which calcium
phosphate determines the bone stiffness (14), and the content of calcium is closely
related to vertebral BMD and bone strength (15), therefore, decalcification can cause
vertebral osteoporosis. Research has shown that the reason behind
vertebral fracture is that the stress exceeds the strength of the
trabecular bone, and the main structure of vertebral body is able
to bear, and the trabecular bone structure is damaged and the local
fracture is further developed, which leads to the vertebral
fracture (16). In conclusion, if
decalcification can be realized rapidly and vertebral compression
fracture with peripheral wall damage can be achieved by external
force, then the peripheral wall damage-type OVCF animal model will
be established successfully.
There are many osteoporosis animal models tht are
currently used in spinal biomechanics research, which are
represented by castrated female animals or medicine-taking animals
(17). It is the living animal
model, and is considered to be the most satisfactory model for
evaluating the efficiency of a chemical agent and investigating the
pathophysiological mechanism of osteoporosis (18); however, it has the disadvantages of
time-consuming modeling, complex operation and vulnerability to
external environment interference (12), and therefore, it is not suitable for
the biomechanical experiments. Now, the most commonly-used model
for evaluating biological materials and efficiency of spinal
internal fixation repair system is the new and ripe calf spinal
specimen (19). Combined with a
large number of study reports, the author used a vertebral
peripheral wall damage-type OVCF model of a new calf (12–14 weeks)
that was treated with a decalcifying agent (Shandon TBD-1) with the
vertebral compression fracture caused by mechanical device and
peripheral wall damage that was determined by the drop weight
method for the experiment. The experimental results showed that
after being decalcified using Shandon TBD-1, the change in
vertebral BMD was obvious with significant differences, and the
vertebral osteoporotic change was achieved completely, which was
characterized by a shorter processing time, less external
disturbance and simple operation; it was also safer than nitric
acid and other decalcifying agents. Moreover, the compression
fracture was caused by the 25% compression of anterior vertebral
body with the knee and slope of force-displacement curve, which was
in accordance with the experimental demand. The height was changed
similarly and the multi-factor influence was reduced.
Comparison of biomechanics of improved PVP and
traditional PKP in treatment of vertebral peripheral wall
damage-type OVCF. The anterior wall damage degree of experimental
models in this study are similar, and the bone cement in both the
improved PVP and traditional PKP exceeds the middle line of the
vertebral body, which indirectly indicates the full dispersion of
both kinds of operations. Bone cement has little impact on the
mechanical equilibrium on either side of the vertebral body, and
the injection of bone cement can enhance the strength and stiffness
of the whole vertebral body. The bone cement filling rate was
similar in the process of experiments without statistical
difference, and therefore, there was no case of bone cement
leakage. The bone cement injection speed and method were the same
in the whole vertebral plasty, which shows that the safety factor
of improved PVP was higher than that of traditional PVP, which
might be associated with the bone cement status in the doughing
stage, or the similar effect of bone cement to balloon dilatation
support in doughing stage.
It is reported in the literature that there is a
close correlation between the stiffness and strength of bone
(20–22), and the detection of vertebral
compressive strength and stiffness can help evaluate the
biomechanical properties of vertebral body or implants (23–25).
This experiment detects the change in the two mechanical indexes of
vertebral models, compressive strength and stiffness, before and
after improved PVP and traditional PKP. The comparison of
compressive strength before and after the two kinds of operations
showed that the bone cement injection could quickly restore and
enhance the strength of injured vertebra, and the comparison of
postoperative strength after the two types of operations indicated
that the strength of injured vertebra after improved PVP was lower
than that after traditional PKP; the difference was not
statistically significant (P>0.05), suggesting that the improved
PVP can determine the strength of injured vertebra similar to
traditional PKP. The comparison of preoperative stiffness before
and after the two types of operations showed that the injection of
bone cement could not fully recover the stiffness of injured
vertebra. The stiffness was reduced compared to that of the normal
osteoporotic vertebral body, and the difference was not
statistically significant, which was considered to be caused by the
vertebral compression-induced vertebral cortex damage (6). The comparison of postoperative
stiffness after two types of operations, indicated that the
difference in postoperative stiffness was not statistically
significant after improved PVP and traditional PKP (P>0.05).
Overall, improved PVP can provide sufficient biomechanical strength
and stiffness and meet the needs of injured vertebra.
In conclusion, in terms of biomechanics, improved
PVP can basically achieve the curative effect of traditional PKP in
the treatment of vertebral peripheral wall damage-type OVCF,
however, considering the non-human specimen and the limited number
of calf specimens in this study, it has certain limitation and
therefore, our results must be used for reference only.
References
|
1
|
Melton LJ and Kallmes DF: Epidemiology of
vertebral fractures: Implications for vertebral augmentation. Acad
Radio1. 13:538–545. 2006. View Article : Google Scholar
|
|
2
|
Oldenhuis CN, Oosting SF, Gietema JA and
de Vries EG: Prognostic versus predictive value of biomarkers in
oncology. Eur J Cancer. 44:946–953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tseng YY, Lo YL, Chen LH, Lai PL and Yang
ST: Percutaneous polymethylmethacrylate vertebroplasty in the
treatment of pain induced by metastatic spine tumor. Surg Neurol.
70 Suppl 1:S78–S83. 2008. View Article : Google Scholar
|
|
4
|
Taylor RS, Taylor RJ and Fritzell P:
Balloon kyphoplasty and vertebroplasty for vertebral compression
fractures: A comparative systematic review of efficacy and safety.
Spine. 31:2747–2755. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Azorit C, Hervas J, Analla M, Carrasco R
and Muñoz-Cobo J: Histological thin-sections: A method for the
microscopic study of teeth in Spanish red deer (Cervus elaphus
hispanicus). Anat Histol Embryol. 31:224–227. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomita S, Kin A, Yazu M and Abe M:
Biomechanical evaluation of kyphoplasty and vertebroplasty with
calcium phosphate cement in a simulated osteoporotic compression
fracture. J Orthop Sci. 8:192–197. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boonen S, Wahl DA, Nauroy L, Brandi ML,
Bouxsein ML, Goldhahn J, Lewiecki EM, Lyritis GP, Marsh D, Obrant
K, et al CSA Fracture Working Group of International Osteoporosis
Foundation, : Balloon kyphoplasty and vertebroplasty in the
management of vertebral compression fractures. Osteoporos Int.
22:2915–2934. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Watts NB, Harris ST and Genant HK:
Treatment of painful osteoporotic vertebral fractures with
percutaneous vertebroplasty or kyphoplasty. Osteoporos Int.
12:429–437. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mathis JM: Percutaneous vertebroplasty or
kyphoplasty: Which one do I choose? Skeletal Radiol. 35:629–631.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lamy O, Uebelhart B and Aubry-Rozier B:
Risks and benefits of percutaneous vertebroplasty or kyphoplasty in
the management of osteoporotic vertebral fractures. Osteoporos Int.
25:807–819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao G, Liu X and Li F: Balloon
kyphoplasty versus percutaneous vertebroplasty for treatment of
osteoporotic vertebral compression fractures (OVCFs). Osteoporos
Int. 27:2823–2834. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anselmetti GC, Muto M, Guglielmi G and
Masala S: Percutaneous vertebroplasty or kyphoplasty. Radiol Clin
North Am. 48:641–649. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Seven A, Yuksel B, Kucur S Kabil, Yavuz G,
Polat M, Unlu BS and Keskin N: The evaluation of hormonal and
psychological parameters that affect bone mineral density in
postmenopausal women. Eur Rev Med Pharmacol Sci. 20:20–25.
2016.PubMed/NCBI
|
|
14
|
Cho AR, Kim HK, Kwon JY, Kim TK, Choi YM
and Kim KH: The incorporation of platelet-rich plasma into calcium
phosphate cement enhances bone regeneration in osteoporosis. Pain
Physician. 17:E737–E745. 2014.PubMed/NCBI
|
|
15
|
Gao J, Mi S and Liu C: Treatment of
various kinds of osteoporotic vertebral fracture with vertebral
plasty. Chin J Bone Jt Inj. 24:35–37. 2009.(In Chinese).
|
|
16
|
Colangelo D, Nasto LA, Genitiempo M,
Formica VM, Autore G, Pambianco V, Tamburrelli FC, Cerulli G and
Pola E: Kyphoplasty vs conservative treatment: a case-control study
in 110 post-menopausal women population. Is kyphoplasty better than
conservative treatment? Eur Rev Med Pharmacol Sci. 19:3998–4003.
2015.PubMed/NCBI
|
|
17
|
Zarrinkalam MR, Beard H, Schultz CG and
Moore RJ: Validation of the sheep as a large animal model for the
study of vertebral osteoporosis. Eur Spine J. 18:244–253. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nayak S, Olkin I, Liu H, Grabe M, Gould
MK, Allen IE, Owens DK and Bravata DM: Meta-analysis: Accuracy of
quantitative ultrasound for identifying patients with osteoporosis.
Ann Intern Med. 144:832–841. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Egermann M, Goldhahn J and Schneider E:
Animal models for fracture treatment in osteoporosis. Osteoporos
Int. 16 Suppl 2:S129–S138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakagi Y, Ito T, Hirooka K, Sugioka Y,
Endo H, Saijo Y, Imai H, Takeda H, Kayama F, Sasaki S, et al:
Association between lifestyle habits and bone mineral density in
Japanese juveniles. Environ Health Prev Med. 15:222–228. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kilincer C, Inceoglu S, Sohn MJ, Ferrara
LA, Bakirci N and Benzel EC: Load sharing within a human thoracic
vertebral body: An in vitro biomechanical study. Turk Neurosurg.
17:167–177. 2007.PubMed/NCBI
|
|
22
|
Giro G, Gonçalves D, Sakakura CE, Pereira
RM, Marcantonio E Júnior and Orrico SR: Influence of estrogen
deficiency and its treatment with alendronate and estrogen on bone
density around osseointegrated implants: Radiographic study in
female rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod.
105:162–167. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shirke SS, Jadhav SR and Jagtap AG:
Methanolic extract of Cuminum cyminum inhibits ovariectomy induced
bone loss in rats. Exp Biol Med. 233:1403–1410. 2008. View Article : Google Scholar
|
|
24
|
Martin-Monge E, Tresguerres IF, Blanco L,
Khraisat A, Rodríguez-Torres R and Tresguerres JA: Validation of an
osteoporotic animal model for dental implant analyses: An in vivo
densitometric study in rabbits. Int J Oral Maxillofac Implants.
26:725–730. 2011.PubMed/NCBI
|
|
25
|
Fyhrie DP and Vashishth D: Bone stiffness
predicts strength similarly for human vertebral cancellous bone in
compression and for cortical bone in tension. Bone. 26:169–173.
2000. View Article : Google Scholar : PubMed/NCBI
|