Introduction
Ischemic heart disease (IHD) is a leading cause of
death and disability globally (1).
IN 2010, >7.0 million fatalities were caused by IHD worldwide
compared with 4.5 million IHD fatalities recorded in 1980 (2). Notably, 25.6% of IHD fatalities
occurred in patients aged <65 years old in 2010.
Perioperatively, cardiac complications, including myocardial
ischemia and infarction, are the primary causes of postoperative
morbidity and mortality (3).
Reperfusion, when initiated early, improves salvage of the
myocardium and limits the final infarct size (4). However, reperfusion itself is capable
of inducing cardiomyocyte death, known as ischemia/reperfusion
(I/R) injury, for which there is still no effective therapy
(5,6).
Dexmedetomidine, a highly selective α-2 adrenoceptor
agonist with sedative, anxiolytic and analgesic effects, has been
widely used for anesthesia and in the intensive care unit (7). Our previous studies have demonstrated
that dexmedetomidine was able to reduce cardiovascular events and
mortality in patients undergoing cardiac surgery (8,9).
Previous experiments on animals have also demonstrated benefits of
dexmedetomidine when administered before ischemia (10–12);
however, its use at reperfusion was reported to increase the
myocardial infarct size (13). The
capacity for dexmedetomidine to protect cardiac tissues against I/R
injury requires further investigation.
Currently, the direct effects of dexmedetomidine on
cardiomyocytes have yet to be examined. The present study was
performed in order to determine the protective effects of
dexmedetomidine on primary cultured neonatal rat cardiomyocytes
under hypoxic/reoxygenation (H/R) conditions at the cellular level.
It was hypothesized that preconditioning, but not postconditioning,
with dexmedetomidine would attenuate H/R injury in primary neonatal
rat cardiomyocytes.
Materials and methods
Reagents and animals
Reagents included dexmedetomidine hydrochloride
(SML0956; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), dimethyl
sulfoxide (DMSO; SHBB0919V; Sigma-Aldrich; Merck KGaA), gelatin
(48722; Sigma-Aldrich; Merck KGaA), sodium hydrosulfite
(Na2S2O4; 80116718; Shanghai
Clinical Research Center, Shanghai, China), fetal bovine serum
(FBS; 16000-044), trypsin (27250–018), collagenase type II
(17101–015), Hanks' balanced salt solution (14025–076; all Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), Dulbecco's
modified Eagle medium (DMEM; SH30022.08) and phosphate-buffered
saline (PBS; SH30028.01B; both HyClone; GE Healthcare Life
Sciences, Logan, UT, USA). For all solutions, dexmedetomidine
hydrochloride was used, as the HCl form has increased
solubility.
A total of 80 neonatal specific-pathogen-free
Sprague-Dawley rats (1–3 days old; weight, 8±2 g; gender was not
determined) were provided by the Experimental Animal Center of
Soochow University (Suzhou, China). All animals were treated in
accordance with the Guide for the Care and Use of Laboratory
Animals published by the United States National Institute of Health
(14). All rats had free access to
food and water (breast-fed by mother rats) and were maintained in
conditions with ventilation and a 12-h light/dark cycle. The room
temperature was maintained at 22–25°C. All experimental procedures
were approved by the Ethics Committee of Experimental Animals of
Soochow University.
Primary cultured neonatal rat
cardiomyocyte isolation and culture
Cardiomyocytes were prepared as previously described
with some modifications (15,16).
Rats were anesthetized by inhalation of 2–3% ether (provided with
an anesthesia apparatus; R510; RWD Industrial Co., Ltd., Shenzhen,
China) and euthanized by cervical dislocation. Subsequently, the
left ventricle was excised under aseptic conditions, attached
tissues were removed and residual blood was washed away with PBS.
The ventricle was then minced into pieces of ~1 mm3 and
digested in 0.07% trypsin and 0.05% collagenase II in a 37°C water
bath for 10 min. The cells released after the first digestion were
discarded and the digestion was repeated four times. The cell
supernatants from these four digestions were transferred to 10-ml
centrifuge tubes containing DMEM supplemented with 10% FBS. Cell
suspensions were centrifuged for 5 min (700 × g) at room
temperature, filtered, and finally resuspended in DMEM supplemented
with 10% FBS at 37°C.
To reduce contamination by fibroblasts and other
cells, cardiomyocytes were purified by the differential wall
adhesion method (15). Resuspended
cells were plated into a T-75 culture flask (BD Biosciences,
Franklin Lakes, NJ, USA) and cultured in an incubator with 5%
CO2 at 37°C for 90 min. Non-adherent cells were
extracted and counted with a hemocytometer (Biotechwell, Shanghai,
China). Subsequently, the cells in the culture medium were
transferred into 96-well gelatin-coated plates at a density of
~1×106 cells/ml. After 96 h at 37°C (5% CO2),
myocyte cultures were used for subsequent experiments under H/R
conditions.
Cytotoxicity of dexmedetomidine
The plasma concentration of dexmedetomidine
clinically used for sedation is ~0.6 ng/ml, and the maximum
tolerated concentration is 10–15 ng/ml (16,17). The
molecular weight of dexmedetomidine is 200.28, therefore, 10–15
ng/ml is equivalent to 3 to 75 nM [molar concentration (mol/l)=mass
concentration (g/l)/molecular weight].
After incubation for 40–42 h, the cultured
cardiomyocytes were exposed to various concentrations of
dexmedetomidine, from 10−9-10−6 M (3, 10, 20,
40, 80, 200 and 500 nM) and 10−6-10−3 M (1,
3, 10, 30, 100, 300 and 1,000 µM), respectively, with three
replicates per group. DMSO was used as the vehicle with a
concentration of 0.1% in all groups. Additionally, cell responses
were continuously monitored and recorded for 72 h.
H/R injury model
An in vitro H/R model was established, as
previously described (18).
Na2S2O4 was used to generate
hypoxia in the H/R injury model.
Na2S2O4 removes oxygen from the
culture medium without injuring the cell membranes, and
reoxygenation may be achieved by replacing the medium. Primary
cultured neonatal rat cardiomyocytes were randomly divided into
seven groups, with three replicates per group. Group 1 cells were
cultured at 37°C in DMEM supplemented with 10% FBS for 24 h and
monitored for the duration. In groups 2–7, the culture media was
replaced with serum-free DMEM and the cells were cultured with 1,
2, 3, 4, 5 and 6 mM Na2S2O4,
respectively. These cardiomyocytes in group 2–7 were exposed to
Na2S2O4 at 37°C with 5%
CO2 for 1 h, and then the culture media was replaced
with DMEM with 10% FBS and the cells were monitored for 24 h.
Preconditioning and postconditioning
with dexmedetomidine
In cultured cardiomyocytes preconditioned with
dexmedetomidine, cells were incubated with DMEM with 10% FBS
containing 3, 10, 20, 40, 80, 200, 500 or 1,000 nM dexmedetomidine
for 2 h at 37°C before hypoxia. The culture medium was replaced
with serum-free DMEM containing
Na2S2O4 for 1 h at 37°C, after
which Na2S2O4 media was replaced
with normal DMEM and cells were cultured for a further 24 h at
37°C. In cultured cardiomyocytes postconditioned with
dexmedetomidine, the reoxygenation media (DMEM supplemented with
10% FBS) was supplemented with 3, 10, 20, 40, 80 or 200 nM
dexmedetomidine. Cells were monitored and recorded for 24 h, and
DMSO was used as the vehicle at a final concentration of 0.1% in
all groups.
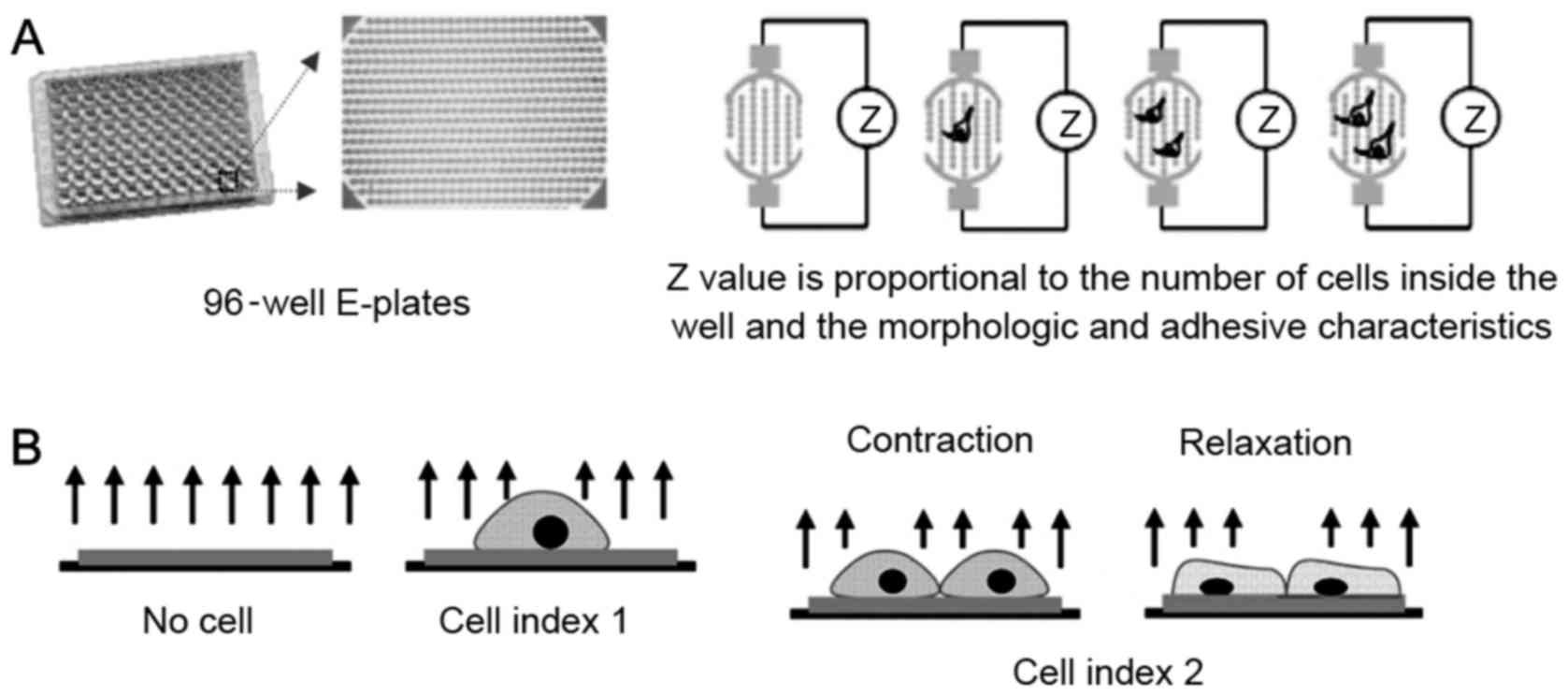
Real-time cell analyzer (RTCA)
assay
The impedance-based RTCA system consisted of a RTCA
single plate station, an RTCA computer with integrated software
(version 1.1; Roche Applied Science, Mannheim, Germany), and
disposable 96-well E-plates of the xCELLigence system (Cardio Plate
96; ACEA Biosciences, San Diego, CA, USA). The RTCA single plate
station fits inside a standard tissue-culture incubator. The cells
were seeded onto the E-plates at a density of ~1×106
cells/ml, and the electronic impedance was measured continuously to
allow monitoring of cellular attachment and growth on the
electrodes (19–26). In brief, the Cardio Plate 96 is a
microtiter plate, which is integrated with gold microelectrodes in
the bottom of each well. The impedance signal is generated by the
application of low-voltage signal, creating a current between the
electrodes. The interaction of cells with the electrodes impedes
the current and generates the impedance signal (Z value), which is
proportional to the number and the morphologic and adhesive
characteristics of cells, as demonstrated in Fig. 1. Changes in rat neonatal primary
cardiomyocytes were detected using methods previously described
(21).
The RTCA system measures the impedance of
cardiomyocytes and transforms the values to cell index (CI) values
to reflect the changes in beating activity. Normalized cell index
(NCI) was used in the analysis to represent baseline cell activity.
The NCI was calculated as the ratio of the CI value at a given
time-point to the initial CI value at the time of exposure.
Dose-effect curve and 50% effective
dose (ED50) calculation
The dexmedetomidine doses were transformed into
logarithmic dose values and non-linear fit analysis was performed
to generate dose-response curves. The model used in the present
study was the following:
Y=Bottom+(Top-Bottom)/[1+10(LogEC50-X)*HillSlope], where
the HillSlope describes the steepness of the family of curves.
An NCI of 0–100% was used in the y-axis of the
dose-effect curve. Based on the dose-response curve, the
ED50 of dexmedetomidine on the NCI was calculated.
Furthermore, the reliability of the ED50 calculated from
a specific dose-effect curve was evaluated by the slope factor.
Statistical analysis
The area under the time-course curves (AUCs) was
used to measure the cumulative effects of
Na2S2O4 and dexmedetomidine. Data
were expressed as the mean ± standard error of the mean, and
one-way analysis of variance with Dunnett's post hoc test was
performed using SPSS v. 19.0 statistical software (IBM SPSS,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
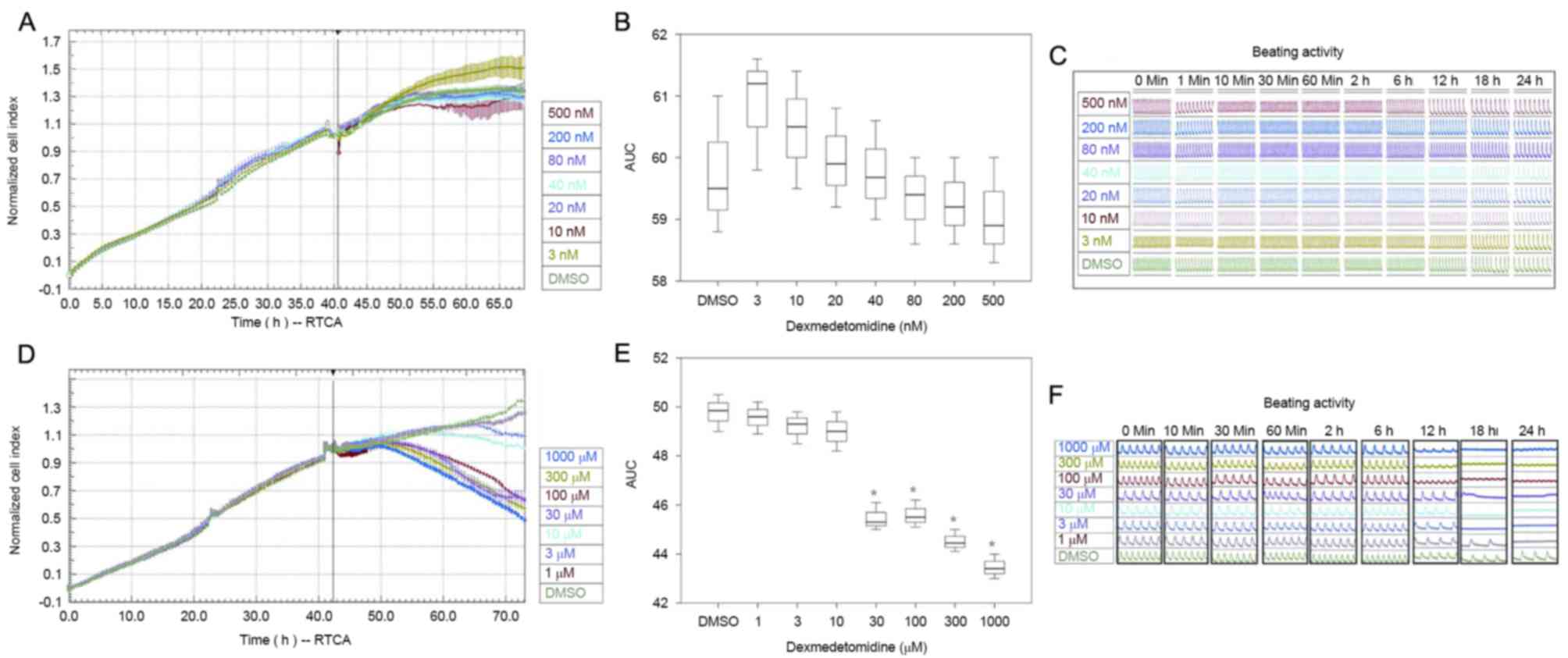
Cytotoxicity of dexmedetomidine
Incubation with 3–500 nM dexmedetomidine did not
significantly alter the NCI (Fig.
2A-C), indicating that dexmedetomidine was not cytotoxic to
cultured cardiomyocytes when used at clinically relevant
concentrations. NCI decreased significantly at dexmedetomidine
concentrations ≥30 µM compared with DMSO (P<0.01; Fig. 2D-F).
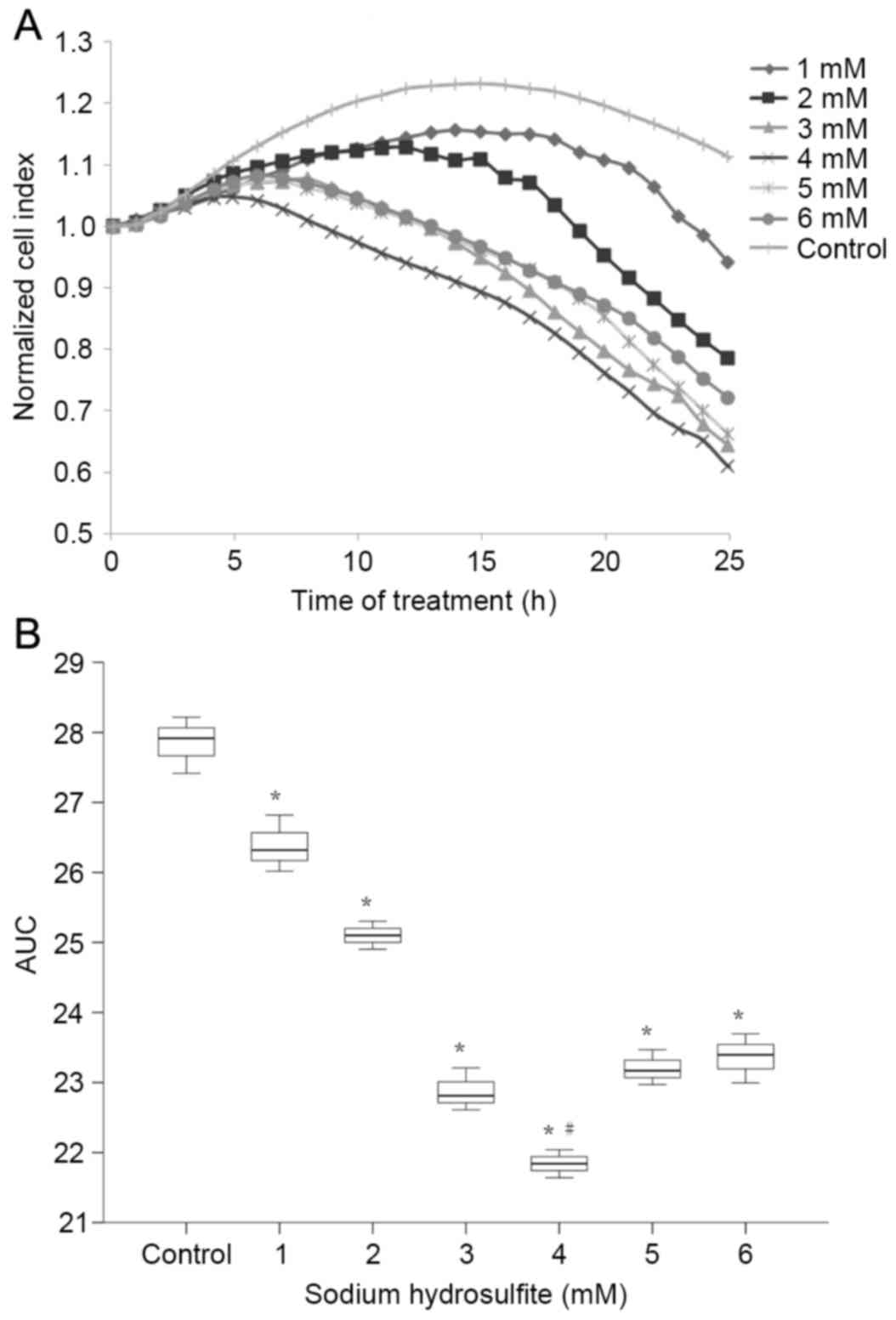
Effect of
Na2S2O4 on cardiomyocytes
As demonstrated in Fig.
3, the NCI of primary cultured neonatal rat cardiomyocytes
markedly decreased following exposure to
Na2S2O4. The AUCs created by all
concentrations of Na2S2O4 were
significantly lower than the control (P<0.01) and cells
incubated with a Na2S2O4
concentration of 4 mM gave the significantly lowest AUC compared to
all other groups (P<0.01). As the most significant reduction in
AUC for the time-course of NCI was observed in cells incubated with
4 mM Na2S2O4, this concentration
was selected for subsequent experiments.
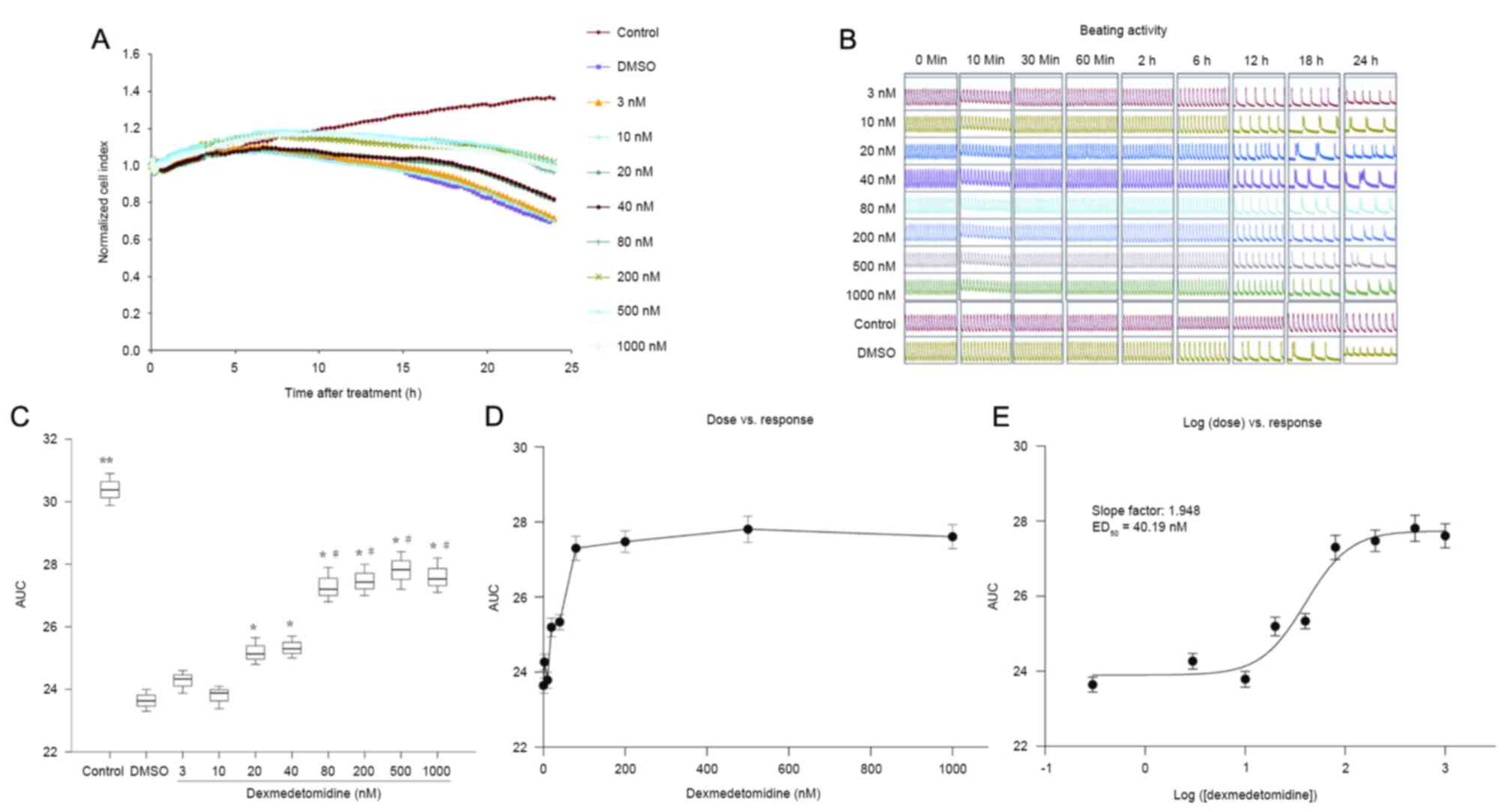
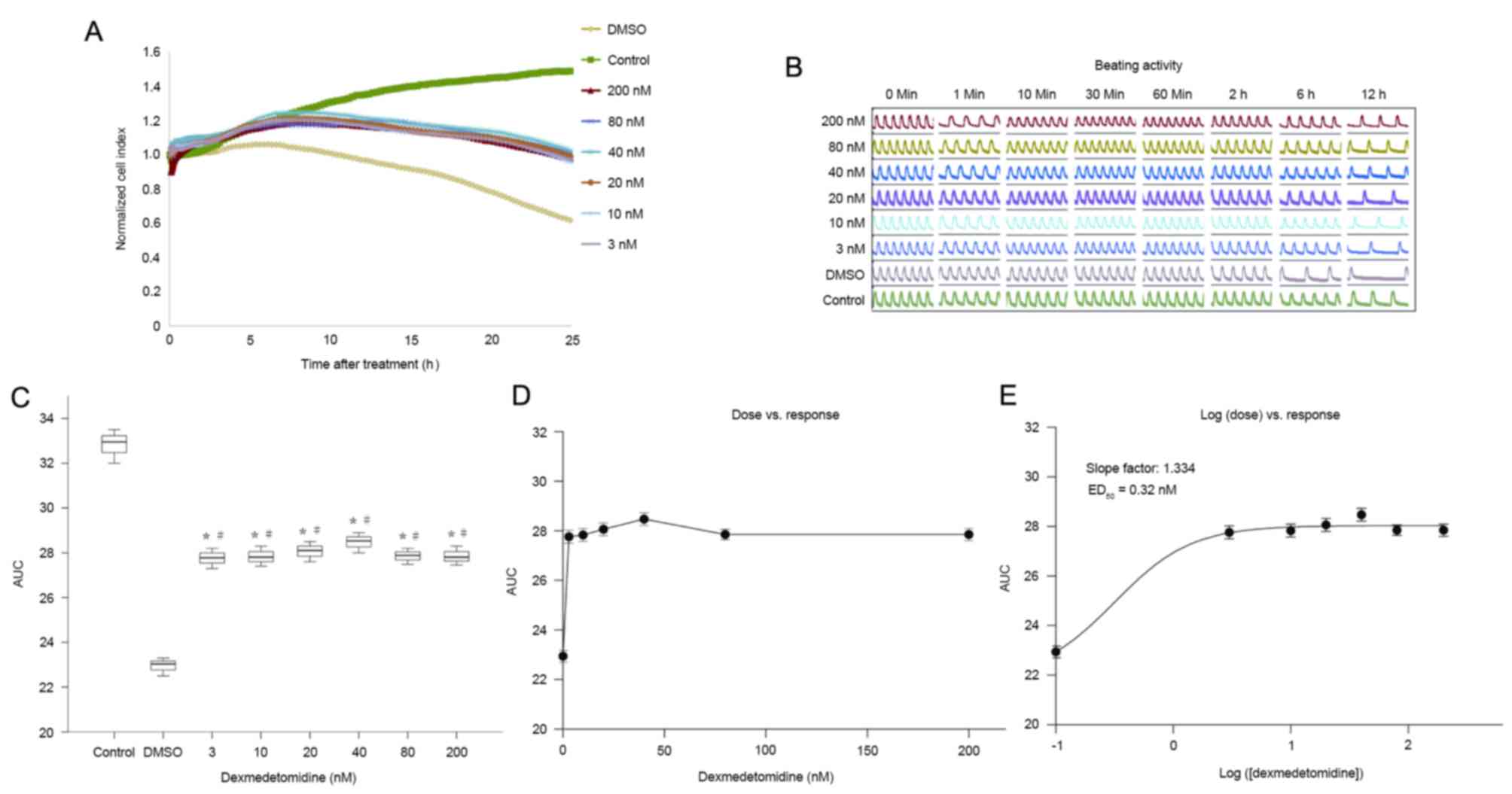
Effects of dexmedetomidine on
cardiomyocytes under H/R injury
As demonstrated in Fig.
4A-C, preconditioning with dexmedetomidine for 2 h before
hypoxia ameliorated reductions in NCI in primary neonatal rat
cardiomyocytes exposed to H/R injury. Dexmedetomidine significantly
increased the AUC of NCI during the 24 h cultivation, in a
concentration-dependent manner (0–10 vs. 20–40 vs. 80–1,000 nM; all
P<0.01; Fig. 4C). The effects of
different concentrations of dexmedetomidine on the NCI were
calculated from the dose vs. response curve (Fig. 4D) based on the log (dose) vs.
response curve (Fig. 4E). The
ED50 of the protective effect of dexmedetomidine on the
NCI was calculated to be 40.19 nM.
Dexmedetomidine postconditioning also ameliorated
reductions in NCI at all concentrations (Fig. 5A-C). The AUCs were significantly
higher in the dexmedetomidine groups than in the hypoxia (DMSO)
group (P<0.01), and the effect did not differ significantly
between dexmedetomidine concentrations. The dose-effect curves are
shown in Fig. 5D and E, but the
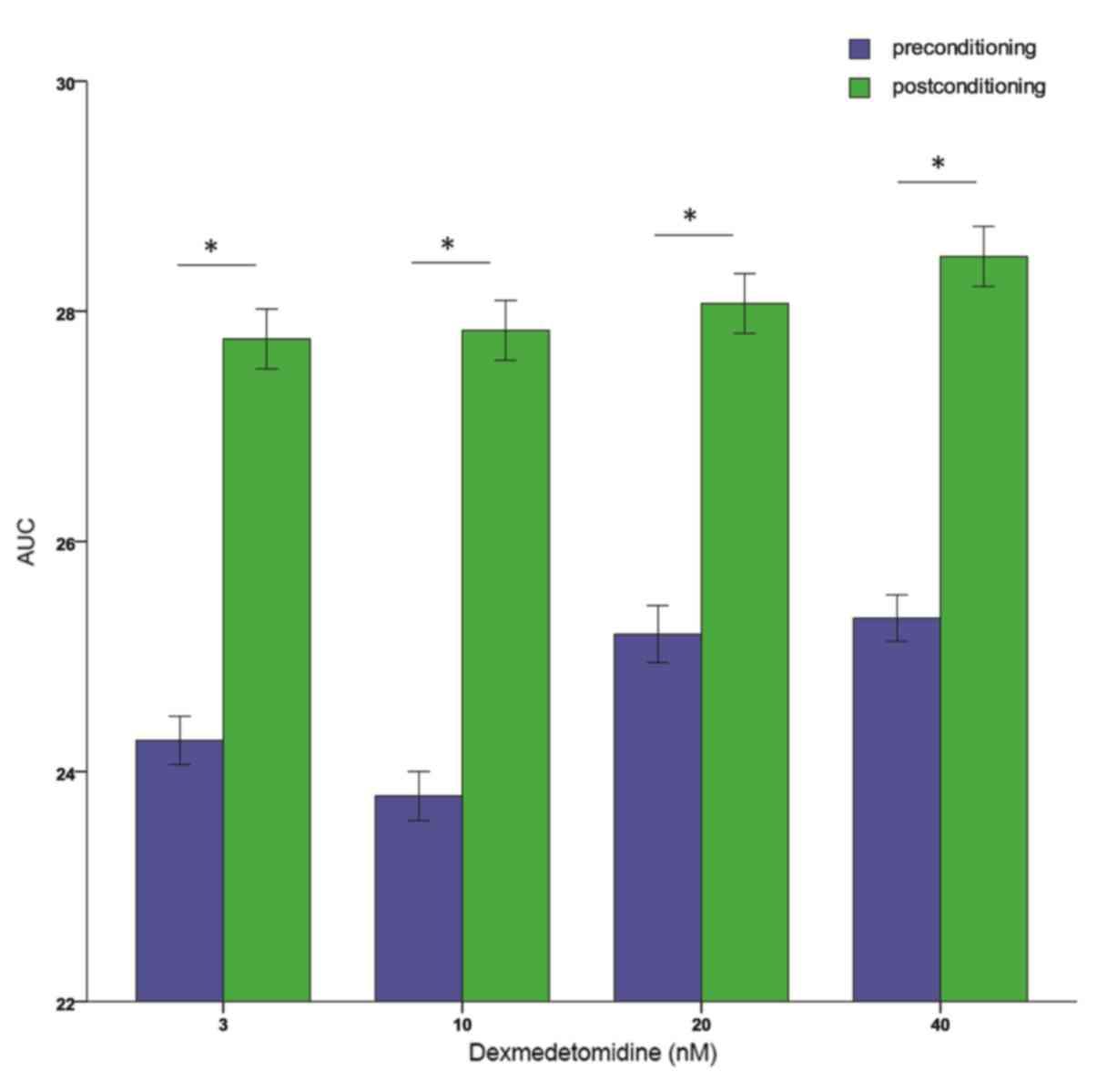
exact ED50 cannot be retrieved. Additionally, 3, 10, 20,
and 40 nM dexmedetomidine postconditioning resulted in
significantly higher AUCs compared with preconditioning at the same
concentration (all P<0.01; Fig.
6). All these results indicated that further studies are
required to explore the effects of dexmedetomidine postconditioning
on primary cultured neonatal rat cardiomyocytes.
Discussion
The present study demonstrated that preconditioning
with dexmedetomidine ameliorated H/R injury-induced reductions in
NCI in primary cultured neonatal rat cardiomyocytes, in a
concentration-dependent manner. When applied during reoxygenation,
however, dexmedetomidine ameliorated reductions in NCI independent
of its concentration. Consistent with this, higher AUCs were
observed in cells postconditioned with 3–40 nM dexmedetomidine than
those preconditioned with equivalent dexmedetomidine
concentrations.
The present study utilized rat neonatal
cardiomyocytes instead of adult cells for the following reasons:
The phenotype of cultured neonatal cardiomyocytes is stable, and
their contractile profile during H/R is comparable with that of
in situ hearts during I/R, whereas the phenotype of isolated
adult cardiomyocytes is quite different from that of in situ
hearts, in part due to the loss of connections among myocytes
(27). Neonatal cells form a
continuous sheet, whereas adult cells are discontinuous.
Furthermore, cultured neonatal rat cardiomyocytes have been
demonstrated to be useful for analyzing states of oxygen- and
volume-restriction, conditions that are known to stimulate anoxia
and ischemia at the cellular level (28). The limitation of using neonatal cells
is that they do not fully recapitulate the adult cell type, and
future studies using an in vivo model are required to build
on the observations of the present report.
Hypoxia was induced using a chemical oxygen
scavenger, and therefore possible side effects of
Na2S2O4 need to be considered. In
previous studies, hydrosulfite was demonstrated to not be
equivalent to authentic hypoxia because of little similarity to
hypoxic vasoconstriction (29,30).
Although hypoxic ventilation and hydrosulfite both lower
PO2, only hydrosulfite generates activated oxygen
species, increases lung weight, and impairs vascular responsiveness
to vasoconstrictors (29,30). According to previous research,
Na2S2O4 may still be a useful
agent for inducing hypoxia in various types of cell models,
including primary neonatal rat cardiomyocytes (18,31–33).
The RTCA is a novel technique for continuous
monitoring of cell adherence, proliferation, migration and
cytotoxicity (21). This technology
allows for uninterrupted, label free, real-time analysis of the
cells over the entire course of the experiment, which provides a
dynamic and sensitive assessment beyond the scope of single
time-point assays (22). A number of
studies have demonstrated that this system is a valid method for
analyzing the effects of compound cytotoxicity, and measuring cell
death of various cell types, including primary cultured neonatal
rat cardiomyocytes (19–26).
The advantages of the RTCA technology include its
capacity for noninvasive measurements that do not require cellular
labeling or overexpression of reporter proteins, and no or minimal
intervention in cell physiology. Additionally, its real-time
sensitive cell-monitoring platform allows for real-time kinetic
evaluation and is easy to set up. The RTCA technology may be used
for pharmacology and toxicology studies with a reasonable
throughput (22,24), and, therefore, represents a powerful
and reliable tool for drug discovery (34).
Generally, the cytotoxicity of the experimental drug
is evaluated prior to analyzing its effect on cell physiology.
Thus, the cytotoxicity of dexmedetomidine was determined before
investigating its capacity to protect cardiomyocytes following H/R
injury. In the present study, a clinically relevant concentration
range of dexmedetomidine was observed to not have significant
adverse effects on cardiomyocyte viability.
Previous studies have reported that systemic
administration of α-2 adrenergic agonists, clonidine or
dexmedetomidine, were able to provide myocardial protection in
vivo by attenuating the catecholamine response to ischemic
stress (35–37) and improving the myocardial oxygen
balance (38,39). Despite these benefits, the direct
effects of α-2 adrenergic agonists on the coronary vasculature are
controversial. Some studies have indicated that α-2 adrenergic
coronary vasoconstriction exerts favorable effects on the ischemic
myocardium, preventing transmural redistribution of blood flow away
from the endocardium and improving the subendocardial to
subepicardial blood flow ratio (40,41).
Other studies have demonstrated that the activation of coronary
vascular α-2 adrenergic receptors induced post-stenotic coronary
vasoconstriction and myocardial ischemia (42). However, the direct effects of
dexmedetomidine on cardiomyocytes have not been determined.
To the best of our knowledge, the present study is
the first to demonstrate the protective effects of dexmedetomidine
on H/R injury in a neonatal rat cardiomyocyte model. As expected,
preconditioning with dexmedetomidine significantly ameliorated the
loss of viability resulting from H/R injury in a
concentration-dependent manner, with no observed cardiomyocyte
toxicity. The in vitro H/R model used in the present study
excluded local in vivo cardiac and central sympathetic
effects, thus revealing the direct and specific effects of
dexmedetomidine on cardiomyocytes. Furthermore, the present study
simulated plasma concentrations of dexmedetomidine that are
frequently used clinically for sedation, and it was demonstrated
that the cell number and activity was better preserved by higher
concentrations of dexmedetomidine. This observation suggested that
dexmedetomidine preconditioning with higher concentrations may
achieve greater protection against H/R injury of
cardiomyocytes.
Cardioprotective therapy following reperfusion is
more clinically feasible than preconditioning, as the onset of
reperfusion is predictable and is under the control of the
clinicians. However, previous studies have reported that
administration of dexmedetomidine after reperfusion did not exert a
direct protective effect on left ventricular dysfunction, and even
increased myocardial infarct size in isolated rat hearts (13,43).
Contrary to our secondary hypothesis, the results of the present
study indicated that postconditioning with dexmedetomidine was also
able to attenuate H/R injury of cardiomyocytes. Notably,
dexmedetomidine applied shortly after the onset of reoxygenation
improved cell survival even more effectively than when applied
before hypoxia. There are two possible explanations for this
observation: i) Preconditioning was applied 2 h before hypoxia,
whereas postconditioning was initiated at the time of reoxygenation
and therefore, the timing of dexmedetomidine administration may
have caused different dose-effects on the cardiomyocytes; and ii)
the culture medium containing dexmedetomidine was replaced with
media containing Na2S2O4 during
preconditioning, which may affect the protective effects of
dexmedetomidine on the cultured cardiomyocytes. As the predominant
conclusion of the present study is that dexmedetomidine attenuates
H/R injury under both preconditioning and postconditioning, a
future direction from here is to determine the lowest efficacious
concentration.
The conclusions of the present study are limited by
its scope. Firstly, an in vitro experimental H/R model was
used to simulate I/R injury, and condition-dependent differences
may exist between models. Secondly, rat neonatal cardiomyocytes
were used instead of adult cardiomyocytes, and the physiological
benefits of dexmedetomidine will need to be investigated using
adult rat or human cells to more accurately reflect the clinical
application of dexmedetomidine. Furthermore, investigation into the
precise mechanisms responsible for the cardioprotective effects of
dexmedetomidine is now in progress. In summary, the present study
is a preliminary investigation that was performed to determine the
effects of preconditioning and postconditioning with
dexmedetomidine on cardiomyocyte protection against H/R injury at
the cellular level, as measured by improved cell physiology
assays.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81471835 and
81471889) and the Science and Technology Development Projects of
Soochow (grant no. SYSD2013073). The present work was a follow-up
study of the abstract presented at the Anesthesiology Annual
Meeting of the American Society of Anesthesiologists Oct 27th 2015
in San Diego, CA, USA as abstract no. A4171.
References
|
1
|
Sluijter JP, Condorelli G, Davidson SM,
Engel FB, Ferdinandy P, Hausenloy DJ, Lecour S, Madonna R, Ovize M,
Ruiz-Meana M, et al: Novel therapeutic strategies for
cardioprotection. Pharmacol Ther. 144:60–70. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moran AE, Forouzanfar MH, Roth GA, Mensah
GA, Ezzati M, Murray CJ and Naghavi M: Temporal trends in ischemic
heart disease mortality in 21 world regions, 1980 to 2010: The
Global Burden of Disease 2010 study. Circulation. 129:1483–1492.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Poldermans D, Hoeks SE and Feringa HH:
Pre-operative risk assessment and risk reduction before surgery. J
Am Coll Cardiol. 51:1913–1924. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keeley EC, Boura JA and Grines CL: Primary
angioplasty versus intravenous thrombolytic therapy for acute
myocardial infarction: A quantitative review of 23 randomised
trials. Lancet. 361:13–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kloner RA: Does reperfusion injury exist
in humans? J Am Coll Cardiol. 21:537–545. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hausenloy DJ and Yellon DM: Myocardial
ischemia-reperfusion injury: A neglected therapeutic target. J Clin
Invest. 123:92–100. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gerlach AT, Murphy CV and Dasta JF: An
updated focused review of dexmedetomidine in adults. Ann
Pharmacother. 43:2064–2074. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji F, Li Z, Nguyen H, Young N, Shi P,
Fleming N and Liu H: Perioperative dexmedetomidine improves
outcomes of cardiac surgery. Circulation. 127:1576–1584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji F, Li Z, Young N, Moore P and Liu H:
Perioperative dexmedetomidine improves mortality in patients
undergoing coronary artery bypass surgery. J Cardiothorac Vasc
Anesth. 28:267–273. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshitomi O, Cho S, Hara T, Shibata I,
Maekawa T, Ureshino H and Sumikawa K: Direct protective effects of
dexmedetomidine against myocardial ischemia- reperfusion injury in
anesthetized pigs. Shock. 38:92–97. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Okada H, Kurita T, Mochizuki T, Morita K
and Sato S: The cardioprotective effect of dexmedetomidine on
global ischaemia in isolated rat hearts. Resuscitation. 74:538–545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ibacache M, Sanchez G, Pedrozo Z, Galvez
F, Humeres C, Echevarria G, Duaso J, Hassi M, Garcia L, Díaz-Araya
G and Lavandero S: Dexmedetomidine preconditioning activates
pro-survival kinases and attenuates regional ischemia/reperfusion
injury in rat heart. Biochim Biophys Acta. 1822:537–545. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mimuro S, Katoh T, Suzuki A, Yu S, Adachi
YU, Uraoka M, Sano H and Sato S: Deterioration of myocardial injury
due to dexmedetomidine administration after myocardial ischaemia.
Resuscitation. 81:1714–1717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guide for the Care and Use of Laboratory
Animals. NIH Publication No. 85 23, . 1996.
|
|
15
|
Zhang C, Lin G, Wan W, Li X, Zeng B, Yang
B and Huang C: Resveratrol, a polyphenol phytoalexin, protects
cardiomyocytes against anoxia/reoxygenation injury via the
TLR4/NF-κB signalling pathway. Int J Mol Med. 29:557–563.
2012.PubMed/NCBI
|
|
16
|
Chrysostomou C and Schmitt CG:
Dexmedetomidine: Sedation, analgesia and beyond. Expert Opin Drug
Metab Toxicol. 4:619–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ebert TJ, Hall JE, Barney JA, Uhrich TD
and Colinco MD: The effects of increasing plasma concentrations of
dexmedetomidine in humans. Anesthesiology. 93:382–394. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ren C, Bao YR, Meng XS, Diao YP and Kang
TG: Comparison of the protective effects of ferulic acid and its
drug-containing plasma on primary cultured neonatal rat
cardiomyocytes with hypoxia/reoxygenation injury. Pharmacogn Mag.
9:202–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Garcia SN, Gutierrez L and McNulty A:
Real-time cellular analysis as a novel approach for in vitro
cytotoxicity testing of medical device extracts. J Biomed Mater Res
Part A. 101:2097–2106. 2013. View Article : Google Scholar
|
|
20
|
Pan T, Khare S, Ackah F, Huang B, Zhang W,
Gabos S, Jin C and Stampfl M: In vitro cytotoxicity assessment
based on KC(50) with real-time cell analyzer (RTCA) assay. Comput
Biol Chem. 47:113–120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xi B, Wang T, Li N, Ouyang W, Zhang W, Wu
J, Xu X, Wang X and Abassi YA: Functional cardiotoxicity profiling
and screening using the xCELLigence RTCA cardio system. J Lab
Autom. 16:415–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Atienzar FA, Tilmant K, Gerets HH,
Toussaint G, Speeckaert S, Hanon E, Depelchin O and Dhalluin S: The
use of real-time cell analyzer technology in drug discovery:
Defining optimal cell culture conditions and assay reproducibility
with different adherent cellular models. J Biomol Screen.
16:575–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kirstein SL, Atienza JM, Xi B, Zhu J, Yu
N, Wang X, Xu X and Abassi YA: Live cell quality control and
utility of real-time cell electronic sensing for assay development.
Assay Drug Dev Technol. 4:545–553. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Quereda JJ, Martínez-Alarcón L, Mendoça L,
Majado MJ, Herrero-Medrano JM, Pallarés FJ, Ríos A, Ramírez P,
Muñoz A and Ramis G: Validation of xCELLigence real-time cell
analyzer to assess compatibility in xenotransplantation with
pig-to-baboon model. Transplant Proc. 42:pp. 3239–3243. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang L, Xie L, Boyd JM and Li XF:
Cell-electronic sensing of particle-induced cellular responses.
Analyst. 133:643–648. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xing JZ, Zhu L, Jackson JA, Gabos S, Sun
XJ, Wang XB and Xu X: Dynamic monitoring of cytotoxicity on
microelectronic sensors. Chem Res Toxicol. 18:154–161. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamashita N, Nishida M, Hoshida S, Kuzuya
T, Hori M, Taniguchi N, Kamada T and Tada M: Induction of manganese
superoxide dismutase in rat cardiac myocytes increases tolerance to
hypoxia 24 h after preconditioning. J Clin Invest. 94:2193–2199.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iwaki K, Chi SH, Dillmann WH and Mestril
R: Induction of HSP70 in cultured rat neonatal cardiomyocytes by
hypoxia and metabolic stress. Circ Res. 87:2023–2032. 1993.
View Article : Google Scholar
|
|
29
|
Archer SL, Hampl V, Nelson DP, Sidney E,
Peterson DA and Weir EK: Dithionite increases radical formation and
decreases vasoconstriction in the lung. Evidence that dithionite
does not mimic alveolar hypoxia. Circ Res. 77:174–181. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu MF, Gorenne I, Su X, Moreland RS and
Kotlikoff MI: Sodium hydrosulfite contractions of smooth muscle are
calcium and myosin phosphorylation independent. Am J Physiol.
275:L976–L982. 1998.PubMed/NCBI
|
|
31
|
Sun J, Li YZ, Ding YH, Wang J, Geng J,
Yang H, Ren J, Tang JY and Gao J: Neuroprotective effects of gallic
acid against hypoxia/reoxygenation-induced mitochondrial
dysfunctions in vitro and cerebral ischemia/reperfusion injury in
vivo. Brain Res. 1589:126–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo L, Lü L, Lu Y, Zhang L, Li B, Guo K,
Chen L, Wang Y, Shao Y and Xu J: Effects of hypoxia on progranulin
expression in HT22 mouse hippocampal cells. Mol Med Rep.
9:1675–1680. 2014.PubMed/NCBI
|
|
33
|
Rigatto H, Fitzgerald SF, Willis MA and Yu
C: In search of the real respiratory neurons: Culture of medullary
fetal cells sensitive to CO2 and low pH. Biol Neonate. 65:149–155.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abassi YA, Xi B, Zhang W, Ye P, Kirstein
SL, Gaylord MR, Feinstein SC, Wang X and Xu X: Kinetic cell-based
morphological screening: Prediction of mechanism of compound action
and off-target effects. Chem Biol. 16:712–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Willigers HM, Prinzen FW, Roekaerts PM, de
Lange S and Durieux ME: Dexmedetomidine decreases perioperative
myocardial lactate release in dogs. Anesth Analg. 96:657–664. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meissner A, Weber TP, van Aken H,
Zbieranek K and Rolf N: Clonidine improve recovery from myocardial
stunning in conscious chronically instrumented dogs. Anesth Analg.
87:1009–1014. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Roekaerts PM, Prinzen FW and De Lange S:
Beneficial effects of dexmedetomidine on ischaemic myocardium of
anaesthetized dogs. Br J Anaesth. 77:427–429. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lawrence CJ, Prinzen FW and de Lange S:
Hemodynamic and coronary vascular effects of dexmedetomidine in the
anesthetized goat. Acta Anaesthesiol Scand. 41:830–836. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jalonen J, Halkola L, Kuttila K, Perttilä
J, Rajalin A, Savunen T, Scheinin M and Valtonen M: Effects of
dexmedetomidine on coronary hemodynamics and myocardial oxygen
balance. J Cardiothorac Vasc Anesth. 9:519–524. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miyamoto MI, Rockman HA, Guth BD, Heusch G
and Ross J Jr: Effect of alpha-adrenergic stimulation on regional
contractile function and myocardial blood flow with and without
ischemia. Circulation. 84:1715–1724. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chilian WM and Ackell PH: Transmural
differences in sympathetic coronary constriction during exercise in
the presence of coronary stenosis. Circ Res. 62:216–225. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Heusch G, Schipke J and Thämer V:
Clonidine prevents the sympathetic initiation and aggravation of
poststenotic myocardial ischemia. J Cardiovasc Pharmacol.
7:1176–1182. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guo H, Takahashi S, Cho S, Hara T,
Tomiyasu S and Sumikawa K: The effects of dexmedetomidine on left
ventricular function during hypoxia and reoxygenation in isolated
rat hearts. Anesth Analg. 100:629–635. 2005. View Article : Google Scholar : PubMed/NCBI
|