Introduction
The occurrence of two or more consecutive
spontaneous miscarriages before the 20th week of gestation is
defined as recurrent spontaneous abortion (RSA), a condition that
affects 1–5% women of reproductive age (1). The etiology mainly involves genetic
disorders, immune diseases, endocrine dysfunctions, infection and
anatomic abnormalities (2). The
cause of unexplained recurrent spontaneous abortion (URSA) remains
unknown. However, it has been suggested that URSA is associated
with the failure of fetal-maternal immunologic tolerance (3).
URSA affects approximately 50% of RSA patients, and
it may be associated with the failure of fetal-maternal immunologic
tolerance. Tolerance of the fetus by the maternal immune system is
considered to depend on the interactions of an array of cytokines.
Cytokines produced by CD4+ T cells may play an important
role in maternal-fetal immunoregulation (2). The CD4+ T cells can be
classified into the following subsets: T helper (Th)1, Th2, Th17,
and regulatory T (Treg) cells according to their functions. A study
indicated that altered immunity in URSA is dominated by the Th1/Th2
hypothesis (4). However, the Th1/Th2
paradigm is not enough to explain the mechanism regarding how the
fetus is increased by maternal immune cells. The Th1/Th2 paradigm
has been expanded to the Th1/Th2/Th17, and Treg cell paradigm. The
Th17 and Treg cells were recently discovered as lymphocyte subsets
with a unique differentiation and growth regulatory mechanism,
which is different from Th1 and Th2 cells. These are known to play
a major role in the development of autoimmune diseases and
infection. Previous studies have shown that the imbalance of
Th17/Treg may be associated with URSA (5–7). Large
doses of immunoglobulin have been administered as immunotherapy to
idiopathic RSA women (8,9). We improved the current methods by using
small doses of immunoglobulin (IG) combined human chorionic
gonadotropin (hCG) hormone for the therapy of URSA women with a
success rate of 95.1% (10).
In this study, we aimed to investigate the effects
of the combined therapy of immunoglobulin plus hCG on Th17/Treg
balance in URSA women.
Materials and methods
Sample selection
The study enrolled 20 URSA patients who had at least
two consecutive spontaneous miscarriages that occurred before 20
weeks gestation and had no chromosomal, anatomic, endocrine
dysfunctions, or infections of the reproductive tract. The
husband's semen analysis was normal. All the subjects were patients
of the Department of Obstetrics and Gynecology at the Affiliated
Hospital of Xuzhou Medical College between June 2013 and April
2014. Their mean age was 26.05±2.31 years. Informed consent to
participate in the study was obtained from the patients. The study
was approved by the Ethics Committee of the Xuzhou Central Hospital
(Jiangsu, China).
Immunoglobulin therapy and sampling
time
IG-combined hCG was administered to the study
subjects as soon as pregnancy was confirmed by a positive urine
β-hCG test. The therapy protocol was as follows: 0.3 g IG were
injected intramuscularly every three weeks until 14 weeks of
gestation, and 2,000 U hCG were injected intramuscularly every two
days. Ultrasound was used to detect whether the embryo was in
normal development between 12 and 13 weeks of pregnancy. The dose
of hCG was decreased after the 13th week of pregnancy and finally
discontinued. After pregnancy, 8 ml peripheral blood was drawn
before treatment and at the 12 weeks of gestation (after treatment)
respectively. Samples included 5 ml of K2EDTA anticoagulant and 3
ml procoagulant. All the pregnancies were ongoing beyond 28 weeks
of gestation.
Drugs and reagents
IG (Franc Group), hCG (Taibang Biological Products
Co. Ltd., Shandong, China), High-purity Total RNA extraction kit
(Generay Biotech Co., Ltd., Shanghai, China), TIANScript RT kit
(Tiangen Biotech Co., Ltd., Shanghai, China), polymerase chain
reaction (PCR) kit of 2X Taq Master Mix (Vazyme Biotech Co., Ltd.,
Nanjing, China), DNA molecular size marker of 100 bp DNA ladder
(Tiangen Biotech Co., Ltd.) and enzyme-linked immunosorbent assay
(ELISA) kit (Suzhou Calvin Biotechnology Co., Ltd., Suzhou, China)
were used. Primers were produced by Sangon Biotech (Shanghai) Co.,
Ltd. (Shanghai, China).
Total RNA preparation and cDNA
synthesis
Total RNA was isolated from EDTA anticoagulated
blood using High-purity Total RNA Extraction kit (Generay Biotech
Co., Ltd.). Synthesis of cDNA was performed using TIANScript RT kit
(Tiangen Biotech Co., Ltd.) with a 20-µl reaction system. The whole
process was carried out on ice.
PCR
Amplification reactions (25 µl) consisted of 2 µl of
cDNA, 12.5 µl of 2X Taq Master Mix, 8.5 µl of ddH2O, 1
µl of forward primer and 1 µl of reverse primer. PCR reaction was
performed as follows: An initial denaturation step of 94°C for 5
min, followed by 94°C for 30 sec, 55°C for 30 sec, 72°C for 60 sec
for 35 cycles and a final extension at 72°C for 10 min. Primer
sequences, the size of PCR product and annealing temperature are
described in Table I. PCR products
were observed on a 2% agarose gel that was stained with ethidium
bromide, electrophoresed for 1.5 h at 40 mA and photographed under
ultra-violet light. The OD ratio of target genes and β-actin was
calculated to measure the mRNA levels of target genes
relatively.
 | Table I.Primer sequences, size of PCR product
and annealing temperature. |
Table I.
Primer sequences, size of PCR product
and annealing temperature.
| Genes | Nucleotide sequences
(5′-3′) | Annealingtemperature
(°C) | Size of PCR product
(bp) |
|---|
| IL-17 |
TGTCCACCATGTGGCCTAAGAG | 60 | 119 |
|
|
GTCCGAAATGAGGCTGTCTTTGA |
|
|
| IL-6 |
CAAAGATGGCTGAAAAAGATGGATG | 58 | 313 |
|
|
GATGAACTAATTAAACCTGTGGGAG |
|
|
| IL-10 |
CTTGTCTGAGATGATCCAGTTTTAC | 58 | 298 |
|
|
AAGAGAAATGAGCAAGAGATCTGAC |
|
|
|
TGF-β1 |
GGGACTATCCACCTGCAAGA | 55 | 239 |
|
|
CCTCCTTGGCGTAGTAGTCG |
|
|
| β-actin |
CGGGAAATCGTGCGTGACAT | 62 | 481 |
|
|
CGGACTCGTCATACTCCTGCTTG |
|
|
ELISA to detect cell factor
The absorbance of serum interleukin (IL)-17, IL-6,
IL-10 and transforming growth factor (TGF)-β1 were
detected using the ELISA kit, the concentration was calculated in
accordance with the absorbance value.
Separation of PBMCs
Peripheral blood mononuclear cells (PBMCs) were
isolated using Ficoll-Hypaque (MP Biomedicals, Solon, OH, USA)
density centrifugation. After washing with Hanks' balanced salt
solution, the cells were adjusted to a final concentration of
1×107 cells/ml in RPMI-1640 supplemented with 10% fetal
bovine serum (FBS) (all from Gibco, Grand Island, NY, USA).
Prepared PBMCs were stored at 4°C in the dark.
Flow cytometry
To activate PBMCs, 1 ml of 1×107/ml of
cell suspension was incubated with 10 ng/ml PMA and 0.5 mM
ionomycin (eBioscience, San Diego, CA, USA) for 5 h at 37°C in a 5%
CO2 humidified incubator. Monensin (1 µl of a ×1,000
solution) (eBioscience) was also applied to enhance intracellular
cytokine staining. After incubation, PBMCs were washed in
phosphate-buffered saline (PBS) with 0.09% (w/v) sodium azide
(eBioscience) twice. This was followed by staining with the
anti-CD3-FITC and anti-CD8-APC (both from eBioscience), and then
incubation for 15 min at 4°C. The cells were washed in PBS and
fixed with fixation buffer (eBioscience). The cells were washed
twice with 1X permeabilization buffer and incubated with 0.25 µg
conjugated anti-human IL-17A-PE (both from eBioscience) at room
temperature for 20 min. After intracellular staining, the cells
were washed with 1X permeabilization buffer and resuspended in 0.5
ml of permeabilization buffer. The proportion of IL-17-producing T
cells in peripheral blood lymphocytes were enumerated by flow
cytometry.
To identify Treg cells, PBMCs were stained with 0.25
µg FITC-conjugated rabbit monoclonal CD4 antibody (dilution, 1:50;
cat. no. 85-11-0048-42) and 0.25 µg APC-conjugated rabbit
monoclonal CD25 antibody (dilution, 1:50; cat. no. 85-17-0259-42)
for surface antigens, and 0.25 µg PE-conjugated rabbit monoclonal
Foxp3 antibody (dilution, 1:50; cat. no. 85-12-4776-42), all
purchased from eBioscience (San Diego, CA, USA), for intracellular
molecules, as per the manufacturer's instructions. PBMC
(1×106) was washed twice in PBS following staining with
the fluorochrome-conjugated antibodies specific for cell surface
antigen markers for 20 min in the dark at 4°C. In order to stain
the intracellular molecule, Foxp3, cells were permeabilized with
permeabilization/fixation buffer and stained with anti-Foxp3
antibody following the surface staining. PE-Rat IgG2a was used as
an isotype control for anti-Foxp3-PE antibody. Cells were
resuspended in 0.5 ml of staining buffer for subsequent flow
cytometry analysis. The prepared cells were analyzed on a
FACSCalibur flow cyto-meter (BD Biosciences, Franklin Lakes, NJ,
USA). CellQuest Pro software (BD Biosciences) was used for data
analysis.
Statistical analysis
Statistical analysis was performed using the SPSS
16.0 statistical program. Numerical data are presented as mean ±
SEM. To compare the results of immunologic studies before and after
hCG plus immunoglobulin treatment, paired t-test was applied.
Correlations between IL-17 and IL-6, IL-10 and TGF-β1
were performed using Pearson's correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Th17 cells, Treg cells and the ratio
of Th17/Treg cells in patients with URSA after the treatment of IG
plus hCG
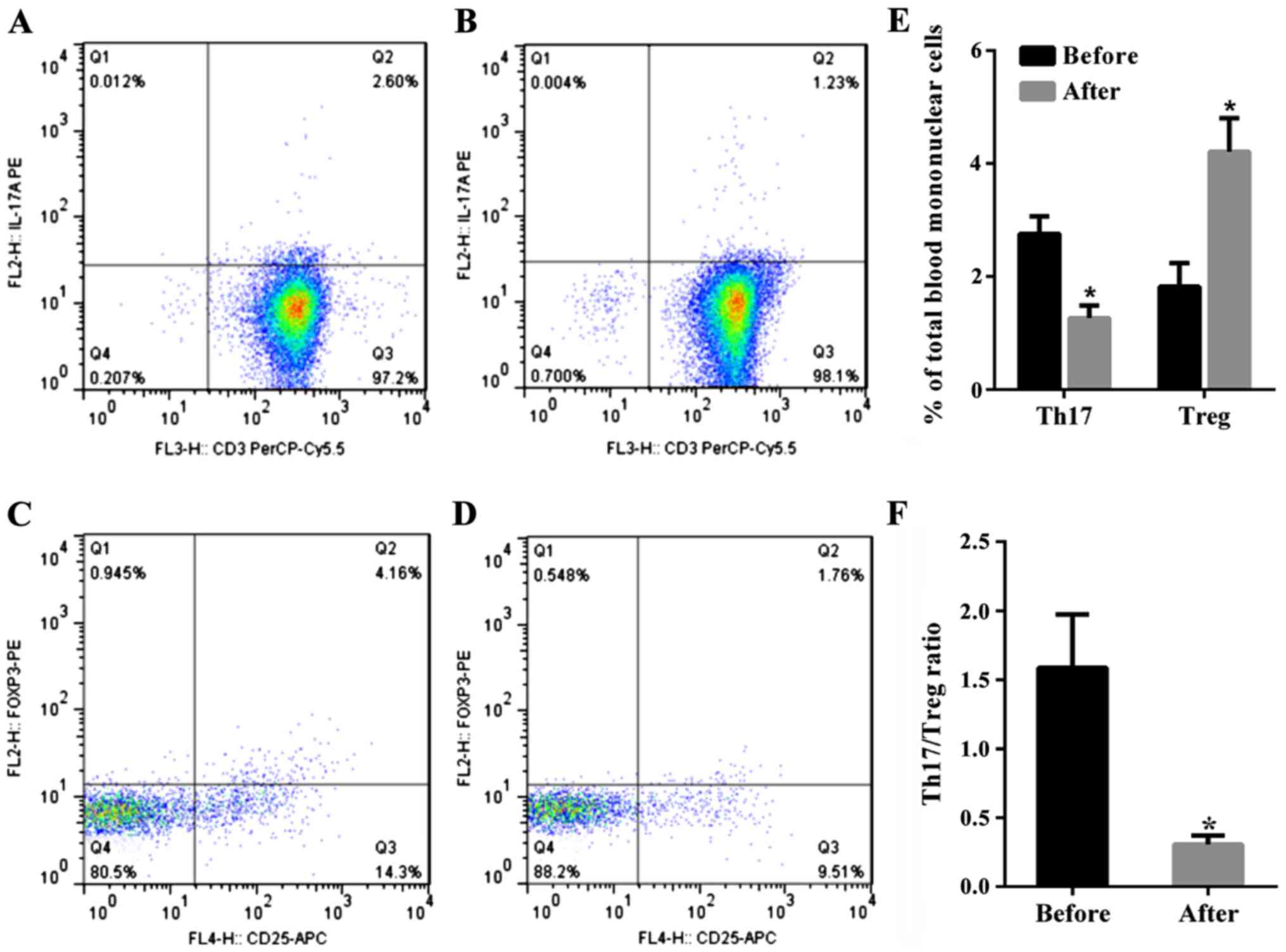
The percentage of Th17 cells
(CD3+CD8+IL-17A+T cells) in PBMC
in the total patient population with URSA before therapy was
2.76±0.31%, and the percentage of this subset after therapy was
1.527±0.22% (P<0.01, paired t-test). Thus, the percentage of
Th17 cells significantly decreased after therapy compared to before
therapy (Fig. 1). The percentage of
Tregs (CD4+CD25+Foxp3+ T cells) in
PBMC significantly increased in patients with URSA following
therapy at 4.21±0.59% as compared to before at 1.82±0.42% therapy
(P<0.01, paired t-test). Thus, the percentage of Tregs
significantly increased after therapy (Fig. 1). The mean Th17/Treg ratio in all
patients with URSA before immunotherapy was 1.57 but was reduced to
0.36 after immunotherapy (P<0.01). Therefore, the mean Th17/Treg
ratio significantly decreased after therapy compared to the ratio
before therapy (P<0.01, paired t-test), as shown in Fig. 1.
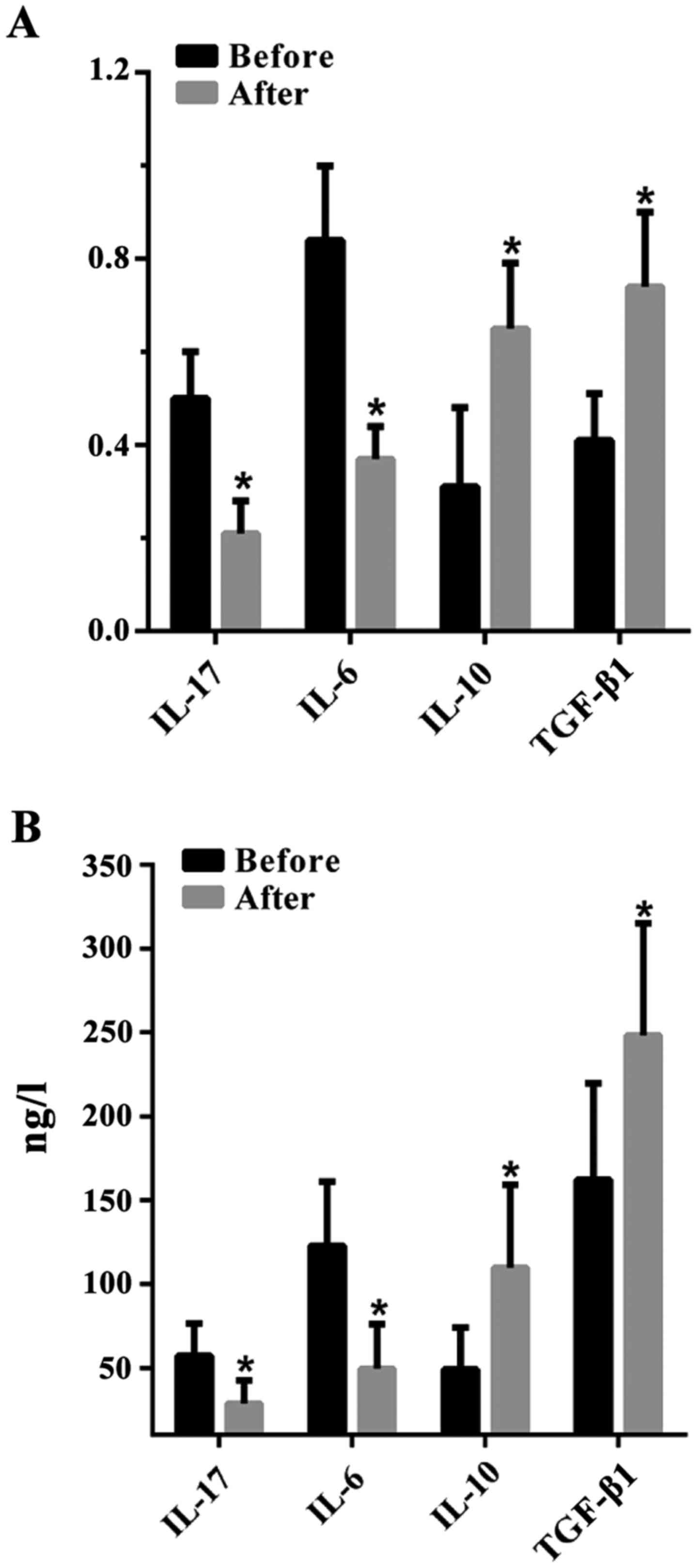
The levels of IL-17, IL-6, IL-10 and
TGF-β1 in the peripheral blood of URSA patients before
and after therapy
The concentration of Th17-type cytokines IL-17 and
IL-6 was significantly decreased in the serum of patients with URSA
after therapy [0.21±0.07 and 0.37±0.07 (RT-PCR); 28.84±13.61 and
49.39±26.72 ng/l (ELISA)] (P<0.01, paired t-test) than before
therapy [0.50±0.10 and 0.84±0.16 (RT-PCR); 57.17±19.31 and
122.66±38.32 ng/l (ELISA)]. Levels of Treg-type cytokines such as
IL-10 and TGF-β1 significantly increased after therapy
[0.65±0.14 and 0.74±0.16 (RT-PCR); 109.64±49.65 and 248.17±66.91
ng/l (ELISA)] than before therapy [0.31±0.17 and 0.41±0.10
(RT-PCR); 48.93±25.15 and 162.05±57.67 ng/l (ELISA)] (P<0.01,
paired t-test) (Fig. 2).
Discussion
Th17 cells and Treg cells are recently discovered
lymphocyte subsets, that are different from Th1 and Th2. Th17 cells
are associated with chronic inflammation through the secretion of
the inflammatory cytokines IL-17A (also known as IL-17), IL-17F,
IL-6, IL-21, IL-22 and TNF-α, which play a major role in the
development of organ transplant rejection (11,12).
Normal pregnancy is similar to an allograft. Nakashima et al
found that there were lower levels of Th17 cells in the peripheral
blood of normal pregnant women (13). Recent studies reported that the
proportions and concentrations of Th17 cells in the peripheral
blood were increased in pregnant women with URSA than when compared
to normal pregnant and non-pregnant women (6). This association correlated with the
secretion of other inflammatory cytokines (14). To study the expression of IL-17 in
women that have undergone abortion, we suggested that the
activation of IL-17 could increase the expression of NF-κB, thereby
reducing the number of progesterone receptors and weakening its
function. Therefore, progesterone cannot combine with a sufficient
number of progesterone receptors, resulting in decidua dysplasia
and inadequate nutrition for the embryo, finally leading to
miscarriage (15). Treg cells
comprise a subset of T-lymphocytes with pivotal immunological
regulation effects in maintaining a stable internal environment and
inducing immune tolerance to graft by inhibiting effector T-cell
responses and conventional T-cell activation by promoting the
secretion of suppressive cytokines (16). The role of Treg cells in immune
tolerance is still unclear. Currently, Treg cells indicate
immunosuppression by secreting immunosuppressive factors, such as
IL-10 and TGF-β1, that contribute to inhibition of
antigen presenting cells and antagonism of effector T cells
(17). Several studies have shown
that proportions of Treg cells in the peripheral blood and decidua
of pregnant women with URSA were decreased when compared to normal
pregnant women; they also presented with lower immunosuppressive
activity than normal pregnant women (18,19). The
adoptive transfer of pregnancy-induced
CD4+CD25+ Treg therapy contributed to
successful pregnancy and reduced the rate of spontaneous abortion
of abortion-prone mice (20).
Our previous studies demonstrated that hCG inhibited
the expression of TNF-α and INF-γ of maternal-fetal interface and
reduced the embryonic resorption rate of abortion-prone mice
(21). Bai et al cultured
human PBMCs with different doses of hCG and found that hCG had
significantly suppressed the effect on IL-6 and TNF-α mRNA
expression, indicating that hCG could inhibit the production of
pro-inflammatory cytokines (22). In
the presence of IL-6, CD4+ T cells differentiated to
Th17 cells by TGF-β1, resulting in autoimmunity and
inflammation (23). Therefore, hCG
can inhibit the differentiation of Th17 cells and increase the
differentiation of Treg cells by inhibiting the expression of IL-6.
Schumacher et al suggested that levels of hCG and Treg in
the decidua and placenta of pregnant women with RSA were lower than
those in normal pregnant women (24). Levels of hCG and Treg were positively
correlated and hCG could upregulate the LH/CG receptor on the
surface of Treg and attract Treg cells into the fetal-maternal
interface, inducing the formation of immune tolerance state
(24).
Immunoglobulin (IG) has been administered as
immunotherapy to URSA women (8,25–29).
Although the therapeutic effect of IG is controversial, recent
meta-analysis of IG treatment revealed that IG has significantly
higher success rate in women with immune abnormalities when
compared to women without any immune abnormalities (30). The therapeutic effect of IG was
achieved mainly by the injection of IgG into the body. Maddur et
al found that Th17 cells and their effector cytokines were
significantly decreased after the addition of IG into cultured T
cells, suggesting that IG could inhibit the differentiation and
amplification of Th17 cells, as well as the production of their
effector cytokines. IG was also able to significantly enhance Treg
cells among memory T cells (31). In
this study, we investigated the levels of Th17 and Treg as well as
the related cytokines in the peripheral blood of pregnant women
with URSA before and after treatment. We found that, after the
treatment of IG plus hCG, IL-17+ T cells and the related
cytokines IL-17 and IL-6 levels were lower than before. However,
Foxp3+ T cells and the related cytokines IL-10 and
TGF-β1 levels were increased. This suggests that the
therapy of immunoglobulin combined hCG is able to inhibit the
differentiation of Th17 cells and increase the differentiation of
Treg, thereby making the Th17/Treg paradigm into the Treg immune
bias, inducing maternal immune tolerance to the embryo and thereby
promoting the development of full-term pregnancy. This study also
indicates that correction of the disorder of Th17/Treg balance may
be one of the mechanisms through which immunoglobulin combined hCG
can be used to treat URSA. Our previous clinical and animal studies
have shown that the therapeutic effect of immunoglobulin plus hCG
was superior to monotherapy (10,21).
However, the mechanisms of combination therapy are still under
exploration.
References
|
1
|
Li Y, Wang XQ, Zhang L, Lv XD, Su X, Tian
S, Liu CM, Ma X and Xia HF: A SNP in pri-miR-10a is associated with
recurrent spontaneous abortion in a Han-Chinese population.
Oncotarget. 7:8208–8222. 2016.PubMed/NCBI
|
|
2
|
Mei S, Tan J, Chen H, Chen Y and Zhang J:
Changes of CD4+CD25high regulatory T cells and FOXP3 expression in
unexplained recurrent spontaneous abortion patients. Fertil Steril.
94:2244–2247. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang WJ, Liu FJ, Qu HM, Hao CF, Qu QL,
Xiong-Wang Bao HC and Wang XR: Regulation of the expression of Th17
cells and regulatory T cells by IL-27 in patients with unexplained
early recurrent miscarriage. J Reprod Immunol. 99:39–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Raghupathy R, Makhseed M, Azizieh F,
Hassan N, Al-Azemi M and Al-Shamali E: Maternal Th1- and Th2-type
reactivity to placental antigens in normal human pregnancy and
unexplained recurrent spontaneous abortions. Cell Immunol.
196:122–130. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang WJ, Hao CF, Yi-Lin Yin GJ, Bao SH,
Qiu LH and Lin QD: Increased prevalence of T helper 17 (Th17) cells
in peripheral blood and decidua in unexplained recurrent
spontaneous abortion patients. J Reprod Immunol. 84:164–170. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sereshki N, Gharagozloo M, Ostadi V,
Ghahiri A, Roghaei MA, Mehrabian F, Andalib AA, Hassanzadeh A,
Hosseini H and Rezaei A: Variations in T-helper 17 and regulatory T
cells during the menstrual cycle in peripheral blood of women with
recurrent spontaneous abortion. Int J Fertil Steril. 8:59–66.
2014.PubMed/NCBI
|
|
7
|
Wu L, Luo LH, Zhang YX, Li Q, Xu B, Zhou
GX, Luan HB and Liu YS: Alteration of Th17 and Treg cells in
patients with unexplained recurrent spontaneous abortion before and
after lymphocyte immunization therapy. Reprod Biol Endocrinol.
12:742014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hutton B, Sharma R, Fergusson D, Tinmouth
A, Hebert P, Jamieson J and Walker M: Use of intravenous
immunoglobulin for treatment of recurrent miscarriage: a systematic
review. BJOG. 114:134–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moraru M, Carbone J, Alecsandru D,
Castillo-Rama M, García-Segovia A, Gil J, Alonso B, Aguarón A,
Ramos-Medina R, Martínez de María J, et al: Intravenous
immunoglobulin treatment increased live birth rate in a Spanish
cohort of women with recurrent reproductive failure and expanded
CD56(+) cells. Am J Reprod Immunol. 68:75–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Liu FM, Du Y, Ye Y and Fang P:
Combined intramuscular immunoglobulin and human chorionic
gonadotropin therapy for prevention of recurrent spontaneous
abortion. Acta Academiae Medicinae Xuzhou. 17:368–370. 1997.(In
Chinese).
|
|
11
|
Loong CC, Hsieh HG, Lui WY, Chen A and Lin
CY: Evidence for the early involvement of interleukin 17 in human
and experimental renal allograft rejection. J Pathol. 197:322–332.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma L, Zhang H, Hu K, Lv G, Fu Y, Ayana DA,
Zhao P and Jiang Y: The imbalance between Tregs, Th17 cells and
inflammatory cytokines among renal transplant recipients. BMC
Immunol. 16:562015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakashima A, Ito M, Yoneda S, Shiozaki A,
Hidaka T and Saito S: Circulating and decidual Th17 cell levels in
healthy pregnancy. Am J Reprod Immunol. 63:104–109. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakashima A, Ito M, Shima T, Bac ND,
Hidaka T and Saito S: Accumulation of IL-17-positive cells in
decidua of inevitable abortion cases. Am J Reprod Immunol. 64:4–11.
2010.PubMed/NCBI
|
|
15
|
Qin XJ, Liu FM and Liu XY: Expression and
significance of IL-17A, NF-κB and PR in uterine decidua of
unexplained early spontaneous abortion patients. Acta Academiae
Medicinae Xuzhou. 33:132–135. 2013.(In Chinese).
|
|
16
|
Paust S and Cantor H: Regulatory T cells
and autoimmune disease. Immunol Rev. 204:195–207. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Somerset DA, Zheng Y, Kilby MD, Sansom DM
and Drayson MT: Normal human pregnancy is associated with an
elevation in the immune suppressive CD25+ CD4+ regulatory T-cell
subset. Immunology. 112:38–43. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arruvito L, Sanz M, Banham AH and Fainboim
L: Expansion of CD4+CD25+ and FOXP3+ regulatory T cells during the
follicular phase of the menstrual cycle: implications for human
reproduction. J Immunol. 178:2572–2578. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu YS, Wu L, Tong XH, Wu LM, He GP, Zhou
GX, Luo LH and Luan HB: Study on the relationship between Th17
cells and unexplained recurrent spontaneous abortion. Am J Reprod
Immunol. 65:503–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zenclussen AC, Gerlof K, Zenclussen ML,
Ritschel S, Bertoja A Zambon, Fest S, Hontsu S, Ueha S, Matsushima
K, Leber J, et al: Regulatory T cells induce a privileged tolerant
microenvironment at the fetal-maternal interface. Eur J Immunol.
36:82–94. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Liu FM, Liu XY and Zhu XW: The
effect of hCG bind immunoglobin G on maternal-fetal interface's
Th1/Th2 type cytokines and pregnancy's outcome of abortion model.
Chinese J Birth Health Heredity. 21:53–55. 2013.(In Chinese).
|
|
22
|
Bai H, Pan J, Jia X and Huang H: The
inhibition effect of human chorionic gonadotropin(hCG) on mRNA
expression of cytokines initiated inflammatory reaction. Chinese J
Immunol. 19:193–203. 2003.(In Chinese).
|
|
23
|
Kimura A and Kishimoto T: IL-6: regulator
of Treg/Th17 balance. Eur J Immunol. 40:1830–1835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schumacher A, Brachwitz N, Sohr S,
Engeland K, Langwisch S, Dolaptchieva M, Alexander T, Taran A,
Malfertheiner SF, Costa SD, et al: Human chorionic gonadotropin
attracts regulatory T cells into the fetal-maternal interface
during early human pregnancy. J Immunol. 182:5488–5497. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carp HJ, Achiron R, Toder V and Mashiach
S: Intravenous immunoglobulin in the prevention of recurrent
miscarriage. Br J Obstet Gynaecol. 102:509–510. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Coulam CB, Krysa L, Stern JJ and Bustillo
M: Intravenous immunoglobulin for treatment of recurrent pregnancy
loss. Am J Reprod Immunol. 34:333–337. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Christiansen OB, Pedersen B, Rosgaard A
and Husth M: A randomized, double-blind, placebo-controlled trial
of intravenous immunoglobulin in the prevention of recurrent
miscarriage: evidence for a therapeutic effect in women with
secondary recurrent miscarriage. Hum Reprod. 17:809–816. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Winger EE and Reed JL: Treatment with
tumor necrosis factor inhibitors and intravenous immunoglobulin
improves live birth rates in women with recurrent spontaneous
abortion. Am J Reprod Immunol. 60:8–16. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim DJ, Lee SK, Kim JY, Na BJ, Hur SE, Lee
M and Kwak-Kim J: Intravenous immunoglobulin G modulates peripheral
blood Th17 and Foxp3(+) regulatory T cells in pregnant women with
recurrent pregnancy loss. Am J Reprod Immunol. 71:441–450. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Clark DA: Immunological factors in
pregnancy wastage: fact or fiction. Am J Reprod Immunol.
59:277–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Maddur MS, Vani J, Hegde P,
Lacroix-Desmazes S, Kaveri SV and Bayry J: Inhibition of
differentiation, amplification, and function of human TH17 cells by
intravenous immunoglobulin. J Allergy Clin Immunol.
127:823–830.e1-7. 2011. View Article : Google Scholar : PubMed/NCBI
|