Introduction
Colorectal cancer (CRC) is the second most common
type of cancer in western countries and has exhibited an increasing
incidence in many Asian countries in recent decades (1,2). A
recent study demonstrated that screening for CRC in average-risk
adults was effective in reducing the mortality rate (3). A satisfactory screening method for CRC
must be safe, non-invasive, cost-effective, easily acceptable and
possess a high diagnostic accuracy (4).
The PillCam colon capsule endoscopy (CCE) may
achieve direct visualization of the entire colon without sedation,
radiation or air insufflation, which represents a non-invasive
imaging system for exploring the colon (5). Although colonoscopy is currently
considered to be the gold standard method for CRC screening and the
diagnosis of most colonic diseases (4), the PillCam colon capsule endoscopy has
been developed as the most promising approach to CRC screening in
recent years, which is an alternative for patients with incomplete
colonoscopy or who are reluctant to accept colonoscopy examination
due to discomfort or embarrassment (6).
The effectiveness of CCE partly depends on the
cleanliness of the colon. An optimal bowel preparation regimen for
CCE is required for a clean intestine, capsule propulsion and
visualization of the whole large intestine. Previous studies have
demonstrated bowel preparation regimens for CCE that generally
consist of a split-dosage of polyethylene glycol (PEG) with the
volume of 4 l, prokinetic agents and sodium phosphate (NaP)
boosters (5,7–9).
However, large volumes of laxatives may reduce patient compliance.
Furthermore, NaP is associated with certain adverse events, such as
electrolyte disturbance, acute nephropathy and kidney failure
(10), and should be avoided in
patients at increased risk of NaP toxicity. Therefore, there is a
requirement for the current bowel preparation regimen to be
improved.
The present study aimed to evaluate a novel
low-volume and NaP-free bowel preparation regimen for CCE. In this
regimen, the volume of PEG was reduced to 2 l and NaP was
substituted with PEG as the booster. Colon cleansing quality and
completion rate were assessed.
Patients and methods
Patients
Between July 2013 and July 2014, a total of 31
patients were enrolled to the current prospective study at the
Department of Gastroenterology, Nanfang Hospital, Southern Medical
University (Guangzhou, China) in accordance with the following
inclusion criteria: Aged 18–75 years, willing to accept CCE
examination and providing signed informed consent. According to the
criteria in a previously published study, exclusion criteria were
as follows: Dysphagia or swallowing disorder, prior major abdominal
surgery of the gastrointestinal tract, known or suspected bowel
obstruction, presence of a cardiac pacemaker or other implanted
electromedical devices and pregnancy (11). The methodology was approved by the
Ethics Committee of Nanfang hospital.
PillCam colon capsule endoscopy
The PillCam CCE (PillCam COLON, Given Imaging;
Medtronic, Dublin, Ireland) was 11×31 mm in size and had two
cameras, one at each end of the capsule, each capturing 2
images/sec. The angle of view for each imager was 156°. The capsule
enters a 1 h delay mode following a 3-min initial function, then
the system automatically restarts and functions for an additional 9
h. Captured images were transmitted to the data recorder via eight
sensors. A rapid real-time viewer allowed a real-time view during a
PillCam procedure. Following the examination, the recorded data was
downloaded into the Given Imaging RAPID 4 workstation (Medtronic).
Captured videos were reviewed by two physicians who had prior
experience with colonoscopy and small intestine capsule endoscopy.
Colon cleansing levels were then assessed, as described below.
Bowel preparation regimens
As shown in Table I,
the conventional regimen of bowel preparation for CCE includes 4 l
of an oral preparation of PEG (Colopeg; Roche Laboratories,
Gaillard, France), 1–2 oral boosters of NaP at a dosage of 30–45 ml
(Fleet Phospho Soda®; Wolf, Fleet, Lynchburg, VA, USA) and a
suppository of bisacodyl as necessary (Dulcolax®, Boehringer
Ingelheim, Scherer, Aprilia, Italy) (4). In order to avoid adverse events
associated with NaP, improve patient compliance and maintain the
quality of bowel cleansing, the current study evaluated a modified
regimen. In the novel bowel preparation regimen, on one day prior
to examination, a low-fiber diet was permitted, 5 mg mosapride
citrate (Gasmotin; Sumitomo Dainippon Pharma Co., Ltd., Osaka,
Japan) was orally administered twice (1 h before lunch and 1 h
before supper) and 1 l of an oral preparation of PEG (Fortrans;
Ipsen, Paris, France) at 6:00-9:00 p.m. On the day of the
examination, an additional 1 l PEG, 5 mg mosapride citrate and 200
mg simethicone (Espumisan; Berlin-Chemie AG, Berlin, Germany) were
orally administered at 2.5, 1 and 0.5 h prior capsule ingestion,
respectively. PEG booster (0.5 l) was orally administered twice, 1
and 4 h following capsule ingestion. The detailed procedure of the
novel regimen is presented in Table
I. Patient education concerning the bowel preparation procedure
for CCE was delivered by physicians prior to the examination, in
order to improve the efficacy of the regimen. Any adverse effects
were recorded, including nausea, vomiting, abdominal pain,
dizziness, headache and allergy.
 | Table I.Conventional (4) and novel bowel preparation regimens for
colon capsule endoscopy. |
Table I.
Conventional (4) and novel bowel preparation regimens for
colon capsule endoscopy.
|
| Regimen |
|---|
|
|
|
|---|
| Time | Conventional | Novel |
|---|
| Day before
examination |
|
|
| All
day | Clear liquids
only | Low-fiber diet |
| 1 h
before lunch |
| 5 mg mosapride
citrate |
| 1 h
before supper |
| 5 mg mosapride
citrate |
| 6:00-9:00
p.m. | 3 l PEG | 1 l PEG |
| Examination day |
|
|
| 6:00
a.m. |
| 1 l PEG |
| 6:00-7:00
a.m. | 1 l PEG |
|
| 7:30
a.m. |
| 5 mg mosapride
citrate |
| 7:45
a.m. | 20 mg
domperidone |
|
| 8:00
a.m. | Capsule
ingestion | 200 mg
simethicone |
| 8:30
a.m. |
| Capsule
ingestion |
| 9:30
a.m. |
| 0.5 l PEG |
| 10:00
a.m. | 45 ml NaP + 1 l
water |
|
| 12:30
p.m. |
| 0.5 l PEG |
| 2:00
p.m. | 30 ml NaP + 1 l
water |
|
| 3.00
p.m. | Snack
(optional) |
|
| 3:30
p.m. |
| Snack
(optional) |
| 4:30
p.m. |
| 10 mg
bisacodyl |
Performance evaluation
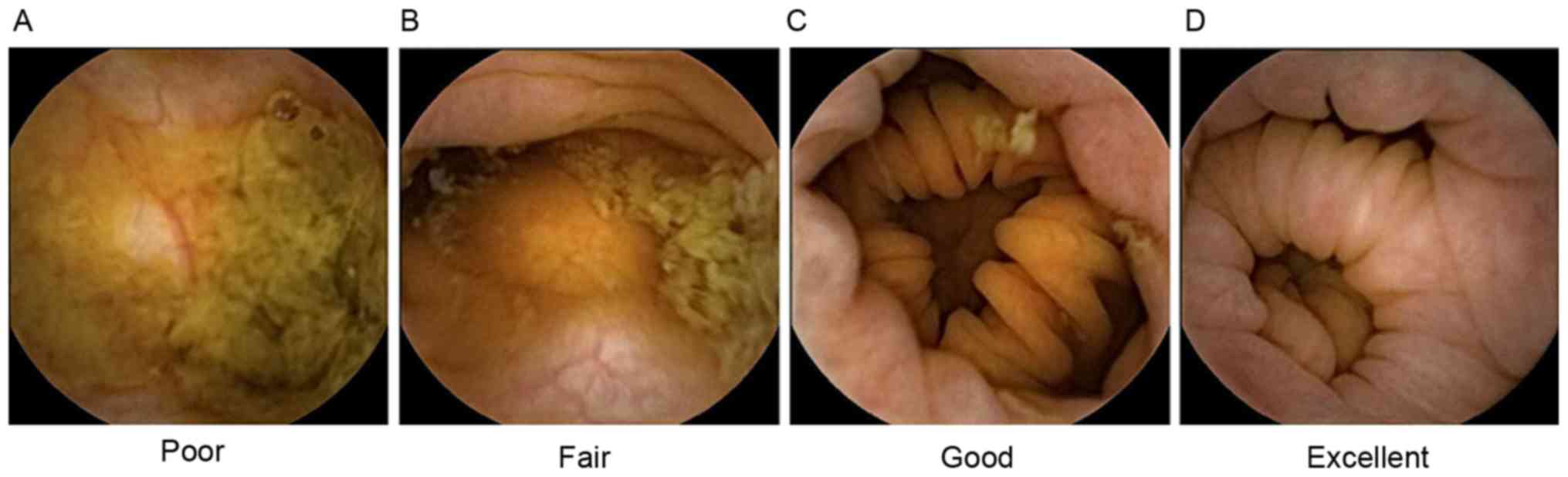
The four-point grading scale system reported by
Leighton and Rex (12) was applied
to evaluate colon cleansing levels. A ‘poor’ level of colon
cleansing was defined as a large amount of fecal residue. ‘Fair’
was defined as a sufficient amount of feces or turbid fluid to
prevent reliable examination. ‘Good’ was defined as a small amount
of feces or turbid fluid not interfering with examination.
‘Excellent’ was defined only small pieces of adherent feces
(Fig. 1). For subsequent analysis,
the grades of excellent and good were defined as ‘adequate
preparation’, whereas fair and poor were defined as ‘inadequate
preparation’. The video footage was divided into five segments:
Cecum, ascending colon, transverse colon, descending colon and
rectosigmoid colon. Subsequently, overall cleansing levels and
cleansing levels of each segment were assessed. Ileocecal valve
transit time was defined as the time from capsule ingestion to the
first image of the cecum. The examination was considered to be
completed when the haemorrhoidal plexus was visualized.
Results
Study participants
A total of 29 patients were included in the final
analysis (male/female, 13/16; mean age, 35 years; age range, 24–61
years). Two patients (6%) were excluded as data was not acquired
due to technical failure. Major clinical indications included
physical examination, constipation, abdominal pain and diarrhea
(Table II). All enrolled patients
complied with the novel bowel preparation. No adverse effects
associated with CCE occurred.
 | Table II.Clinical indications of patients
undergoing colon capsule endoscopy (n=33)a. |
Table II.
Clinical indications of patients
undergoing colon capsule endoscopy (n=33)a.
| Clinical
indication | n | Percentage |
|---|
| Physical
examination | 8 | 24.2 |
| Constipation | 6 | 18.2 |
| Abdominal pain | 5 | 15.2 |
| Diarrhea | 4 | 12.1 |
| Abdominal
distension | 3 |
9.1 |
| Hematochezia | 2 |
6.1 |
| Acid reflux and
heartburn | 2 |
6.1 |
| Recent change of
bowel habits | 1 |
3.0 |
| Prior ulcer in
terminal ileum | 1 |
3.0 |
| Crohn's
disease | 1 |
3.0 |
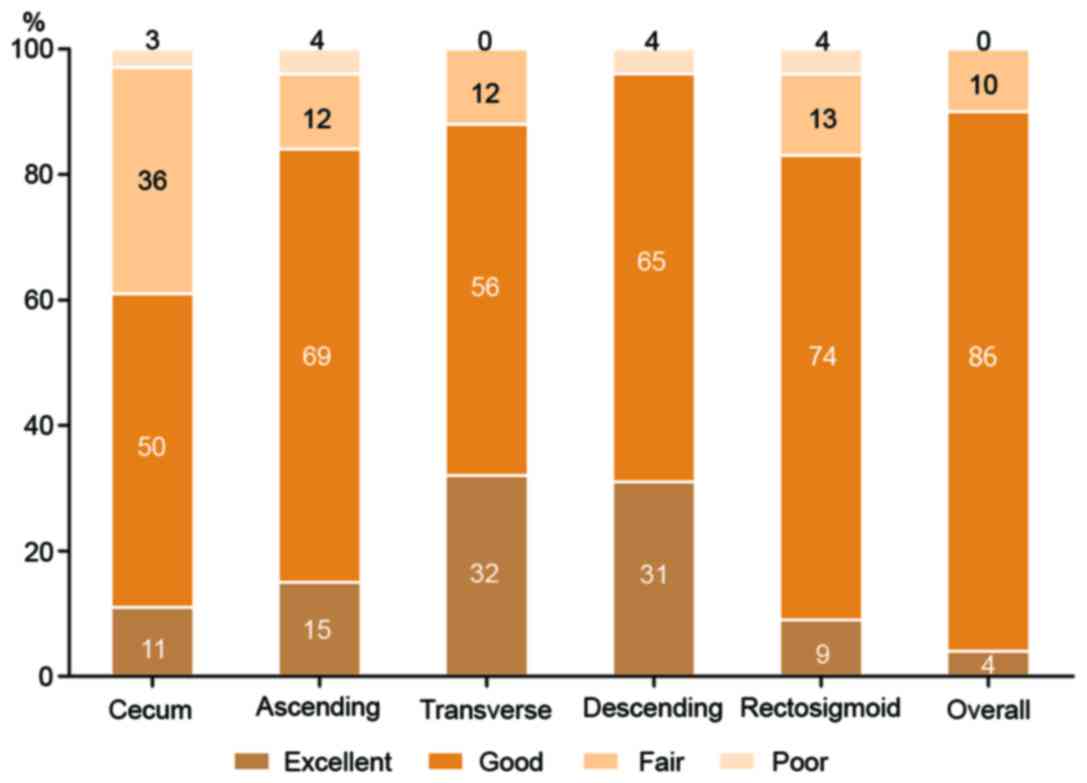
Colon cleansing levels
According to the four-point scale grading system,
the results indicated that overall colon cleansing levels were
rated as excellent in 1 patient (4%), good in 25 patients (86%) and
fair in 3 patients (10%). No patients were rated as poor (Fig. 2). In total, 90% of the patients
exhibited adequate colon preparation (ratings of excellent or
good). In the individual colon segments, the descending colonic
mucosa exhibited the highest quality of cleansing (adequate
cleansing level in 96% of patients), whereas the cecum exhibited
the poorest quality (adequate cleansing level in 61%). As for
bubbles in the large intestine, overall the images were not
evidently affected by bubbles and only a small number were observed
(Fig. 2).
Colon capsule transmit time and
completion rate
Ileocecal valve transit time (time from capsule
ingestion to the first image of cecum) was 2.35±0.82 h.
Furthermore, 93.1% (27/29) of capsules were located in the small
intestine when they restarted following the 1 h delay mode. Two
capsules were still in the stomach at this point and successfully
travelled into the small intestine after the patients drank some
water. Examination completion occurred in 79.3% (23/29) of
patients. At the end of CCE examination in 2 cases, the capsule was
in the descending colon, in 1 case it was in the transverse colon,
in 2 cases it was in the ascending colon and in 1 case it was in
the cecum (Table III).
 | Table III.Location of colon capsule endoscopy
at different time points (n=29). |
Table III.
Location of colon capsule endoscopy
at different time points (n=29).
| Time | Location | Patients (n) | Percentage |
|---|
| Restart after 1 h
delay | Stomach | 2 |
6.9 |
|
| Small
intestine | 27 | 93.1 |
| End of
examination | Haemorrhoidal
plexus (visible) | 23 | 79.3 |
|
| Descending
colon | 2 |
6.9 |
|
| Transverse
colon | 1 |
3.4 |
|
| Ascending
colon | 2 |
6.9 |
|
| Cecum | 1 |
3.4 |
Discussion
At present, a standardized bowel preparation regimen
is still not available. In previous studies, large volumes of PEG
combined with a NaP booster regimen was most commonly applied
(4,5,7–9). However, this remains controversial due
to low patient compliance and safety concerns surrounding NaP
(13,14). In the present study, a novel
low-volume and NaP-free bowel preparation regimen was provided for
CCE, which was demonstrated to be feasible.
Different bowel preparation regimens for CCE in
previous studies have reported variable results; adequate colon
cleansing ranged from 35 to 84% and completion rates ranged from 64
to 100% (4,7–9,14–24).
More recently, modified regimens with low dosage PEG have been
researched. Hartmann et al (14) reported that a 2 l PEG plus ascorbic
acid regimen yielded adequate cleansing levels in 82% of patients
and Kakugawa et al (23)
reported that a 2.3–2.6 l polyethylene glycol electrolyte lavage
solution (PEG-ELS) regimen yielded adequate cleansing levels in 94%
of patients. However, Usui et al (24) evaluated a lower volume regimen with
0.7 l PEG in second-generation colon capsule endoscopy, which
yielded lower adequate cleansing levels of 60%. According to the
four-point grading scale system, 90% of patients achieved adequate
colon cleansing level in the present study with a 2 l PEG regimen.
Consistent with the results reported by Spada et al
(19), the current data revealed
that the cecum was the segment with the lowest quality of cleansing
(adequate level in 61%) and the descending colon had the highest
quality of cleansing (adequate level in 94%). The current study
demonstrated that a low-volume regimen could also achieve a high
quality of colon cleansing.
A booster is necessary for cleaning the colonic
mucosa and improving capsule excretion. The efficacy of NaP as
booster has been reported in previous studies (5,19,22),
however it has associated adverse effects, including electrolyte
disturbance, acute nephropathy and kidney failure. Guidelines
established in Europe (UK National Patient Safety Agency and
European Society of Gastrointestinal Endoscopy) advised against the
routine use of NaP for bowel preparation due to safety concerns
(13,25), and an equivalent recommendation is
suggested by the Guidelines published by Chinese society of
digestive endoscopy in China (26).
Therefore, there is an urgent requirement for a safe substitute for
NaP. Without side effects such as electrolyte disturbance and renal
damage, PEG has been demonstrated to be a safe booster. Spada et
al (21) and Hartmann et
al (14) reported PEG booster
regimens obtained completion rate in 75 and 76% of cases,
respectively. The regimen in the current study achieved a
completion rate in 79.3% of cases. Compared with NaP-based
regimens, the completion rate is lower, yet the application of PEG
as booster is still promising for its safety and further studies
should be performed to evaluate how completion rate may be
increased. Previously, magnesium citrate was applied as an emerging
booster with completion rates of 55–85% (23,24).
These fluctuating results emphasize the need for further research
on magnesium citrate as a booster.
There are other strategies that are favorable for
improving the quality of colon cleansing and increasing completion
rate. Several types of prokinetic agent have been used, such as
tegaserod (8), domperidone (4,6,15,18),
metoclopramide (22) and mosapride
(23,27). Mosapride is an emerging prokinetic
agent, working as a 5-hydroxytryptamine receptor 4 agonist and
accelerating both gastric emptying and peristalsis of the small
intestine (28). Wei et al
(29) and Ida et al (30) recommended that patients received 10
mg mosapride citrate 30–60 min prior to capsule ingestion. However,
the dosage and administration time of mosapride remain to be
evaluated. In the present study, to assist bowel cleansing and
reduce the dosage of PEG, 5 mg mosapride citrate was administered 1
h prior to lunch and supper on the day prior to examination and 1 h
prior to capsule ingestion. The present results revealed that in
93.1% (27/29) of patients, capsules were located in the small
intestine when the capsules activated following the 1 h delay and
the average ileocecal valve transit time was 2.35 h. Although
evidence from a controlled study is not available, this time was
notably shorter than the 5–8 h exhibited in small intestine capsule
endoscopy (31). In addition,
simethicone is also considered to have an auxiliary effect for
bowel preparation. In the studies of Kakugawa et al
(23) and Usui et al
(24), simethicone was applied, but
its necessity was not discussed. In the present study, to decrease
the number of bubbles in the large intestine, 200 mg simethicone
was ingested 30 min prior to capsule endoscopy. Ultimately,
bisacodyl suppository was optional in previous studies (4,14). In
order to reduce the embarrassment of patients and maintain patient
compliance, bisacodyl was not administered.
The present study demonstrated that a low-volume and
NaP-free bowel preparation regimen was effective for colon capsule
endoscopy. However, there were some limitations to the study,
including the limited sample size and the absence of a controlled
comparison with conventional colonoscopy. Furthermore, the study
would be optimized if consecutive patients were included.
In conclusion, a novel low-volume and NaP-free bowel
preparation regimen for CCE has been demonstrated to be feasible,
with adequate colon cleansing levels and completion rate, and could
therefore be used as an alternative regimen. Further studies should
be performed in order to evaluate whether the completion rate can
be increased, and the accuracy of CCE with the novel regimen should
be compared with traditional colonoscopy in a randomized,
controlled trial.
References
|
1
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeoh KG, Ho KY, Chiu HM, Zhu F, Ching JY,
Wu DC, Matsuda T, Byeon JS, Lee SK, Goh KL, et al: The Asia-Pacific
Colorectal Screening score: A validated tool that stratifies risk
for colorectal advanced neoplasia in asymptomatic Asian subjects.
Gut. 60:1236–1241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qaseem A, Denberg TD, Hopkins RH Jr,
Humphrey LL, Levine J, Sweet DE and Shekelle P; Clinical Guidelines
Committee of the American College of Physicians, : Screening for
colorectal cancer: A guidance statement from the American College
of Physicians. Ann Intern Med. 156:378–386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schoofs N, Devière J and Van Gossum A:
PillCam colon capsule endoscopy compared with colonoscopy for
colorectal tumor diagnosis: A prospective pilot study. Endoscopy.
38:971–977. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spada C, Hassan C, Galmiche JP, Neuhaus H,
Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK, et al:
Colon capsule endoscopy: European society of gastrointestinal
endoscopy (ESGE) Guideline. Endoscopy. 44:527–536. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Triantafyllou K, Viazis N, Tsibouris P,
Zacharakis G, Kalantzis C, Karamanolis DG and Ladas SD: Colon
capsule endoscopy is feasible to perform after incomplete
colonoscopy and guides further workup in clinical practice.
Gastrointest Endosc. 79:307–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van Gossum A, Munoz-Navas M,
Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG,
Ponchon T, Neuhaus H, Philipper M, et al: Capsule endoscopy versus
colonoscopy for the detection of polyps and cancer. N Engl J Med.
361:264–270. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eliakim R, Fireman Z, Gralnek IM, Yassin
K, Waterman M, Kopelman Y, Lachter J, Koslowsky B and Adler SN:
Evaluation of the PillCam Colon capsule in the detection of colonic
pathology: Results of the first multicenter, prospective,
comparative study. Endoscopy. 38:963–970. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Eliakim R, Yassin K, Niv Y, Metzger Y,
Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman
Z, et al: Prospective multicenter performance evaluation of the
second-generation colon capsule compared with colonoscopy.
Endoscopy. 41:1026–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wexner SD, Beck DE, Baron TH, Fanelli RD,
Hyman N, Shen B and Wasco KE: American Society of Colon and Rectal
Surgeons; American Society for Gastrointestinal Endoscopy; Society
of American Gastrointestinal and Endoscopic Surgeons: A consensus
document on bowel preparation before colonoscopy: Prepared by a
task force from the American society of colon and rectal surgeons
(ASCRS), the American society for gastrointestinal endoscopy
(ASGE), and the Society of American gastrointestinal and endoscopic
surgeons (SAGES). Gastrointest Endosc. 63:894–909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ladas SD, Triantafyllou K, Spada C,
Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R and Costamagna
G: ESGE Clinical Guidelines Committee: European society of
gastrointestinal endoscopy (ESGE): Recommendations (2009) on
clinical use of video capsule endoscopy to investigate small-bowel,
esophageal and colonic diseases. Endoscopy. 42:220–227. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Leighton JA and Rex DK: A grading scale to
evaluate colon cleansing for the PillCam COLON capsule: A
reliability study. Endoscopy. 43:123–127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hassan C, Bretthauer M, Kaminski MF,
Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green
S, Kuiper T, et al: Bowel preparation for colonoscopy: European
Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy.
45:142–150. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartmann D, Keuchel M, Philipper M,
Gralnek IM, Jakobs R, Hagenmüller F, Neuhaus H and Riemann JF: A
pilot study evaluating a new low-volume colon cleansing procedure
for capsule colonoscopy. Endoscopy. 44:482–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gay G, Delvaux M, Frederic M and Fassler
I: Could the colonic capsule PillCam Colon be clinically useful for
selecting patients who deserve a complete colonoscopy?: Results of
clinical comparison with colonoscopy in the perspective of
colorectal cancer screening. Am J Gastroenterol. 105:1076–1086.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pilz JB, Portmann S, Peter S, Beglinger C
and Degen L: Colon capsule endoscopy compared to conventional
colonoscopy under routine screening conditions. BMC Gastroenterol.
10:662010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sacher-Huvelin S, Coron E, Gaudric M,
Planche L, Benamouzig R, Maunoury V, Filoche B, Frédéric M, Saurin
JC, Subtil C, et al: Colon capsule endoscopy vs. colonoscopy in
patients at average or increased risk of colorectal cancer. Aliment
Pharmacol Ther. 32:1145–1153. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sieg A, Friedrich K and Sieg U: Is PillCam
COLON capsule endoscopy ready for colorectal cancer screening? A
prospective feasibility study in a community gastroenterology
practice. Am J Gastroenterol. 104:848–854. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Spada C, Hassan C, Ingrosso M, Repici A,
Riccioni ME, Pennazio M, Pirozzi GA, Pagano N, Cesaro P, Spera G,
et al: A new regimen of bowel preparation for PillCam colon capsule
endoscopy: A pilot study. Dig Liver Dis. 43:300–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spada C, Hassan C, Munoz-Navas M, Neuhaus
H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME, et
al: Second-generation colon capsule endoscopy compared with
colonoscopy. Gastrointest Endosc. 74:581–589.e1. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spada C, Riccioni ME, Hassan C,
Petruzziello L, Cesaro P and Costamagna G: PillCam colon capsule
endoscopy: A prospective, randomized trial comparing two regimens
of preparation. J Clin Gastroenterol. 45:119–124. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sung J, Ho KY, Chiu HM, Ching J, Travis S
and Peled R: The use of Pillcam Colon in assessing mucosal
inflammation in ulcerative colitis: A multicenter study. Endoscopy.
44:754–758. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kakugawa Y, Saito Y, Saito S, Watanabe K,
Ohmiya N, Murano M, Oka S, Arakawa T, Goto H, Higuchi K, et al: New
reduced volume preparation regimen in colon capsule endoscopy.
World J Gastroenterol. 18:2092–2098. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Usui S, Hosoe N, Matsuoka K, Kobayashi T,
Nakano M, Naganuma M, Ishibashi Y, Kimura K, Yoneno K, Kashiwagi K,
et al: Modified bowel preparation regimen for use in
second-generation colon capsule endoscopy in patients with
ulcerative colitis. Dig Endosc. 26:665–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Connor A, Tolan D, Hughes S, Carr N and
Tomson C: Consensus guidelines for the safe prescription and
administration of oral bowel-cleansing agents. Gut. 61:1525–1532.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chinese Society of Digestive Endoscopy:
Chinese guidelines for bowel preparation of digestive endoscopy
(Draft). Chin J Dig Endosc. 30:481–483. 2013.
|
|
27
|
Hosoe N, Matsuoka K, Naganuma M, Ida Y,
Ishibashi Y, Kimura K, Yoneno K, Usui S, Kashiwagi K, Hisamatsu T,
et al: Applicability of second-generation colon capsule endoscope
to ulcerative colitis: A clinical feasibility study. J
Gastroenterol Hepatol. 28:1174–1179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okamura K, Sasaki N, Yamada M, Yamada H
and Inokuma H: Effects of mosapride citrate, metoclopramide
hydrochloride, lidocaine hydrochloride, and cisapride citrate on
equine gastric emptying, small intestinal and caecal motility. Res
Vet Sci. 86:302–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei W, Ge ZZ, Lu H, Gao YJ, Hu YB and Xiao
SD: Effect of mosapride on gastrointestinal transit time and
diagnostic yield of capsule endoscopy. J Gastroenterol Hepatol.
22:1605–1608. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ida Y, Hosoe N, Imaeda H, Bessho R,
Ichikawa R, Naganuma M, Kanai T, Hibi T and Ogata H: Effects of the
oral administration of mosapride citrate on capsule endoscopy
completion rate. Gut Liver. 6:339–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Selby W: Complete small-bowel transit in
patients undergoing capsule endoscopy: Determining factors and
improvement with metoclopramide. Gastrointest Endosc. 61:80–85.
2005. View Article : Google Scholar : PubMed/NCBI
|