Introduction
Bisphosphonates (BPs) are the most commonly
prescribed medication for the management of osteoporosis (1). Zoledronic acid (ZA) is a new generation
BP that can be administered intravenously once yearly. It comprises
a double nitrogen-containing structure (an imidazole ring) that
results in a high affinity for hydroxyapatite and induces a 10-fold
increased inhibition of bone resorption compared with daily oral
BPs (2,3). However, acute-phase response (APR) is
more common with ZA compared with other BPs (4). APR is characterized by the following:
Transient mild fever, malaise, flu-like symptoms, digestive
symptoms, headache and dizziness, all occurring within 3 days of
the initial drug infusion (5–8). The
molecular mechanisms of APR have recently been elucidated. It has
been suggested that ZA leads to the accumulation of isopentenyl
pyrophosphate and dimethylallyl pyrophosphate, which are potent
agonists of the γδT cell receptor, as upstream metabolites of
farnesyl phyrophosphate synthase in the mevalonate pathway
(9–16). These agonists are naturally
recognized by γδT cells, inducing the activation and release of
tumor necrosis factor (TNF)-α, TNF-β, interferon (IFN)-γ,
interleukin (IL)-2 and IL-6, which are pro-inflammatory cytokines
involved in the development of APR (9–16).
It has been observed by the present authors that
patients treated with ZA in the early post-operative period develop
APR more readily. To the best our knowledge, no previous studies
have reported the risk factors for APR associated with the early
administration of ZA post-surgery. However, some studies have
suggested that advanced age, supplementation of vitamin D, prior
use of oral BPs and nonsteroidal anti-inflammatory drugs (NSAIDs)
are relieving factors for APR (5–8,16).
Previous studies have reported that NSAIDs, such as
acetaminophen, effectively alleviate the symptoms of APR (5–8),
however, the protective efficacy of NSAIDS was found to be
unsatisfactory in patients who underwent ZA therapy soon after
surgery. At present, there is no evidence suggesting that low-dose
glucocorticoids (GC), such as methylprednisolone (MP), have strong
anti-inflammatory properties for the prevention of APR. Therefore,
further investigation is required to obtain data to ascertain
whether MP may be an effective alternative to acetaminophen.
The aim of the present study was to evaluate the
effects of surgical trauma on the development of APR and to
investigate the efficacy of low-dose MP in preventing APR
associated with the early post-surgical administration of ZA using
clinical data from the orthopedics and endocrinology departments at
a single hospital.
Materials and methods
Patients
A total of 482 patients aged from 49 to 89 years
were recruited in this hospital-based, retrospective study
including 77 males and 405 females at the First Affiliated Hospital
of Nanjing Medical University (Nanjing, China) from January 2012 to
December 2014. Patients receiving their initial ZA infusion for
osteoporosis (17), some of whom
received ZA at 1 week after orthopedic surgery, were included in
the present study. Patients with absorption fever or infection
post-surgery were excluded, as this had the potential to affect the
observation of APR symptoms. Exclusion criteria included a history
of cancer, hyperparathyroidism, and previous use of parathyroid
hormone (PTH), strontium, sodium fluoride, intravenous or oral BPs,
recent or chronic corticosteroid therapy, statins or immune
therapy. Furthermore, patients with severe injuries, liver or
kidney disease, or recent or scheduled dental surgery were
ineligible. The present study was approved by the Ethics Committee
of the First Affiliated Hospital of Nanjing Medical University and
written informed consent was obtained from all patients.
Study design and procedures
All subjects were treated with cholecalciferol
(Roche Diagnostics, Basel, Switzerland) 800 IU/day and Caltrate
(Pfizer, Inc., New York, NY, USA) 1,200 mg/day orally from the time
of hospitalization as vitamin D and calcium supplements,
respectively. This treatment was continued following ZA infusion.
All patients received 5 mg ZA (Novartis International AG, Basel,
Switzerland) via intravenous infusion over 15 min. Prior to the
administration of ZA, patients were supplemented with 1 g calcium
gluconate (Qingdau Huanghai Pharmaceutical Co., Ltd., Qingdao,
China) in 0.9% saline to avoid acute hypocalcemia.
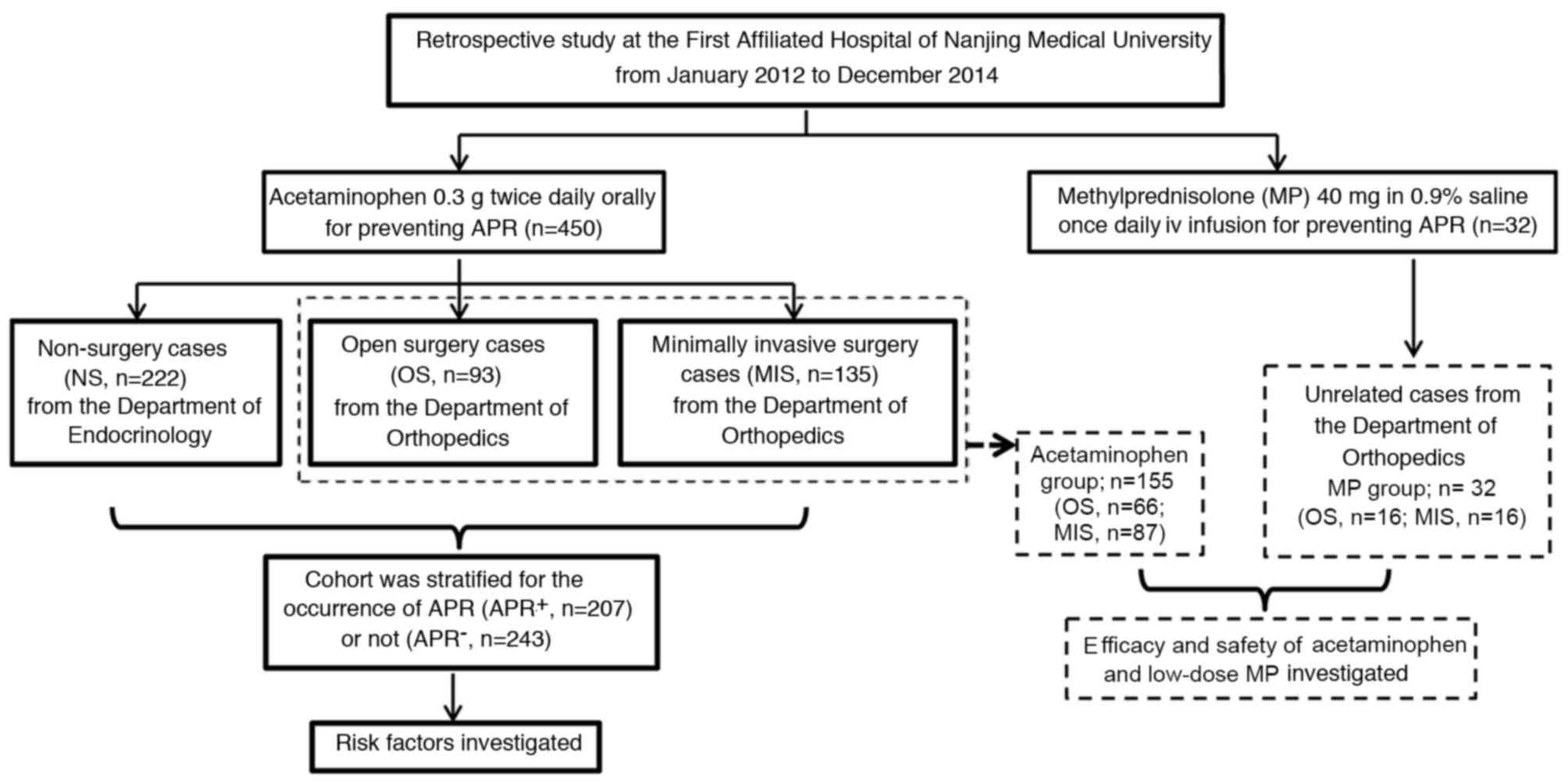
In the first part of the study, 450
acetaminophen-treated patients were divided into the following
groups: Open surgery (OS; n=93), minimally invasive surgery (MIS;
n=135) and non-surgery (NS; n=222) according to the degree of
surgical trauma. Percutaneous vertebroplasty, percutaneous
kyphoplasty and percutaneous cannulated screw fixation were
considered as MIS approaches. OS approaches comprised other
orthopedic surgery methods associated with spinal and articular
implants. Patients in the endocrinology department who had not
experienced fresh fractures due to fragility within the previous 6
months, including vertebral compression fractures and hip
fractures, were defined as NS subjects. All 450 patients received
0.3 g acetaminophen (Bayer AG, Leverkusen, Germany) orally twice
daily on the day of the infusion and as required for the next 3
days (5,8) (Fig.
1).
In the second part of the study, 187 patients who
had undergone orthopedic surgery, which included 155 of the 450
patients from the first part of the study and 32 unrelated cases
from the orthopedics department, were further evaluated to compare
the efficacy and safety of acetaminophen and low-dose MP. Patients
without GC contraindications, such as active ulcer or glaucoma, and
who completed the 1-year follow-up were included. The 155 patients
that met the above criteria from the OS and MIS subgroups (66 and
89 patients, respectively) constituted the acetaminophen group. The
32 unrelated cases had been immediately treated with 40 mg MP
(Pfizer, Inc.) in 0.9% saline daily for 2 days after ZA infusion
subsequent to surgery, and constituted the MP group. The MP group
included 16 patients who had undergone OS and 16 who had undergone
MIS. Fever occurring subsequently was treated with acetaminophen as
required in the acetaminophen and MP groups (Fig, 1).
Biochemical measurements and bone mass
density (BMD) assessment
Samples of fasting peripheral blood at rest were
taken as a baseline on the morning prior to ZA infusion. The
biochemical parameters analyzed in this study were calcitonin (CT),
type I collagen cross-linked C-telopeptide (CTx), N-telopeptide
(NTx), bone gla protein (BGP), PTH, 25-hydroxy-vitamin D3
[25(OH)D], serum Ca2+, C-reactive protein (CRP) and
erythrocyte sedimentation rate (ESR). CT levels were assessed using
a radioimmunoassay kit (CT-10161; Amresco LLC, Solon, OH, USA)
according to the manufacturer's protocol. CTx and NTx were assessed
using electrochemiluminescence immunoassay kits (PP-US-07407 and
PP-US-05294, respectively; Roche Diagnostics) according to the
manufacturer's protocol. BGP levels were assessed using a
chemiluminescent microparticle immunoassay (MAGLUMI 2000 Plus;
Shenzhen New Industries Biomedical Engineering Co., Ltd., Shenzhen,
China). PTH levels were evaluated via chemiluminescence immunoassay
(Immulite 2000; Siemens AG, Munich, Germany). 25(OH)D levels were
assessed using an ELISA kit (LIAISON; cat. no. 310980; DiaSorin,
Saluggia, Italy). Ca2+ levels were analyzed using the
calcium ion complexation EDTA titration method as previously
described (18). CRP levels were
assessed using immunoturbidimetry on a Sat450 analyzer (AMS s.r.l,
Marcianise, Italy). ESR was recorded using an automatic analyzer
(Monitor 100; ELITechGroup, Inc., Smithfield, RI, USA). In
addition, the levels of TNF-α, IL-2 and IL-6 at baseline prior to
administration of ZA were measured in a subgroup of 40 patients (16
OS patients, 14 MIS patients and 10 NS patients) using ELISA kits
(CSB-E04740 h, CSB-E04626 h and CSB-E04638 h, respectively; Cusabio
Biotech Co., Ltd., Wuhan, China).
Dual-energy X-ray absorptiometry (Hologic, Bedford,
MA, USA) of the spine or hip was used to measure BMD prior to and 1
year following the administration of ZA and the neck and spine of
T-score were recorded.
Clinical observation
Post-surgery, all symptoms including APR and serious
adverse events, such as delayed healing, incision infection,
cardiovascular events and stroke, were assessed via the continuous
monitoring of vital signs and physical assessments. Adverse events
were categorized according to codes from the Medical Dictionary for
Regulatory Activities (19) as
previously described (6). APR
symptoms and the axillary body temperature were recorded eight
times per day. APR onset was categorized as the presence of fever
(temperature >37.3°C) or the presence of at least one other APR
symptom, such as myalgia, headache, dizziness, nausea, vomiting or
eye pain. The highest post-dose body temperatures recorded in the 3
days following infusion were used as an indicator of the severity
of APR.
Patients were monitored via telephone interviews for
1 year post-treatment. The incidence of bisphosphonate-related
osteonecrosis of jaw (BRONJ) and new/re-fracture within this time
was recorded. X-ray examinations were necessary for the diagnosis
of BRONJ, and magnetic resonance imaging was performed to confirm
new/re-fracture.
Statistical analysis
Analysis was performed using SPSS 19.0 (IBM SPSS,
Armonk, NY, USA). All data are expressed as the mean ± standard
deviation for each group. Categorical variables and proportions
were analyzed using the Chi-square test. The demographic
characteristics, bone turnover bio-parameters and inflammatory
cytokines were compared according to the APR group
(APR+, APR−) using analysis of variance and
the Wilcoxon rank test for continuous variables. Logistic
regression analysis was performed to determine the potential risk
factors of experiencing at least one APR. The odds ratio (OR)
included 95% confidence intervals (95% CIs). Associations between
continuous variables were examined using simple and multivariate
linear regression.
A paired t-test was used to compare BMD prior to and
following treatment. Increases in BMD were compared using
independent t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
Risk factors for APR
The demographic, clinical and biochemical
characteristics of the 450 patients immediately treated with
acetaminophen are presented in Table
I. APR+ patients had significantly lower levels of
25(OH)D (P<0.001; Table I) and
significantly higher levels of CRP, TNF-α, IL-2 and IL-6 compared
with APR− patients (P<0.001; Table I). The ESR was significantly higher
in APR+ patients compared with APR− patients
(P<0.001; Table I). No
significant differences in gender, age, body mass index (BMI), CT,
CTx, NTx, BGP, PTH or BMD was observed between the APR+
and APR− groups.
 | Table I.Demographic, clinical and biochemical
characteristics of the study population according to APR
development (APR+/APR−). |
Table I.
Demographic, clinical and biochemical
characteristics of the study population according to APR
development (APR+/APR−).
| Characteristic | Total cases |
APR+ |
APR− | P-value |
|---|
| Patients (n) | 450 | 207 | 243 |
|
| Sex (M/F) | 72/378 | 38/169 | 34/209 |
0.25 |
| Age (years) | 66.8±9.3 | 66.7±8.5 | 66.8±9.9 |
0.94 |
| BMI
(kg/m2) | 23.4±2.8 | 23.5±2.9 | 23.4±2.7 |
0.75 |
| Ca2+
(mmol/l) | 2.26±0.13 | 2.27±0.12 | 2.26±0.13 |
0.59 |
| CT (pg/ml) | 43.11±27.21 | 43.62±28.52 | 42.67±26.03 |
0.72 |
| CTx (ng/ml) | 0.50±0.36 | 0.52±0.38 | 0.49±0.34 |
0.38 |
| NTx (ng/ml) | 39.78±32.50 | 41.34±34.27 | 38.46±30.85 |
0.32 |
| BGP (ug/l) | 19.46±11.18 | 20.12±11.48 | 18.89±10.89 |
0.26 |
| PTH (pg/l) | 32.09±13.07 | 32.64±14.13 | 31.63±12.07 |
0.48 |
| 25(OH)D
(nmol/l) | 41.85±20.37 | 36.12±17.26 | 46.74±21.51 | <0.001 |
| T-score neck
(SD) | −3.17±0.82 | −3.25±0.75 | −3.11±0.87 |
0.071 |
| T-score spine
(SD) | −2.81±0.73 | −2.77±0.73 | −2.84±0.73 |
0.311 |
| CRP (mg/l) | 9.29±12.47 | 14.07±15.32 | 5.22±7.22 | <0.001 |
| ESR (mm/h) | 19.29±13.36 | 23.85±15.52 | 15.41±9.63 | <0.001 |
| TNF-α
(ng/ml)a | 0.622±0.237 | 0.747±0.214 | 0.452±0.144 | <0.001 |
| IL-2
(ng/ml)b | 11.44±5.06 | 13.92±4.52 | 8.08±3.68 | <0.001 |
| IL-6
(pg/ml)c | 194.1±64.1 | 222.3±63.4 | 154.7±40.1 | <0.001 |
In the APR+ group, 25(OH)D levels were
36.12±17.26 nmol/l, which is considerably below the normal
reference range and considered to indicate a vitamin D deficiency
(20). Based on this result, the
baseline characteristics influencing a patient's likelihood of
suffering APR were investigated by performing single-factor and
multi-factor logistic regression. Variables considered included age
(≥65 or <65 years), gender, BMI (≥24 or < 24
kg/m2), 25(OH)D levels (≥50 or <50 nmol/l), surgical
approaches (MIS and OS) and disease history (diabetes mellitus,
hypertension and chronic obstructive pulmonary disease). The OR for
APR in 25(OH)D-deficient (<50 nmol/l) compared with 25(OH)D
insufficient or sufficient (≥50 nmol/l) patients was 2.57
(P<0.001) unadjusted, or 2.12 (P=0.007) after multiple
adjustments; the OR in the MIS group compared with the NS group was
3.87 (P<0.001) unadjusted, or 3.54 (P<0.001) after multiple
adjustments; and in the OS group compared with the NS group was
6.40 (P<0.001) unadjusted, and 5.71 (P<0.001) after multiple
adjustments. The results of this analysis revealed that
25(OH)D-deficient and surgical patients had an elevated risk of
APR, particularly following major trauma (Fig. 2).
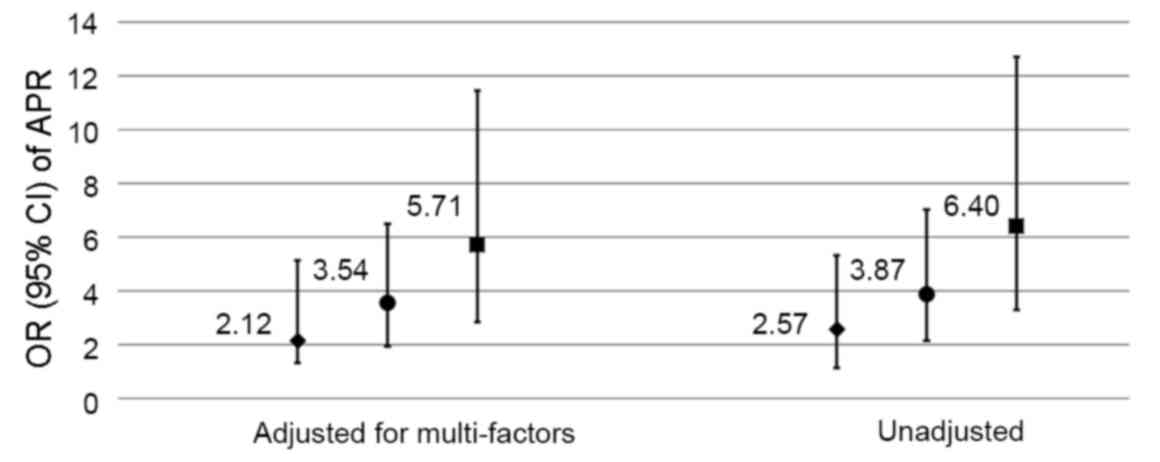 | Figure 2.Risk of APR development. The
multi-factors adjusted for are age, gender, body mass index,
25(OH)D levels, surgical approach and disease history (diabetes,
hypertension and chronic obstructive pulmonary disease). The
diamonds represent the OR of APR in 25(OH)D-deficiency (<50
nmol/l) compared with 25(OH)D insufficiency or sufficiency (≥50
nmol/l). The circles represent the OR of APR in open surgery
compared with NS. The squares represent the OR of APR in minimally
invasive surgery compared with NS. The bars represent the 95% CI.
APR, acute-phase response; 25(OH)D, 25-hydroxyvitamin D3; OR, odds
ratio; CI, confidence interval; NS, non-surgery. |
Table II lists
simple and multiple linear regression coefficients between
continuous variables including age, BMI, levels of Ca2+,
CT, CTx, NTx, BGP, PTH and 25(OH)D and pre-dose CRP levels, with
elevated body temperature. 25(OH)D levels were significantly
negatively correlated (P<0.05) with the presence of fever (a
marker of APR), whereas pre-dose CRP levels were positively
correlated with fever (P<0.001).
 | Table II.Simple and multiple linear regression
coefficients between continuous variables and fever. |
Table II.
Simple and multiple linear regression
coefficients between continuous variables and fever.
| Variable | Regression
coefficient |
|---|
| Age | −0.08 |
| BMI |
0.036 |
|
Ca2+ |
0.137 |
| CT |
0.102 |
| CTx |
0.041 |
| NTx |
0.009 |
| BGP |
0.063 |
| PTH | −0.107 |
| 25(OH)D |
−0.006a |
| CRP |
0.023b |
These results suggest that surgical trauma is
associated with APR incidence, and the association between CRP,
which served to quantify the increased levels of inflammation in
the early phase following surgery, and fever has a dose-response
relationship. These results also indicate that clinically
significant differences in the severity of APR may be associated
with vitamin D deficiency.
Efficacy assessments
To minimize the potential side effects of GC, 40 mg
MP was administered, which is the minimum injectable dose to
prevent APR (21). No significant
difference was observed in baseline demographic and clinical
characteristics between groups. The incidence of APR was
significantly lower in the MP group compared with the acetaminophen
group (P<0.001; Table III).
Similar results were observed for fever morbidity (P<0.05;
Table III).
 | Table III.Demographic characteristics and APR
symptoms in the MP and acetaminophen groups. |
Table III.
Demographic characteristics and APR
symptoms in the MP and acetaminophen groups.
| Characteristic | Total cases
(n=187) | MP group
(n=32) | Acetaminophen group
(n=155) | P-value |
|---|
| Age | 68.9±8.6 | 70.6±9.1 | 68.6±8.5 |
0.230 |
| Gender (M/F) | 27/160 | 5/27 | 22/133 |
0.509 |
| BMI
(kg/m2) | 23.6±3.0 | 22.7±3.5 | 23.8±2.9 |
0.082 |
| 25(OH)D
(nmol/l) | 42.01±21.18 | 39.48±18.43 | 42.64±21.72 |
0.397 |
| CRP (mg/l) | 20.87±13.11 | 23.07±19.32 | 20.42±11.45 |
0.459 |
| OS/MIS | 82/105 | 16/16 | 66/89 |
0.558 |
| APR (all
symptoms) | 99 (52.9%) | 2 (6.3%) | 97 (62.6%) | <0.001 |
| Fever | 97 (51.9%) | 2 (6.3%) | 95 (61.3%) | <0.001 |
| Other symptoms | 28 (15.0%) | 1 (3.1%) | 27 (17.4%) |
0.053 |
Safety assessments
ZA administration did not appear to induce adverse
effects in any participants. At the 12-month follow-up, few cases
of new/re-fracture were reported, and the frequency was similar in
each group (1 case in the MP group and 2 cases in the acetaminophen
group). There were no reports of spontaneous BRONJ.
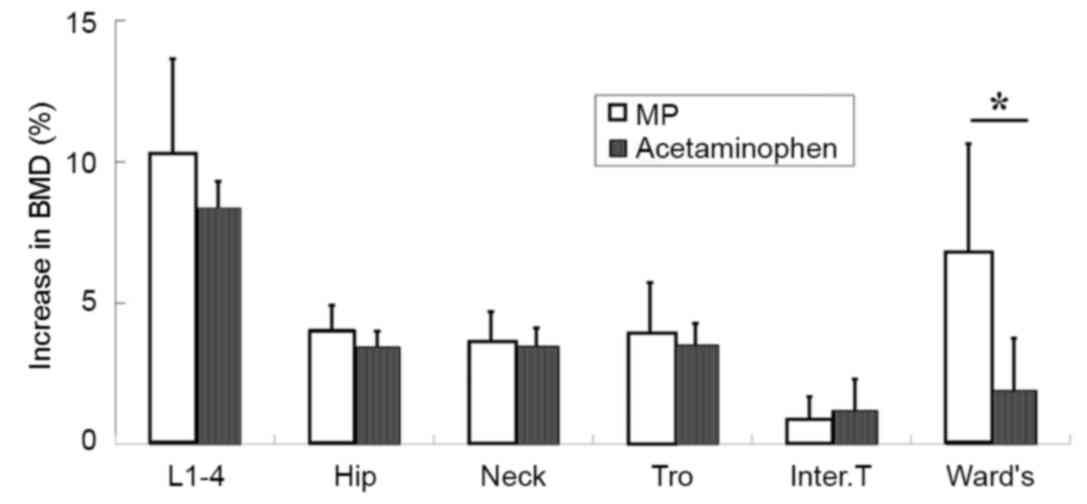
No significant differences were observed in baseline
BMD between groups. At the 12-month follow-up, no significant
difference in BMD increase was observed for the lumbar vertebra or
hip, including the femoral neck, trochanteric or intertrochanteric
region (Fig. 3). However, BMD in
Ward's region showed a significantly greater increase in the MP
group compared with the acetaminophen group (P<0.05; Fig. 3).
Discussion
The results of the present study demonstrate that
the surgical method used and subsequent baseline levels of
inflammation are crucial determinants of the development of an APR
in patients with osteoporosis. As a major acute-phase protein, CRP
is secreted by liver cells following trauma; therefore, CRP levels
may be used to assess inflammatory levels and quantify the degree
of surgical trauma with high sensitivity (22–24).
Additionally, cell cytokines such as IL-2, IL-6 and TNF-α are
upregulated by surgical trauma, and their activation subsequently
upregulates the expression of other inflammatory factors in
activated adjacent γδT cells (22,23). In
the present study, high levels of CRP post-surgery and prior to
treatment were correlated with an elevation in body temperature,
i.e., the severity of APR in patients treated with ZA. Plasma
inflammatory cytokine concentrations following a moderate trauma
stimulus typically recover to basal levels within 1 week (23); however, in the present study the
incidence of APR was notably higher when ZA was administered within
1 week of surgery than has been reported in other literature
(6,7).
It has been reported that vitamin D is able to
modulate immunological function and reduce levels of IFN-γ, TNF-β
and IL-2 in T cells (25,26), and it is believed that that 25(OH)D
deficiency may lead to the development of APR (27). This provides an explanation for the
finding in the present study that 25(OH)D is an important risk
factor for APR. It has been suggested that the attainment of
adequate levels of 25(OH)D (>100 nmol/l) prior to the first
infusion may reduce the incidence of fever (27).
The present study demonstrated that acetaminophen
was unable to control or prevent APR within 1 week post-surgery due
to its limited anti-inflammatory function; however, low-dose MP
administration effectively ameliorated the ZA-induced increase in
body temperature. To the best of our knowledge, no clinical
observation data for low-dose MP and APR in ZA-naïve patients in
the early postoperative period are available. In a GC-induced
osteoporosis subpopulation treated with ZA, the frequency of APR
was reported to be slightly lower than that in primary osteoporosis
(6,28,29),
which may be attributable to the use of GC during therapy. No
significant differences were reported in the incidence of serious
adverse events, such as arrhythmia or serious atrial fibrillation,
BRONJ or re/new fractures between the two groups (6,28,29). The
most common adverse event reported in the present study was fever,
together with associated symptoms such as chills and flushes, which
occurred in 61.3% of acetaminophen-treated subjects after ZA
infusion, compared with <6.3% of the MP group. In addition, the
patients in the acetaminophen group reported a number of other
symptoms, such as myalgia, headache, malaise, and fatigue, which
are probably a reflection of widespread inflammatory changes.
Compared with orally administered BPs, ZA is
advantageous due to decreased incidence of gastrointestinal tract
reactions, a higher absorption rate, and a regimen of once-yearly
infusions which guarantees medication compliance and adherence
(30–33). A previous study reported that ~78.7%
patients prefer a once-yearly infusion regimen (34). A number of studies have demonstrated
that ZA is associated with a significant improvement in clinical
outcomes following orthopedic surgery in patients with osteoporosis
(35–41). However, it is unclear whether BPs are
helpful or harmful in acute fracture healing or spinal fusion. Two
meta-analyses of randomized controlled trials reported that even
early administration of BPs post-surgery did not delay fracture
healing or spinal fusion time, either radiologically or clinically
(42,43). Animal studies have demonstrated that
ZA treatment immediately post-surgery may have a positive effect on
spinal fusion in patients with osteoporosis, with no adverse
effects on the healing process of bone grafts (44). Considering the beneficial aspects of
BP treatment for patients with osteoporosis, ZA infusion following
fracture fixation surgery, spine fusion surgery or arthroplasty
appears to be a viable treatment option.
In the present study, ZA administration was not
tolerated in some acetaminophen-treated patients, inducing a higher
incidence of influenza-like illness and pyrexia events
postoperatively. The ability to reduce the incidence and severity
of APR symptoms with low-dose MP ensures that any post-treatment
effects are kept to an acceptable level post-surgery.
The present study had several limitations. Firstly,
its retrospective nature may have resulted in selection bias of the
patients. Secondly, the small sample size may have have
insufficient statistical power to detect slight effects. Finally,
changes in bone metabolism markers, renal function and hypocalcemia
were not assessed.
In conclusion, surgical trauma is an important
determining factor for the occurrence of APR. For patients
undergoing surgery, low-dose MP appears to have potential as a
therapeutic application for preventing APR induced by ZA infusion.
These findings may provide a feasible and safe treatment for the
management of APR symptoms.
Acknowledgements
The authors of the present study would like to thank
Dr. Chengqiang Yin, Department of Gastroenterology of the First
Affiliated Hospital of Nanjing Medical University, for technical
assistance in this study. This study was supported by National
Natural and Science Foundation (grant no. 81271988) and Jiangsu
Natural and Science Foundation (grant no. BK2012876).
References
|
1
|
Stafford RS, Drieling RL and Hersh AL:
National trends in osteoporosis visits and osteoporosis treatment,
1988–2003. Arch Intern Med. 164:1525–1530. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nancollas GH, Tang R, Phipps RJ, Henneman
Z, Gulde S, Wu W, Mangood A, Russell RG and Ebetino FH: Novel
insights into actions of bisphosphonates on bone: Differences in
interactions with hydroxyapatite. Bone. 38:617–627. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gasser JA, Ingold P, Venturiere A, Shen V
and Green JR: Long-term protective effects of zoledronic acid on
cancellous and cortical bone in the ovariectomized rat. J Bone
Miner Res. 23:544–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saag K, Lindsay R, Kriegman A, Beamer E
and Zhou W: A single zoledronic acid infusion reduces bone
resorption markers more rapidly than weekly oral alendronate in
postmenopausal women with low bone mineral density. Bone.
40:1238–1243. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lyles KW, Colón-Emeric CS, Magaziner JS,
Adachi JD, Pieper CF, Mautalen C, Hyldstrup L, Recknor C,
Nordsletten L, Moore KA, et al: Zoledronic acid and clinical
fractures and mortality after hip fracture. N Engl J Med.
357:1799–1809. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Black DM, Delmas PD, Eastell R, Reid IR,
Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, et al:
Once-yearly zoledronic acid for treatment of postmenopausal
osteoporosis. N Engl J Med. 356:1809–1822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reid IR, Gamble GD, Mesenbrink P, Lakatos
P and Black DM: Characterization of and risk factors for the
acute-phase response after zoledronic acid. J Clin Endocrinol
Metab. 95:4380–4387. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wark JD, Bensen W, Recknor C, Ryabitseva
O, Chiodo J III, Mesenbrink P and de Villiers TJ: Treatment with
acetaminophen/paracetamol or ibuprofen alleviates post-dose
symptoms related to intravenous infusion with zoledronic acid 5 mg.
Osteoporos Int. 23:503–512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schweitzer DH, Oostendorp-van de Ruit M,
van der Pluijm G, Löwik CW and Papapoulos SE: Interleukin-6 and the
acute phase response during treatment of patients with Paget's
disease with the nitrogen-containing bisphosphonate
dimethylaminohydroxypropylidene bisphosphonate. J Bone Miner Res.
10:956–962. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sauty A, Pecherstorfer M, Zimmer-Roth I,
Fioroni P, Juillerat L, Markert M, Ludwig H, Leuenberger P,
Burckhardt P and Thiebaud D: Interleukin-6 and tumor necrosis
factor alpha levels after bisphosphonates treatment in vitro and in
patients with malignancy. Bone. 18:133–139. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thiébaud D, Sauty A, Burckhardt P,
Leuenberger P, Sitzler L, Green JR, Kandra A, Zieschang J and de
Palacios P Ibarra: An in vitro and in vivo study of cytokines in
the acute-phase response associated with bisphosphonates. Calcif
Tissue Int. 61:386–392. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dicuonzo G, Vincenzi B, Santini D,
Avvisati G, Rocci L, Battistoni F, Gavasci M, Borzomati D, Coppola
R and Tonini G: Fever after zoledronic acid administration is due
to increase in TNF-alpha and IL-6. J Interferon Cytokine Res.
23:649–654. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson K and Rogers MJ: Statins prevent
bisphosphonate-induced gamma, delta-T-cell proliferation and
activation in vitro. J Bone Miner Res. 19:278–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sato K, Kimura S, Segawa H, Yokota A,
Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H and
Maekawa T: Cytotoxic effects of gammadelta T cells expanded ex vivo
by a third generation bisphosphonate for cancer immunotherapy. Int
J Cancer. 116:94–99. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Galluzzo S, Santini D, Vincenzi B, Caccamo
N, Meraviglia F, Salerno A, Dieli F and Tonini G: Immunomodulating
role of bisphosphonates on human gamma delta T cells: An intriguing
and promising aspect of their antitumour activity. Expert Opin Ther
Targets. 11:941–954. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rossini M, Adami S, Viapiana O, Ortolani
R, Vella A, Fracassi E and Gatti D: Circulating γδ T cells and the
risk of acute-phase response after zoledronic acid administration.
J Bone Miner Res. 27:227–230. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
NIH Consensus Development Panel on
Osteoporosis Prevention, Diagnosis, and Therapy: Osteoporosis
prevention, diagnosis, and therapy. JAMA. 285:785–795. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Copp DH: Simple and precise micromethod
for EDTA titration of calcium. J Lab Clin Med. 61:1029–1037.
1963.PubMed/NCBI
|
|
19
|
2003 Medical dictionary for regulatory
activities (MedDRA). Reston, VA: Northrop Grumman;
|
|
20
|
Holick MF: Vitamin D deficiency. N Engl J
Med. 357:266–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Modi H, Chung KJ, Yoon HS, Yoo HS and Yoo
JH: Local application of low-dose Depo-Medrol is effective in
reducing immediate postoperative back pain. Int Orthop. 33:737–743.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gabay C and Kushner I: Acute-phase
proteins and other systemic responses to inflammation. N Engl J
Med. 340:448–454. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Desborough JP: The stress response to
trauma and surgery. Br J Anaesth. 85:109–117. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ceciliani F, Giordano A and Spagnolo V:
The systemic reaction during inflammation: The acute-phase
proteins. Protein Pept Lett. 9:211–223. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Penna G, Roncari A, Amuchastegui S, Daniel
KC, Berti E, Colonna M and Adorini L: Expression of the inhibitory
receptor ILT3 on dendritic cells is dispensable for induction of
CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood.
106:3490–3497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Cencioni MT, Angelini DF,
Borsellino G, Battistini L and Brosnan CF: Transcriptional
profiling of gamma delta T cells identifies a role for vitamin D in
the immunoregulation of the V gamma 9V delta 2 response to
phosphate-containing ligands. J Immunol. 174:6144–6152. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bertoldo F, Pancheri S, Zenari S, Boldini
S, Giovanazzi B, Zanatta M, Valenti MT, Carbonare L Dalle and Lo
Cascio V: Serum 25-hydroxyvitamin D levels modulate the acute-phase
response associated with the first nitrogen-containing
bisphosphonate infusion. J Bone Miner Res. 25:447–454. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Reid DM, Devogelaer JP, Saag K, Roux C,
Lau CS, Reginster JY, Papanastasiou P, Ferreira A, Hartl F, Fashola
T, et al: Zoledronic acid and risedronate in the prevention and
treatment of glucocorticoid-induced osteoporosis (HORIZON): A
multicentre, double-blind, double-dummy, randomised controlled
trial. Lancet. 373:1253–1263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grossman JM, Gordon R, Ranganath VK, Deal
C, Caplan L, Chen W, Curtis JR, Furst DE, McMahon M, Patkar NM, et
al: American College of Rheumatology 2010 recommendations for the
prevention and treatment of glucocorticoid-induced osteoporosis.
Arthritis Care Res (Hoboken). 62:1515–1526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siris ES, Harris ST, Rosen CJ, Barr CE,
Arvesen JN, Abbott TA and Silverman S: Adherence to bisphosphonate
therapy and fracture rates in osteoporotic women: Relationship to
vertebral and nonvertebral fractures from 2 US claims databases.
Mayo Clin Proc. 81:pp. 1013–1022. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cramer JA, Gold DT, Silverman SL and
Lewiecki EM: A systematic review of persistence and compliance with
bisphosphonates for osteoporosis. Osteoporos Int. 18:1023–1031.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Briesacher BA, Andrade SE, Yood RA and
Kahler KH: Consequences of poor compliance with bisphosphonates.
Bone. 41:882–887. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seeman E, Compston J, Adachi J, Brandi ML,
Cooper C, Dawson-Hughes B, Jönsson B, Pols H and Cramer JA:
Non-compliance: The Achilles' heel of anti-fracture efficacy.
Osteoporos Int. 18:711–719. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
McClung M, Recker R, Miller P, Fiske D,
Minkoff J, Kriegman A, Zhou W, Adera M and Davis J: Intravenous
zoledronic acid 5 mg in the treatment of postmenopausal women with
low bone density previously treated with alendronate. Bone.
41:122–128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bhandari M, Bajammal S, Guyatt GH,
Griffith L, Busse JW, Schünemann H and Einhorn TA: Effect of
bisphosphonates on periprosthetic bone mineral density after total
joint arthroplasty. A meta-analysis. J Bone Joint Surg Am.
87:293–301. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Friedl G, Radl R, Stihsen C, Rehak P,
Aigner R and Windhager R: The effect of a single infusion of
zoledronic acid on early implant migration in total hip
arthroplasty. A randomized, double-blind, controlled trial. J Bone
Joint Surg Am. 91:274–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eriksen EF, Lyles KW, Colón-Emeric CS,
Pieper CF, Magaziner JS, Adachi JD, Hyldstrup L, Recknor C,
Nordsletten L, Lavecchia C, et al: Antifracture efficacy and
reduction of mortality in relation to timing of the first dose of
zoledronic acid after hip fracture. J Bone Miner Res. 24:1308–1313.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Prieto-Alhambra D, Javaid MK, Judge A,
Murray D, Carr A, Cooper C and Arden NK: Association between
bisphosphonate use and implant survival after primary total
arthroplasty of the knee or hip: Population based retrospective
cohort study. BMJ. 343:d72222011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li C, Wang HR, Li XL, Zhou XG and Dong J:
The relation between zoledronic acid infusion and interbody fusion
in patients undergoing transforaminal lumbar interbody fusion
surgery. Acta Neurochir (Wien). 154:731–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Park YS, Kim HS, Baek SW, Kong DY and Ryu
JA: The effect of zoledronic acid on the volume of the fusion-mass
in lumbar spinal fusion. Clin Orthop Surg. 5:292–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen F, Dai Z, Kang Y, Lv G, Keller ET and
Jiang Y: Effects of zoledronic acid on bone fusion in osteoporotic
patients after lumbar fusion. Osteoporos Int. 27:1469–1476. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li YT, Cai HF and Zhang ZL: Timing of the
initiation of bisphosphonates after surgery for fracture healing: A
systematic review and meta-analysis of randomized controlled
trials. Osteoporos Int. 26:431–441. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Xue D, Li F, Chen G, Yan S and Pan Z: Do
bisphosphonates affect bone healing? A meta-analysis of randomized
controlled trials. J Orthop Surg Res. 9:452014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yasen M, Li X, Jiang L, Yuan W, Che W and
Dong J: Effect of zoledronic acid on spinal fusion outcomes in an
ovariectomized rat model of osteoporosis. J Orthop Res.
33:1297–1304. 2015. View Article : Google Scholar : PubMed/NCBI
|