Introduction
Pancreatic cancer is a highly lethal human
gastrointestinal cancer (1).
Although increasing methods are being applied for pancreatic cancer
treatment, such as surgical resection and radiotherapy, the 5-year
relative survival rate remains very dismal. A potential reason for
the failure of the classical therapeutic approach may be explained
by its high metastatic potential (2). Thus, it is critical to reveal the
metastasis mechanism of pancreatic cancer. Epithelial-mesenchymal
transition (EMT), the conversion from an epithelial to a
mesenchymal phenotype, is a vital process for cancer invasion to
surrounding tissues or metastasis to other organs (3). During the process of EMT, typical
morphological changes occur, such as cell invasion and motility
(4). The molecular indicators for
EMT are the decrease of epithelial markers, such as E-cadherin, and
the increase in the levels of mesenchymal markers, such as
N-cadherin and vimentin (5).
Significant efforts are required to investigate the mechanism of
EMT for cancer control and the improvement of cure rate.
Response gene to complement 32 (RGC-32), first
identified in 1998, is induced by complement and involved in cell
cycle activation (6). RGC-32 is
comprehensively expressed in the placenta, skeletal muscle, kidney,
pancreas and aortic endothelial cells (7). It was reported that RGC-32 was also
overexpressed in various types of cancer, such as colon cancer
(8); however, had various complex
roles in different cancer types (9).
Transforming growth factor-β (TGF-β) and its downstream signal
molecules have been demonstrated to have an essential role in the
EMT of various types of cancer (9).
In human renal proximal tubular cells (10) and pancreatic cancer cell line BxPC-3
(11), RGC-32 mediated TGF-β-induced
EMT. To the best of our knowledge, a hypoxic microenvironment is
common in the majority of solid tumors and is associated with the
EMT of tumors (12). A vast number
of clinical studies have suggested that hypoxia and hypoxia-induced
signaling pathways are closely related to the poor outcome of tumor
patients (13,14). Although increasing evidence has
indicated that hypoxia may induce EMT (15), the relationship between RGC-32 and
hypoxia-induced EMT is not fully understood.
Hypoxia-inducible factor 1 (HIF-1) is a
transcriptional activator and is involved in a lot of
pathophysiological processes under hypoxia (16). HIF-1 consists of an oxygen-sensitive
α subunit (HIF-1α) and a constitutively expressed β subunit
(HIF-1β) (17). Under hypoxia,
HIF-1α regulates the expression of target genes by binding to the
core sequence at the promoter region of the target genes (18). For example, it was reported that
renalase, an amine oxidase secreted by the proximal tubule, was
upregulated by hypoxia via a HIF-1α-dependent mechanism (19,20).
HIF-1α is closely associated with the invasion, metastasis and
prognosis of tumors (21).
In the present study, a cell model of
hypoxia-induced EMT was constructed and it was demonstrated that
repression of HIF-1α with HIF-1α inhibitor or small interfering
(si)RNA transfection suppressed hypoxia-induced HIF-1α, RGC-32,
N-cadherin and vimentin, but increased the expression of E-cadherin
and cytokeratins inhibited by hypoxia. Furthermore, it was also
observed that inhibition of RGC-32 by siRNA transfection
upregulated the expression of E-cadherin, but impaired the protein
expression level of vimentin. These data suggested that hypoxia
activated the expression of HIF-1α, then increased the levels of
RGC-32, in turn to modulate the EMT-related proteins for EMT. These
findings increased the understanding about the function of RGC-32
in hypoxia-induced EMT and may have identified a novel target for
pancreatic cancer treatment.
Materials and methods
Reagents
RPMI-1640 and α-minimal essential medium were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Negative control siRNA (NC siRNA, CCT ACA TCC CGA TCG ATG ATG
TT), HIF-1α-Homo-488 siRNA (CTG ATG ACC AG CAA CTT GA),
HIF-1α-Homo-1216 siRNA (CCT ATA TCC CAA TGG ATG ATG TT) and RGC-32
siRNA (siRGC-32, CAG ATT CAC TTT ATA GGA A) were purchased from
GenePharma Technology Co., Ltd. (Shanghai, China). Lipofectamine
RNAi MAX reagent (cat. no. 13778-150) was purchased from Invitrogen
(Thermo Fisher Scientific, Inc.). RNA isolation kit (cat. no.
74104) was purchased from Qiagen GmbH (Hilden, Germany). SuperReal
PreMix Color (cat. no. FP215-02) was purchased from Tiangen Biotech
Co., Ltd., (Beijing, China). Antibodies against HIF-1α (cat. no.
ab51608), E-cadherin (cat. no. ab40772), N-cadherin (cat. no.
ab98952) and vimentin (cat. no. ab8978) were purchased from Abcam
(Cambridge, MA, USA). RGC-32 antibody (cat. no. sc-84222) was
purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA).
GAPDH antibody (cat. no. AP0063) was purchased from Bioworld
Technology Inc., (St. Louis Park, MN, USA). Horseradish peroxidase
(HRP)-labeled secondary antibody (cat. no. LK-GAR007) was purchased
from MultiSciences (Lianke) Biotechnology Co. Ltd. (Hangzhou,
China). Radioimmunoprecipitation assay (RIPA) buffer (cat. no.
PP1901) was purchased from BioTeke Corp., (Beijing, China). HIF-1α
inhibitor (cat. no. HY12033) was purchased from MedChem Express
company (Monmouth Junction, NJ, USA).
Cell culture and siRNA
transfection
Human pancreatic cancer BxPC-3 cell line was
purchased from Hibio Bio-tech Co., Ltd (Hangzhou, China). BxPC-3
cells were seeded into 6-well plates at a density of
1×105/well with RPMI-1640 medium supplemented with 10%
fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.). When
the confluence reached 70–80%, siRNA transfections were performed
according to the manufacturer's instructions. Briefly, HIF-1α siRNA
and Lipofectamine RNAi MAX reagent was diluted with Opti-MEM
(Gibco; Thermo Fisher Scientific, Inc.) at room temperature for 5
min. The diluted Lipofectamine was added into siRNA dilution and
placed at room temperature for an additional 5 min. Subsequently,
the mixture was added into the wells with a final concentration of
20 nM siRNA. Cells were incubated at 37°C in a humidified
atmosphere with 5% CO2 overnight. The following morning,
cells were placed in fresh RPMI-1640 medium supplemented with 10%
fetal bovine serum and incubated in hypoxic conditions, at
5%CO2+1%O2+94%N2 for 48 h at 37°C.
Control cells received the same amount of negative control (NC)
siRNA.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from BxPC-3 cells with TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). The concentration of
RNA was determined by measuring the absorbance at 260 and 280 nm
using Merinton SMA4000 (Merinton, Inc., Beijing, China). For the RT
reaction, random-primed cDNA was synthesized from 1 mg of total RNA
using a PrimeScript™ RT reagent kit (Takara Biotechnology Co.,
Ltd., Dalian, China), according to the manufacturer's instructions.
qPCR analysis was performed using SuperReal PreMix Color
(SYBR-Green), according to the manufacturer's instructions, and
detected on a CFX96™ Real-Time system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) with the following thermal conditions: 50°C for
3 min, 95°C for 15 min, followed by 40 cycles each of 95°C for 10
sec, 57°C for 10 sec and 72°C for 40 sec. Reactions were performed
in triplicate. Quantification was calculated using the
2−∆∆CT method (22) with
GAPDH mRNA as an endogenous control. The primers for GAPDH, HIF-1α,
RGC-32, E-cadherin, cytokeratins, vimentin and N-cadherin were
synthesized by Sunny Biotech Co., Ltd. (Shanghai, China) and are
listed in Table I.
 | Table I.Primers used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primers used in reverse
transcription-quantitative polymerase chain reaction.
| Target gene | Direction | Sequence |
|---|
| Hypoxia-inducible
factor 1α | Forward |
5′-CACCACAGGACAGTACAGGAT-3′ |
|
| Reverse |
5′-CGTGCTGAATAATACCACTCACA-3′ |
| Response gene to
complement 32 | Forward |
5′-CGCTGTGCGAGTTTGACG-3′ |
|
| Reverse |
5′-TCCAGGTGCTCCTCGT-3′ |
| E-cadherin | Forward |
5′-AATGCCGCCATCGCTTAC-3′ |
|
| Reverse |
5′-CCACCAGGGTATACGTAGGGA-3′ |
| N-cadherin | Forward |
5′-GAGGCTTCTGGTGAAATCGC-3′ |
|
| Reverse |
5′-GGAAAGCTTCTCACGGCATAC-3′ |
| Vimentin | Forward |
5′-CCGCTTCGCCAACTACATC-3′ |
|
| Reverse |
5′-GGTTAGCTGGTCCACCTGCC-3′ |
| Cytokeratins | Forward |
5′-ACTACAGCCACTACTACACGACCA-3′ |
|
| Reverse |
5′-AGCCTGTTCCGTCTCAAACTT-3′ |
| GAPDH | Forward |
5′-AGAAGGCTGGGGCTCATTTG-3′ |
|
| Reverse |
5′-AGGGGCCATCCACAGTCTTC-3′ |
Western blotting
BxPC-3 cells were divided into five groups: a) No
treatment and cells under normoxia (5% CO2) at 37°C; b)
cells under hypoxia (5% CO2 + 1% O2 + 94%
N2) at 37°C; c) cells pretreated with HIF-1α inhibitor
for 30 min, then incubated under hypoxia (5% CO2 +
1%O2 + 94% N2) at 37°C; d) cells transfected
with negative control siRNA, then incubated under hypoxia (5%
CO2 + 1% O2 + 94% N2) at 37°C; e)
cells transfected with HIF-1α-Homo-1216 siRNA then cultured under
hypoxia (5% CO2 + 1% O2 + 94% N2)
at 37°C. After 48 h, cells were harvested and lysed with RIPA
buffer. Cells were shaken repeatedly until completed lysed,
followed by centrifugation at 12,000 × g for 20 min at 4°C.
Concentrations of proteins from the above five groups were
determined using the bicinchoninic acid method. Protein samples
were boiled for 5–10 min for further experiments. Proteins (30–50
µg) per sample were subjected to 12% SDS-PAGE and transferred to
polyvinylidene fluoride membranes. Subsequent to blocking using 5%
milk in Tris-buffered saline with 0.1% Tween-20 (TBST) at room
temperature for 2 h, the blots were probed with antibodies specific
for the proteins of interest, including RGC-32 (1:250) E-cadherin,
cytokeratins, vimentin and N-cadherin (all 1:1,000) at 4°C
overnight. Respective membranes were incubated with HRP-labeled
secondary antibody (1:5,000) for 1 h at room temperature after the
membranes were washed with TBST three times. Finally, the
expression signals were detected with an enhanced chemiluminescence
detection system (Pierce; Thermo Fisher Scientific, Inc.) and
captured with a ChemiDoc XRS + System (Bio-Rad Laboratories, Inc.).
To measure the protein levels, the bands of the western blots were
measured with ImageJ Plus software v1.63 (National Institutes of
Health, Bethesda, MD, USA), and the gray value of each target
protein was calculated by comparison with GAPDH expression.
Statistical analysis
All experiments were repeated at least three times,
and representative experiments were demonstrated. Data were
expressed as the mean ± standard deviation. Statistical analysis
was performed using SPSS v. 13.0 software (SPSS, Inc., Chicago, IL,
USA). Student's two-tailed t-tests were applied for comparison of
two independent experimental groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
siRNA targeted to HIF-1α successfully
inhibits the expression of HIF-1α
To screen the effectiveness of HIF-1α siRNA, siRNA
transfection experiments were conducted and the protein expression
level of HIF-1α was detected by western blotting. As demonstrated
in Fig. 1A and B, compared with the
no treatment control group (CK), negative control (NC) siRNA
incubation had no significant effect on the protein expression
level of HIF-1α (P>0.05). However, various siRNA targeted to
HIF-1α had a different effect on the expression of HIF-1α. Compared
with the NC siRNA group, both HIF-1α-Homo-488 siRNA and
HIF-1α-Homo-1216 siRNA significantly suppressed the expression of
HIF-1α (P<0.01); however, HIF-1α-Homo-1553 siRNA did not
significantly affect the protein expression level of HIF-1α.
Furthermore, HIF-1α-Homo-1216 siRNA had a more significant effect
on the expression of HIF-1α. Therefore, HIF-1α-Homo-1216 siRNA was
selected for further experiments.
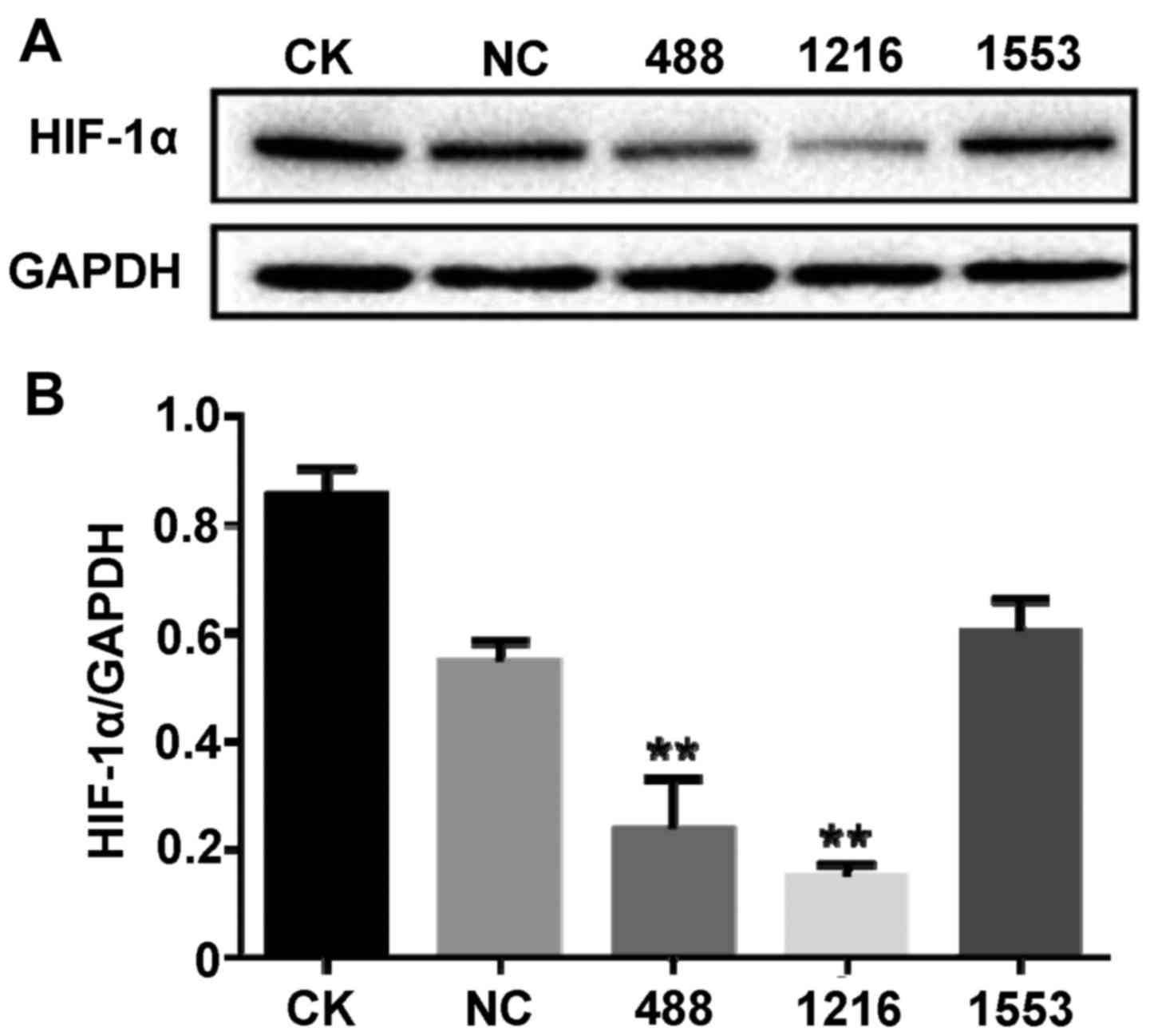 | Figure 1.siRNA targeted to HIF-1α successfully
inhibits the expression of HIF-1α. Cells were transfected with NC
siRNA, HIF-1α-Homo-488 siRNA, HIF-1α-Homo-1216 siRNA and
HIF1A-Homo-1553 siRNA, respectively. After 48 h, proteins were
extracted from cells. (A) HIF-1α-Homo-488 siRNA and
HIF-1α-Homo-1216 siRNA suppressed the expression of HIF-1α detected
by western blot with anti-HIF-1α antibody. (B) The bands of the
western blots were quantified with ImageJ Plus software. Data are
presented as the mean ± standard deviation. **P<0.01 vs. NC.
siRNA, small interfering RNA; HIF-1α, hypoxia-inducible factor 1α;
CK, no treatment group; NC, negative control siRNA; 488,
HIF-1α-Homo-488 siRNA; 1216, HIF-1α-Homo-1216; 1553,
HIF-1α-Homo-1553. |
HIF-1α regulates mRNA and protein
expression levels of RGC-32 and EMT-associated genes induced by
hypoxia
To determine the role of HIF-1α in hypoxia-induced
EMT in BxPC-3 cells, the expression of HIF-1α was blocked
withHIF-1α-Homo-1216 siRNA and the expression of EMT markers was
assessed. As demonstrated in Fig. 2,
hypoxia incubation significantly increased the mRNA expression
level of HIF-1α, RGC-32, N-cadherin and vimentin compared with no
treatment cells under normoxia (P<0.05). However, the HIF-1α
inhibitor pretreatment significantly suppressed the upregulation of
these genes induced by hypoxia (P<0.05). HIF-1α-Homo-1216 siRNA
significantly inhibited the mRNA expression levels of HIF-1α,
RGC-32, N-cadherin and vimentin compared with the NC siRNA group
(P<0.01). It was also demonstrated that hypoxia significantly
suppressed the expression of E-cadherin and cytokeratins compared
with no treatment cells under normoxia (P<0.05), and this
inhibition was significantly released by HIF-1α inhibitor
(P<0.05). Compared with the NC siRNA group, HIF-1α-Homo-1216
siRNA transfection significantly upregulated the transcripts of
E-cadherin and cytokeratins (P<0.05 and P<0.01, respectively;
Fig. 2).
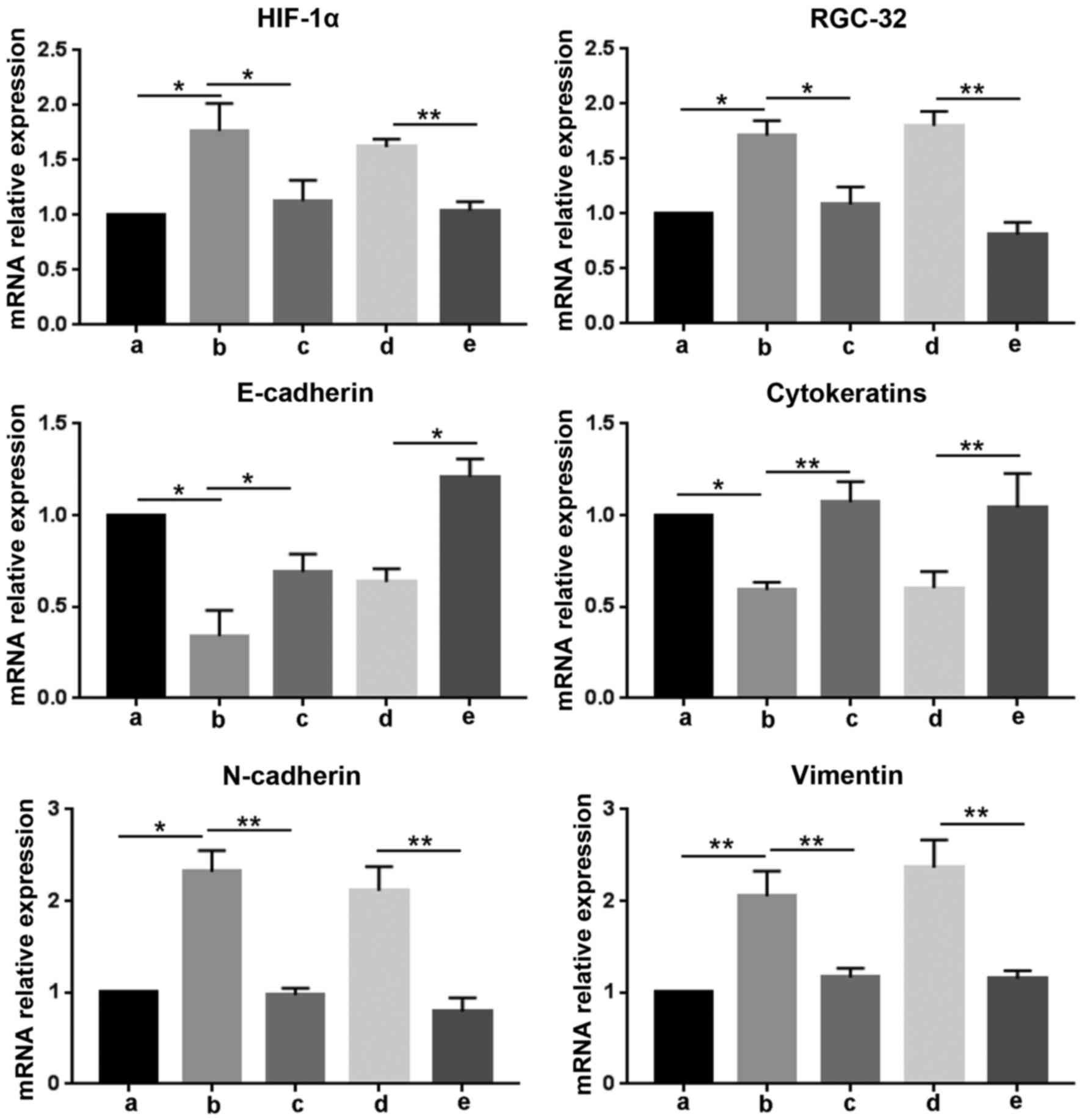 | Figure 2.HIF-1α regulates the mRNA levels of
RGC-32 and epithelial-mesenchymal transition-associated genes
induced by hypoxia. RNA was isolated from cells after different
treatments for reverse transcription-quantitative polymerase chain
reaction to detect the mRNA expression levels of HIF-1α, RGC-32,
E-cadherin, cytokeratins, N-cadherin and vimentin relative to
GAPDH. Data are presented as the mean ± standard deviation.
*P<0.05 and **P<0.01, as indicated. siRNA, small interfering
RNA; HIF-1α, hypoxia-inducible factor 1α; RGC-32, response gene to
complement 32; a, no treatment cells under normoxia; b, cells under
hypoxia; c, cells were pretreated with HIF-1α inhibitor for 30 min,
and then incubated under hypoxia; d, cells were transfected with
negative control siRNA, and then incubated under hypoxia; e, cells
were transfected with HIF-1α-Homo-1216 siRNA and then cultured
under hypoxia. |
To further examine the effect of HIF-1α on the
protein levels of RGC-32 and EMT-related proteins, western blotting
was conducted following siRNA transfection, as demonstrated in
Fig. 3. The results were similar to
the RT-qPCR data. HIF-1α inhibitor and siRNA significantly
suppressed the expression of HIF-1α, RGC-32, N-cadherin and
vimentin induced by hypoxia, but released the protein expression
levels of E-cadherin and cytokeratins inhibited by hypoxia
(P<0.01). These results demonstrated that HIF-1α regulated the
expression of EMT markers, and hypoxia induced the expression of
RGC-32 via HIF-1α.
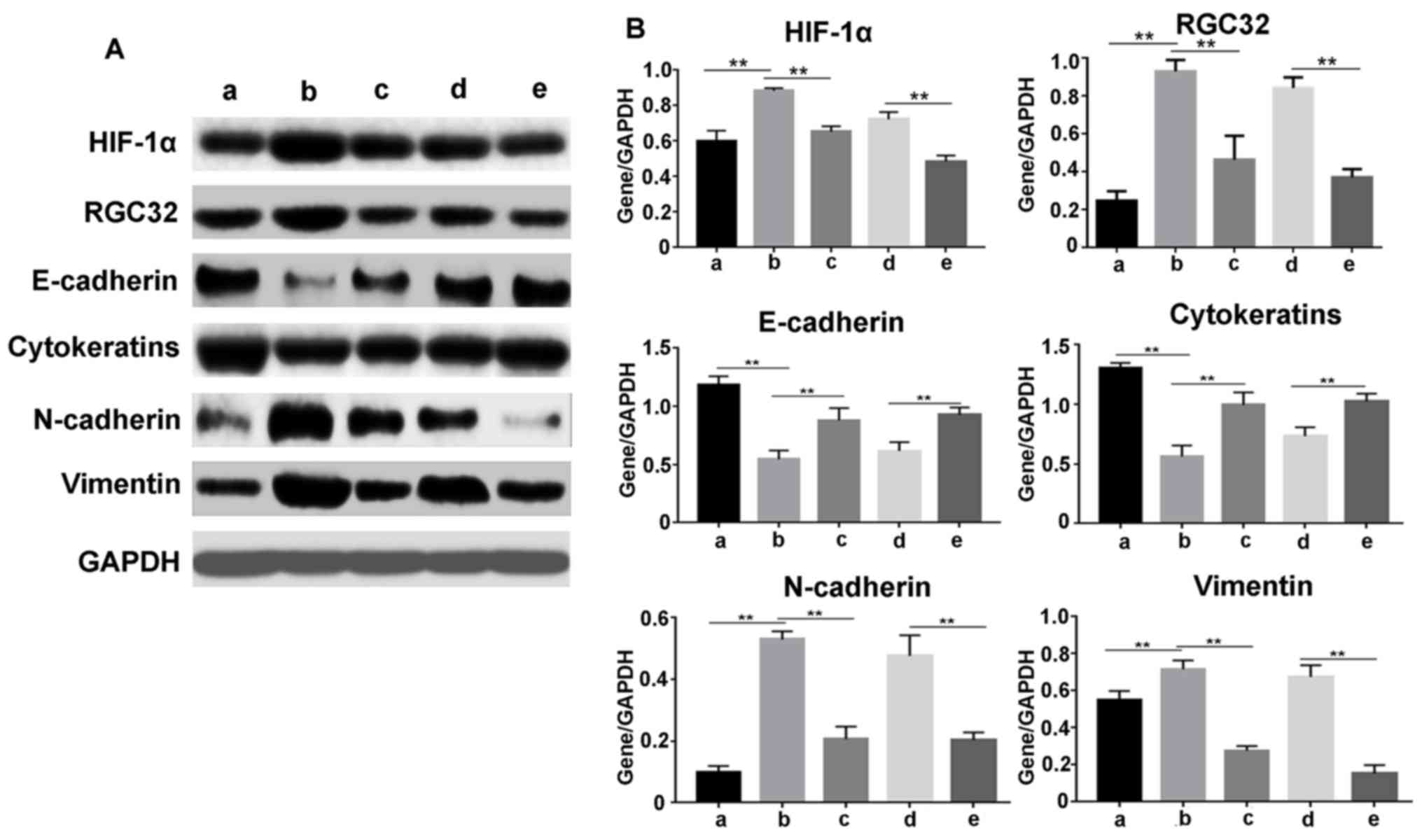 | Figure 3.HIF-1α regulates the protein levels of
RGC-32 and epithelial-mesenchymal transition-associated genes
induced by hypoxia. Proteins were extracted from cells after
different treatments. (A) Protein levels of HIF-1α, RGC-32,
E-cadherin, cytokeratins, N-cadherin and vimentin were detected by
western blotting. (B) The western blot bands were quantified using
ImageJ Plus software. Data are presented as the mean ± standard
deviation. **P<0.01 as indicated. siRNA, small interfering RNA;
HIF-1α, hypoxia-inducible factor 1α; RGC-32, response gene to
complement 32; a, no treatment cells under normoxia; b, cells under
hypoxia; c, cells were pretreated with HIF-1α inhibitor for 30 min,
and then incubated under hypoxia; d, cells were transfected with
negative control siRNA, and then incubated under hypoxia; e, cells
were transfected with HIF-1α-Homo-1216 siRNA and then cultured
under hypoxia. |
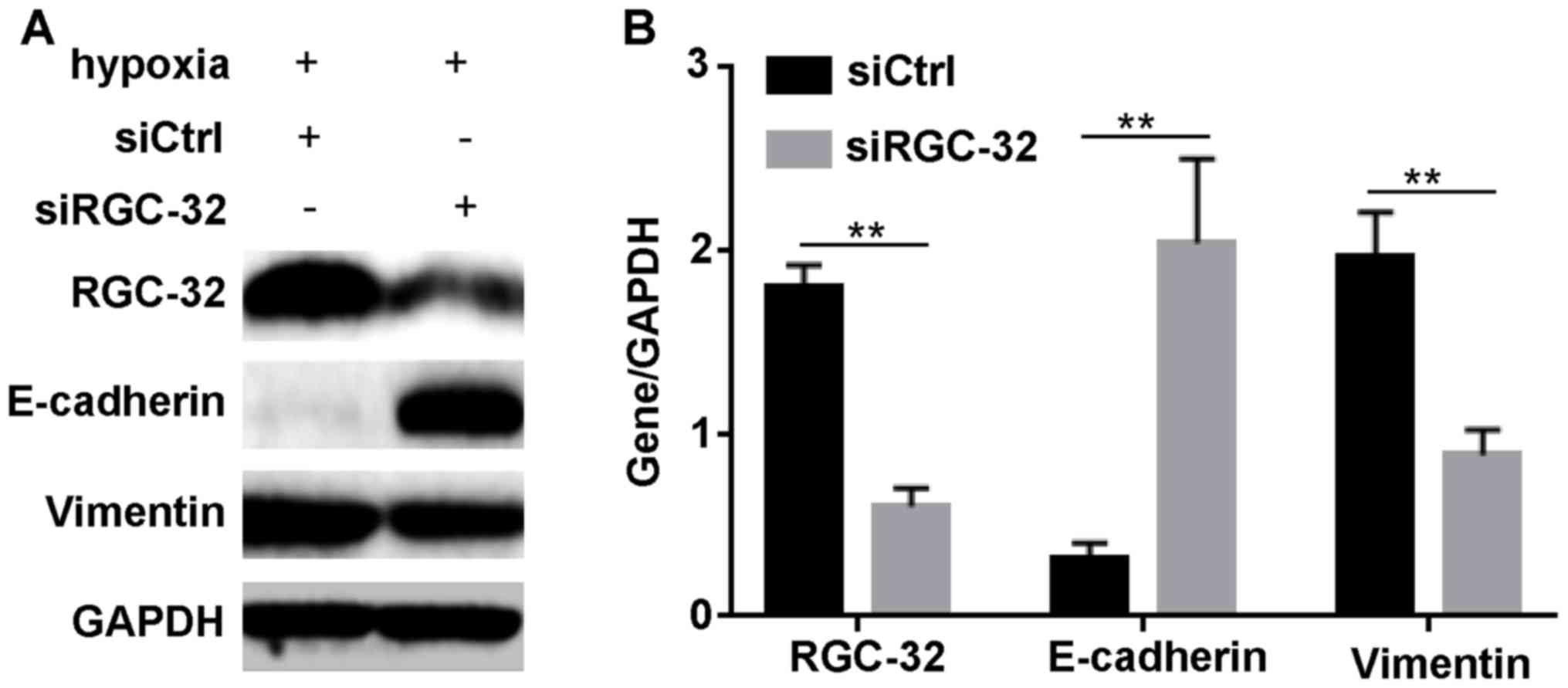
Inhibition of RGC-32 modulates
expression levels of EMT-related proteins
According to the aforementioned results, it was
hypothesized that HIF-1α regulated BxPC-3 cell EMT through RGC-32.
To validate this hypothesis, the effect of the inhibition of RGC-32
on the expression of EMT-associated proteins after hypoxia
induction was examined. As demonstrated in Fig. 4, RGC-32 was successfully diminished
by siRGC-32 under hypoxia. Compared with siCtrl incubation,
siRGC-32 transfection significantly increased the expression of
epithelial marker, E-cadherin (P<0.01); however, it
significantly diminished the hypoxia-induced changes in the
interstitial marker, vimentin (P<0.01). These data indicated
that RGC-32 regulated the expression of EMT markers under
hypoxia.
Discussion
Pancreatic cancer is a human malignancy with one of
the highest mortality rates and little progress has been achieved
in its treatment in recent decades. The molecular mechanism
underlying HIF-1α and RGC-32 function in hypoxia-induced EMT
remains largely unknown. The present study investigated the role of
HIF-1α in hypoxia-induced EMT by siRNA transfection in human
pancreatic cell line BxPC-3. Repression of HIF-1α modulated the
expression of EMT-related proteins under hypoxia induction. In
addition, knockdown of RGC-32 upregulated E-cadherin and
downregulated vimentin. Therefore, the upregulation of HIF-1α
induced by hypoxia increased the expression of RGC-32 to modulate
the levels of EMT-associated proteins for EMT. These results
indicated the function of RGC-32 in hypoxia-induced EMT.
HIF-1 acts as a master regulator of oxygen-regulated
gene expression in response to hypoxia (23). Under hypoxia, HIF-1α homodimerizes
with HIF-1b to mediate nuclear translocation and to activate the
expression of target genes by binding to hypoxic responsive
elements in the promoter regions (24). The high expression of HIF-1α was
reported to correlate with tumor metastasis and poor clinical
outcomes (13). Research has
demonstrated that hypoxia induced the expression of HIF-1α to
activate the process of EMT (25). A
variety of biomarkers have been used to demonstrate EMT, such as
epithelial markers (E-cadherin and cytokeratin) and mesenchymal
markers [N-cadherin, vimentin, fibronectin and α smooth muscle
actin (α-SMA)] (26). In human lens
epithelial cells, the inhibition of HIF-1α downregulated the
expression of two EMT early markers, fibronectin and α-SMA
(27). In follicular thyroid cancer
FTC133 cells, hypoxia induced HIF-1α expression was demonstrated to
regulate the Twist signal and to induce EMT (17). Hypoxia induced EMT in mesothelial
cells occurs via the activation HIF-1α and regulation of the
expression of E-cadherin and vimentin (28). In the present study, it was indicated
that the inhibition of HIF-1α by HIF-1α siRNA or HIF-1α inhibitor
impaired the upregulation of RGC-32, N-cadherin and vimentin
induced by hypoxia, and increased the expression of E-cadherin and
cytokeratins, which were similar to the previous reports. These
findings revealed the mechanism of HIF-1α involvement in
hypoxia-induced EMT and provided novel insight into a possible
therapeutic strategy to prevent hypoxia-induced EMT in pancreatic
cancer.
TGF-β and hypoxia are believed to be the two major
inducers of EMT (29). RGC-32 was
revealed to be involved in the TGF-β-induced EMT process for tumor
invasion (11). However, little was
revealed about the function of RGC-32 in hypoxia-induced EMT in
tumor cells. In the present study, it was demonstrated that hypoxia
induced the expression of RGC-32 and the silencing of HIF-1α
suppressed the expression of RGC-32 activated by hypoxia in BxPC-3
cells. These data were similar to a previous study, which indicated
that HIF-1α and vascular endothelial growth factor significantly
increased RGC-32 expression in hypoxia and ischemia (30). In addition, the present study
indicated that the knockdown of RGC-32 significantly inhibited the
expression of vimentin and upregulated E-cadherin under hypoxia. In
BxPC-3 cells, TGF-β stimuli upregulated RGC-32 expression to
suppress the level of E-cadherin for EMT (11). It was believed that RGC-32 was
involved in hypoxia-induced EMT. As RGC-32 participated in hypoxia
and TGF-β induced EMTs, RGC-32 was able to provide a novel link
about the study on the relationship between two types of EMT.
In conclusion, the present study demonstrated that
RGC-32, as a downstream gene of HIF-1α, induced by hypoxia, is a
promoter of hypoxia-induced EMT in human pancreatic cancer BxPC-3
cells. RGC-32 activates hypoxia-induced EMT through mediating the
expression of EMT-related proteins. Therefore, RGC-32 may be a
potential therapeutic target for the treatment of pancreatic
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81302152) and the
Project of Health and Family Planning Commission of Jiangxi
Province (grant no. 20155207).
Glossary
Abbreviations
Abbreviations:
|
EMT
|
epithelial-mesenchymal transition
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
RGC-32
|
response gene to complement 32
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
TGF-β
|
transforming growth factor-β
|
References
|
1
|
Muniraj T, Jamidar PA and Aslanian HR:
Pancreatic cancer: A comprehensive review and update. Dis Mon.
59:368–402. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keane MG, Bramis K, Pereira SP and Fusai
GK: Systematic review of novel ablative methods in locally advanced
pancreatic cancer. World J Gastroenterol. 20:2267–2278. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan YL, Zheng M, Tang YL and Liang XH: A
new perspective of vasculogenic mimicry: EMT and cancer stem cells
(Review). Oncol Lett. 6:1174–1180. 2013.PubMed/NCBI
|
|
4
|
Lima J Felipe, Nofech-Mozes S, Bayani J
and Bartlett JM: EMT in breast carcinoma-A review. J Clin Med.
5(pii): E652016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogers CD, Saxena A and Bronner ME: Sip1
mediates an E-cadherin-to-N-cadherin switch during cranial neural
crest EMT. J Cell Biol. 203:835–847. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Badea TC, Niculescu FI, Soane L, Shin ML
and Rus H: Molecular cloning and characterization of RGC-32, a
novel gene induced by complement activation in oligodendrocytes. J
Biol Chem. 273:26977–26981. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Badea T, Niculescu F, Soane L, Fosbrink M,
Sorana H, Rus V, Shin ML and Rus H: RGC-32 increases p34CDC2 kinase
activity and entry of aortic smooth muscle cells into S-phase. J
Biol Chem. 277:502–508. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fosbrink M, Cudrici C, Niculescu F, Badea
TC, David S, Shamsuddin A, Shin ML and Rus H: Overexpression of
RGC-32 in colon cancer and other tumors. Exp Mol Pathol.
78:116–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vogelmann R, Nguyen-Tat MD, Giehl K, Adler
G, Wedlich D and Menke A: TGFbeta-induced downregulation of
E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN.
J Cell Sci. 118:4901–4912. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang WY, Li ZG, Rus H, Wang X, Jose PA
and Chen SY: RGC-32 mediates transforming growth
factor-beta-induced epithelial-mesenchymal transition in human
renal proximal tubular cells. J Biol Chem. 284:9426–9432. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu L, Qin H, Li PY, Xu SN, Pang HF, Zhao
HZ, Li DM and Zhao Q: Response gene to complement-32 enhances
metastatic phenotype by mediating transforming growth factor
beta-induced epithelial-mesenchymal transition in human pancreatic
cancer cell line BxPC-3. J Exp Clin Cancer Res. 31:292012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen X, Xue Y, Si Y, Wang Q, Wang Z, Yuan
J and Zhang X: The unfolded protein response potentiates
epithelial-to-mesenchymal transition (EMT) of gastric cancer cells
under severe hypoxic conditions. Med Oncol. 32:4472015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ackerman D and Simon MC: Hypoxia, lipids,
and cancer: Surviving the harsh tumor microenvironment. Trends Cell
Biol. 24:472–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choudhry H, Albukhari A, Morotti M, Haider
S, Moralli D, Smythies J, Schödel J, Green CM, Camps C, Buffa F, et
al: Tumor hypoxia induces nuclear paraspeckle formation through
HIF-2α dependent transcriptional activation of NEAT1 leading to
cancer cell survival. Oncogene. 34:45462015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang J, Tang YL and Liang XH: EMT: A new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zepeda AB, Pessoa A Jr, Castillo RL,
Figueroa CA, Pulgar VM and Farias JG: Cellular and molecular
mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell
Biochem Funct. 31:451–459. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang YJ, Na HJ, Suh MJ, Ban MJ, Byeon HK,
Kim WS, Kim JW, Choi EC, Kwon HJ, Chang JW and Koh YW: Hypoxia
induces epithelial-mesenchymal transition in follicular thyroid
cancer: Involvement of regulation of twist by hypoxia inducible
factor-1α. Yonsei Med J. 56:1503–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weidemann A and Johnson RS: Biology of
HIF-1alpha. Cell Death Differ. 15:621–627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang F, Yin J, Lu Z, Zhang G, Li J, Xing
T, Zhuang S and Wang N: Limb ischemic preconditioning protects
against contrast-induced nephropathy via renalase. EBioMedicine.
9:356–365. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang F, Zhang G, Xing T, Lu Z, Li J, Peng
C, Liu G and Wang N: Renalase contributes to the renal protection
of delayed ischaemic preconditioning via the regulation of
hypoxia-inducible factor-1α. J Cell Mol Med. 19:1400–1409. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tian Q, Xue Y, Zheng W, Sun R, Ji W, Wang
X and An R: Overexpression of hypoxia-inducible factor 1α induces
migration and invasion through Notch signaling. Int J Oncol.
47:728–738. 2015.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Semenza GL: Oxygen sensing,
hypoxia-inducible factors, and disease pathophysiology. Annu Rev
Pathol. 9:47–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao T, Li JZ, Lu Y, Zhang CY, Li Q, Mao J
and Li LH: The mechanism between epithelial mesenchymal transition
in breast cancer and hypoxia microenvironment. Biomed Pharmacother.
80:393–405. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balamurugan K: HIF-1 at the crossroads of
hypoxia, inflammation, and cancer. Int J Cancer. 138:1058–1066.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cammarata PR, Neelam S and Brooks MM:
Inhibition of hypoxia inducible factor-1α downregulates the
expression of epithelial to mesenchymal transition early marker
proteins without undermining cell survival in hypoxic lens
epithelial cells. Mol Vis. 21:1024–1035. 2015.PubMed/NCBI
|
|
28
|
Morishita Y, Ookawara S, Hirahara I, Muto
S and Nagata D: HIF-1α mediates Hypoxia-induced
epithelial-mesenchymal transition in peritoneal mesothelial cells.
Ren Fail. 38:282–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heldin CH, Landström M and Moustakas A:
Mechanism of TGF-beta signaling to growth arrest, apoptosis, and
epithelial-mesenchymal transition. Curr Opin Cell Biol. 21:166–176.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
An X, Jin Y, Guo H, Foo SY, Cully BL, Wu
J, Zeng H, Rosenzweig A and Li J: Response gene to complement 32, a
novel hypoxia-regulated angiogenic inhibitor. Circulation.
120:617–627. 2009. View Article : Google Scholar : PubMed/NCBI
|