Introduction
Tendon repair is an important step in hand surgery
and other types of plastic surgery. For a long time period
post-surgery, tendons cannot be subjected to strain and their
biomechanical properties are aberrant; micro tears, which may
ultimately lead to full-thickness injury of tendon, can be
characterized by microscopy (1,2). Common
tendon injuries include the Achilles tendon, rotator cuff tendon
and elbow lateral condyle, and due to their similar features and
poor prognosis, numerous studies have assessed strategies to
promote tendon healing, mainly including platelet-rich plasma,
whole-blood injections, growth hormones and stem cells (3). Tendon healing after injury is usually
affected by inflammation, collagen production and reshaping of. In
the early stages after tendon damage, mononuclear cells and
macrophages are activated, which release biomolecular factors
promoting tendon cell proliferation and increase in collagen
synthesis; these processes occurring as early as possible may
provide sufficient mechanical strength to enable patients to
perform basic activities. Studies have shown that early activity is
of great significance for tendon performance and associated
prognosis; early healing after tendon injury is therefore key
(4–6). Tendon-derived extracellular matrix
hydrogels containing connective tissue-specific proteins, including
collagen I, can deliver tendon cells to the damage location and
provide an environment supporting cell proliferation (7,8).
Previous studies on rats showed that subcutaneous hydrogel
injection was well tolerated with no nodules or allergic reaction
and gradual degradation after 4 weeks (7,8). Based
on these preliminary studies, the present study explored the
ability of a tendon-derived hydrogel to promote tendon healing in
rats after injury. The present study paved the road for the
implementation of hydrogels for tendon healing in clinical
practice.
Materials and methods
Tissue decellularization
The musculi flexor digitorum profundus, musculus
flexor digitorum superficialis and flexor pollicis longus tendon
were obtained from 36 rats (male, average weight, 350 g, 6–8 weeks)
cadavers frozen directly after sacrifice. This study was approved
by the ethics committee of the Ninth People's Hospital of Wuxi
City, Wuxi, China. According to a previously described method
(8), the epitenon, vaginae
synoviales and musculature were extracted. The superficial flexor
tendon was cut near the bifurcation at 2 cm from the end. The
musculus flexor digitorum superficialis and flexor pollicis longus
tendon were cut off near the bone tendon junction at 1 cm from the
end. The tendons obtained were subjected to acellular treatment and
subsequently immersed in 0.1% EDTA solution for 4 h (8). The tendons were then incubated in 0.1%
lauryl sodium sulfate and 0.1% EDTA solution at room temperature
for 24 h with stirring, followed by washing with phosphate buffer.
The tendons were then frozen at −80°C. The acellular tendon tissue
was then freeze-dried, ground into powder and kept at 4°C until
use.
Preparation of tendon matrix
hydrogel
The tendon powder was incubated with 1 mg/ml pepsin
(Sigma-Aldrich, Merck KGaG, Darmstadt, Germany), 0.02M hydrochloric
acid and aquae sterilisata (pH 2.2; powder concentration, 20 mg/ml)
to digest the extracellular matrix. Following incubation at room
temperature for 24 h with constant stirring, the sample was placed
on ice and sodium carbonate hydrate was added to increase the pH to
8.0 to inactivate pepsin. Finally, 10 volumes of phosphate-buffered
saline (PBS, adjusting pH 7.4) were added to obtain an isoosmotic
solution.
Operative procedure and tendon
treatment
A total of 36 Wistar rats (male, average weight, 350
g, 6–8 weeks) were obtained from the animal center of Ninth
People's Hospital, and were fed at room temperature and 40–50%
humidity, and with 12-h light/dark, and ad libitum access to
food and water. The rats were fixed in the supine position after
being anaesthetized by 2% isoflurane (Sigma-Aldrich; Merck KGaG).
This study was approved by the Ethics Committee of the Ninth
People's Hospital of Wuxi City (Wuxi, China). Following
anti-bacterial treatment with buprenorphine and enrofloxacin
(Sigma-Aldrich; Merck KGaG), a 1-cm curve cut was made around the
Achilles tendon to ensure the completeness of the tendon. The
tendon was carefully separated to avoid damaging the nervus
cutaneus surae medialis and major vessels. Cyanoacrylate was used
to bond two #15 surgical knives together to keep the incision
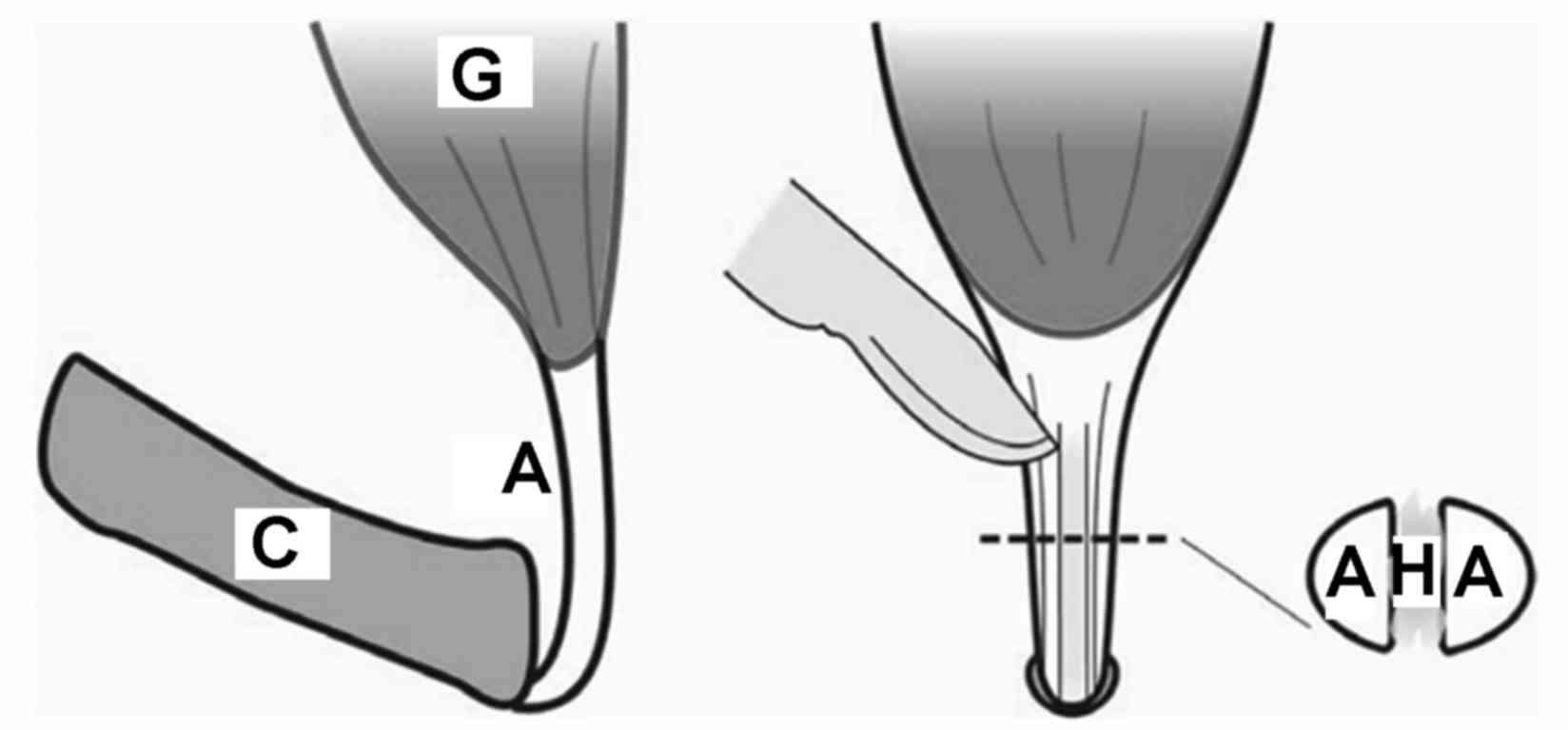
parallel. Under a microscope, a damaged area of 0.5 mm in width and
5 mm in length was generated, reaching from the calcaneus to the
central tendon of the bilateral lower extremities of each rat
(Figs. 1 and 2). The site of injury on the left side was
injected with 0.05 ml tendon hydrogel, while the right-hand side
was injected with 0.05 ml phosphate-buffered saline as the control.
The wound was then sutured with #4 absorb string (Ethicon, Inc.,
Somerville, NJ, USA). According to the findings of a preliminary
experiment (8), animals were
anaesthetized using 1 ml 2% isoflurane (20 mg/rat; Sigma-Aldrich;
Merck KGaG) and then were sacrificed by decapitation at 2, 4 and 8
weeks after the surgery to obtain the tendons. A total of 10 tendon
specimens were used for biomechanical performance tests at each
time-point and 2 specimens were used for histopathological
analysis.
Biomechanical performance test
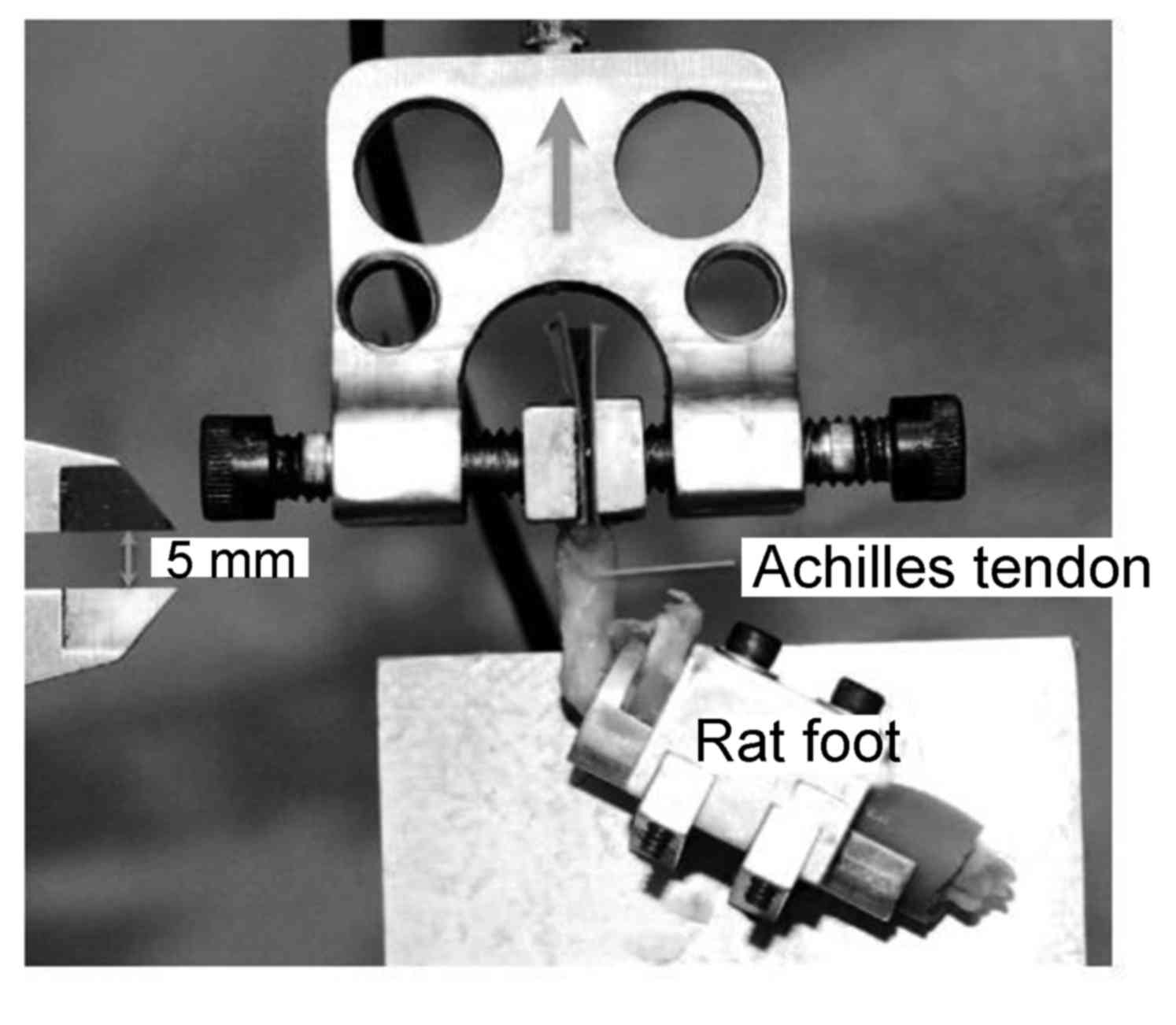
The mini Bionix tset system (MTS Systems Corp., Eden
Prairie, MN, USA) was used as a test load cell. From the rat feet
obtained, the muscle was removed to expose the tendon junction. The
foot was fixed, and abrasive paper and cyanoacrylate were used to
fix the muscle-tendon junction (Fig.
3). Orthogonal images were captured with a digital camera (8.2
megapixels; Canon EOS 30D; Canon, Tokyo, Japan) and a digital
caliper was used to determine the size of the tendon. Image J
software version 2.0 (National Institutes of Health, Bethesda, MD,
USA) was used to calculate the cross-sectional area and ultimate
tensile stress of each tendon. At a tensile speed of 0.5 mm/sec,
the load was increased until the destruction of the tendon and the
ultimate failure load was recorded. The ultimate tensile stress was
determined by limiting load and cross-sectional area (8). Tendon toughness was evaluated via a
force/displacement curve.
Histopathological analysis
The obtained tendon tissue was fixed for 10 min in
10% formalin (Sigma-Aldrich; Merck KGaG) at room temperature and
embedded in ceresin wax and then cut into slices (6 µm). The
morphological characteristics of the tendons were evaluated by
hematoxylin and eosin staining. Adjacent sections were stained with
Sirius red solution (Sigma-Aldrich; Merck KGaG) to determine the
content of type-I and type-III collagen protein. Images were
captured under a fluorescence microscope (Model: D-35578; Leica
Microsystems, Wetzlar, Germany).
Statistical analysis
All of the data were analyzed by using the SPSS
software 18.0 (SPSS Inc., Chicago, IL, USA). Comparisons among
groups were performed by the paired Student's t-test. Values are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
Biomechanical test
For every biomechanical test, one rat tendon was
rolled out from the forceps holder. The connection of the tendon
muscle was broken, so that it was excluded. The results showed that
2 weeks after the operation, almost all tendon rupture positions
were located in the tendon itself. By contrast at 4 and 8 weeks
post-surgery, ruptures of the muscle-tendon junction were common
(Table I). At 2 weeks after the
operation, no obvious difference in ultimate failure load, ultimate
tensile stress and toughness was observed between the two groups
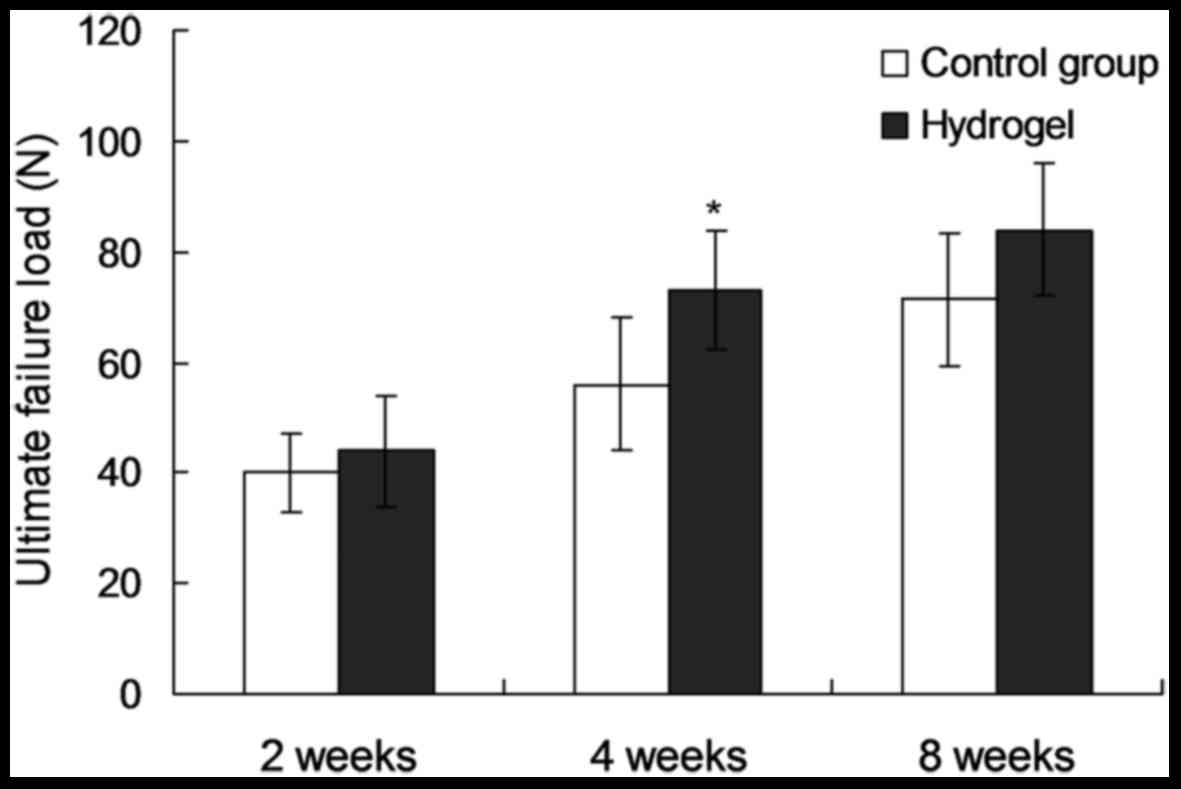
(P=0.151, 0.424 and 0.760, respectively). At 4 weeds after the
operation, the ultimate failure load in the experimental group was
increased by 28% of that of the control group (74.8±11.6 vs.
58.4±14.2 N; P=0.016; Fig. 4), while
the ultimate tensile stress and toughness were not significantly
different between the two groups (P=0.634 and 0.082, respectively).
At 8 weeks post-surgery, the ultimate failure load, ultimate
tensile stress and toughness did not show any significantly
differences between the two groups (P=0.156, 0.385 and 0.748,
respectively).
 | Table I.Results of biomechanical tests. |
Table I.
Results of biomechanical tests.
| Group | Treatment | Mechanical
tests/tissue analyses (n) | Ultimate failure load
(N) | Ultimate tensile
stress (N/mm2) | Toughness (N/mm) | Tendon damage
location |
|---|
| 1 | 2 w, hydrogel | 4/1 |
45.2±8.6 |
1.6±0.6 |
11.4±3.7 | 1TBI/8T/0B |
|
| 2 w, no hydrogel | 9/2 |
39.8±6.3 |
1.4±0.2 |
10.9±2.7 | 2TBI/7T/0B |
| 2 | 4 w, hydrogel | 9/2 |
74.8±11.6a |
3.0±0.7 |
17.9±3.5 | 5TBI/4T/0B |
|
| 4 w, no hydrogel | 9/2 |
58.4±14.2a |
2.8±1.0 |
14.7±3.9 | 7TBI/2T/0B |
| 3 | 8 w, hydrogel | 9/2 |
83.5±13.7 |
2.9±0.7 |
17.7±2.7 | 5TBI/4T/0B |
|
| 8 w, no hydrogel | 9/2 |
74.0±13.2 |
2.6±0.7 |
17.3±4.5 | 6TBI/3T/0B |
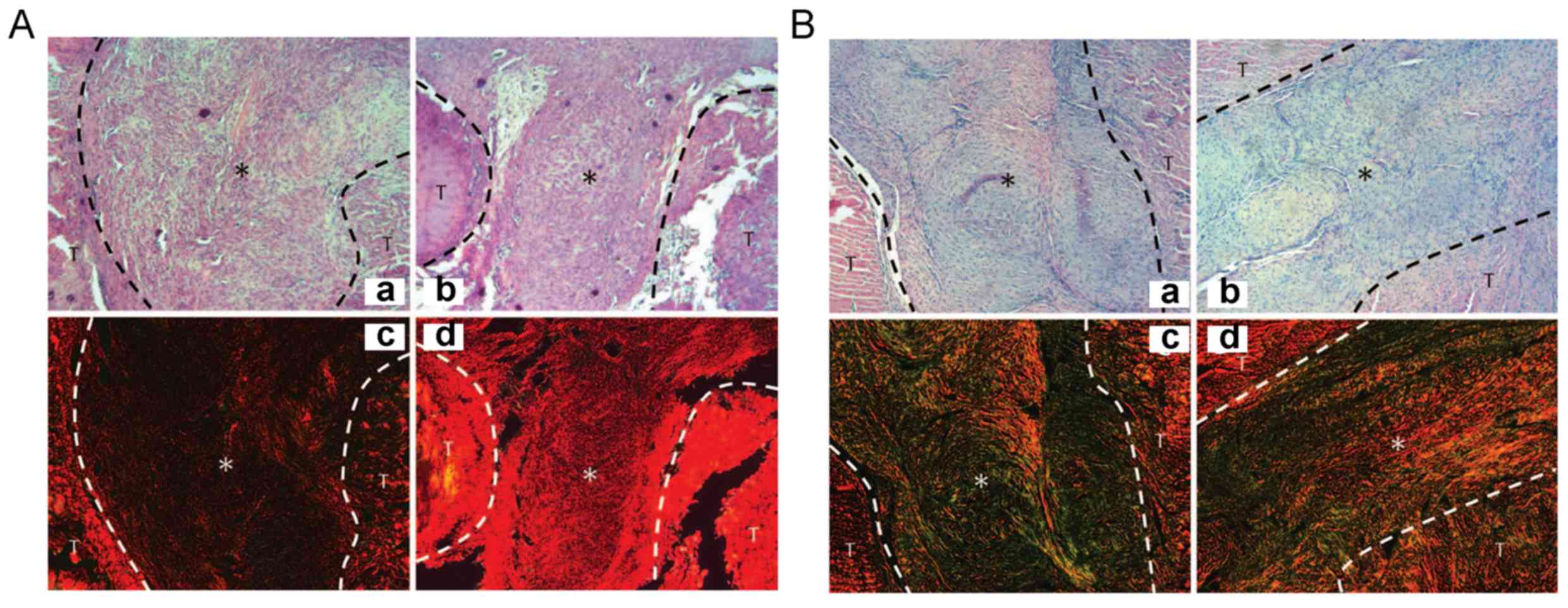
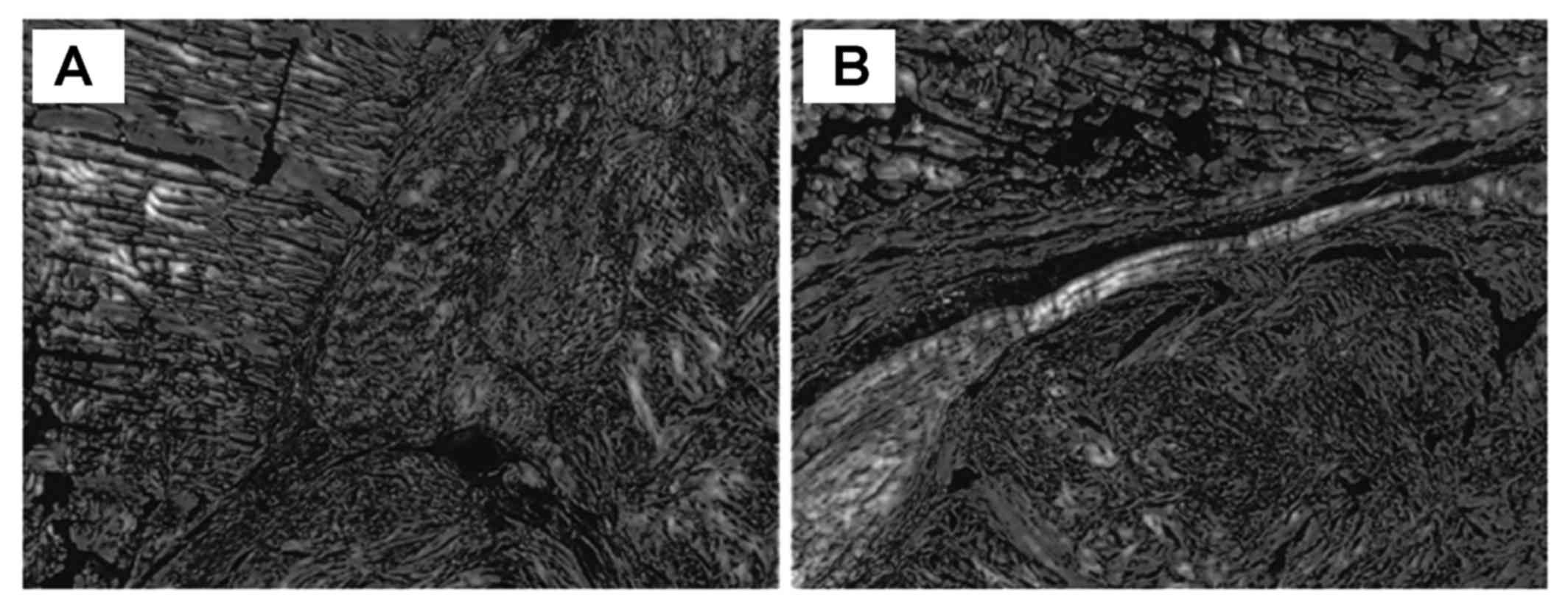
Histopathological analysis
At 2 and 4 weeks after the operation, the
cross-section of the tendons of the two groups was almost
identical. In the hydrogel-treated group, the collagen aggregation
in the injury position was visible (Figs. 5 and 6). At 2 weeks post-surgery, Sirius red
staining showed that the type I collagen content in the
experimental group was obviously higher than that in the control
group (Fig. 5A). At week 4, the
difference in collagen content in hydrogel group was more
significant compared to control group, which suggests that hydrogel
applied immediately after tendon injury can enhance the type-I
collagen fibers (P<0.05, Fig.
5B). Meanwhile, type III collagen protein (green and yellow) in
the control group was obvious higher than that in the experimental
group (P<0.05, Fig. 5).
Furthermore, type I collagen protein (red) in the experimental
group was higher than that in the control (P<0.05, Fig. 5). At 8 weeks, no obvious difference
was found in the collagen content between the two groups (Fig. 6).
Discussion
The results of the present study confirmed the
former hypothesis that a tendon-derived extracellular matrix
hydrogel can improve tissue regeneration and promote tendon
healing. The use of tissue-derived hydrogel has been previously
reported. Ventricle-derived extracellular matrix hydrogel has been
applied in a model of cardiac damage, which improved the repair of
myocardial cells and local metabolism, and skeletal muscle-derived
extracellular matrix hydrogel has been applied in a model hindlimb
ischemia which enhanced the distribution of skeletal muscle cells
along the neovascularization (8,9).
However, collagen hydrogel synthesized in vitro did not
obviously improve the healing of tendon injury (10). In the present study, a hydrogel not
only containing the matrix component of tendons, but also its
proteins and biological factors, was prepared and applied. To the
best of our knowledge, the present study was the first to prepare
tendon matrix hydrogels and apply them to an animal model of tendon
injury.
The biocompatibility of the extracellular matrix
hydrogel has been shown to be good with degradation occurring after
4 weeks. The results of the present study showed that extracellular
matrix hydrogel provides a suitable environment for cell metabolism
and healing. For instance, strengthening of the phagocytic function
of macrophages is beneficial for tissue remodeling (9). In the first stage of tendon healing,
macrophages destroy and take up necrotic tissue, and release
extracellular matrix, oligosaccharide derivatives and other
factors. Macrophages can be used as a vasoactive and chemotactic
factor to promote the aggregation of stem and progenitor cells.
Furthermore, collagen synthesis is increased in the early stages of
injury to repair damaged tissue (11). Certain studies have indicated that
weakening of the tendon strength during the healing process is due
to the natural collagen protein I being replaced by the thinner
type III collagenous fibers (12,13).
Sirius red staining at 4 weeks post-surgery showed that in the
hydrogel group, the ratio of type I vs. type III collagen was
obviously higher than that in the control group, which explains for
the higher ultimate failure load in the hydrogel group. The type I
collagen content was therefore consistent with the mechanical
strength of the tendon. 4 weeks later, the hydrogel-treated tendon
was thicker and more solid in hydrogel group (one animal) and the
intersecting surface was greater compared to the control group. The
toughness of the tendon arises from cross-linked collagenous fibers
in the final stage of healing (14).
Therefore, in the present study, not all of the differences were
significant at different time-point (such as 4 and 8 weeks). The
effect of the hydrogel was mainly shown in the early stages of
injury and the hydrogel, as it was degraded after 4 weeks.
Therefore, its effect of promoting tissue repair gradually
decreased with time. The operation wound in the two groups was
still visible at 8 weeks and the scars were covered by type I
collagen, however, only one animal was assessed. The results
indicated that the process of tissue repair and remodeling was
almost finished at 8 weeks after tendon injury.
In clinical practice, the main application
advantages of hydrogel may be that it accelerates the healing
process of tendon injury in the early stage, enabling patients to
perform early functional exercise. Numerous studies have shown that
application of an appropriate mechanical force can promote
fibroblast proliferation and the synthesis and arrangement of the
original collagen protein (15,16). The
hydrogel can be gathered at the physiological temperature, so that
the targeted application to part of the tendon injury can be used
by minimally invasive injection to improve the prognosis.
Furthermore, substances including growth factors, platelet-rich
plasma and stem cells can be mixed with hydrogel for injection; the
efficacy of such preparations requires evaluation by further
studies.
Of note, the present study had certain limitations.
Sirius red staining used for the quantitative assessment of
collagen content only provided one possible explanation for the
differences in tendon strength demonstrated by the biomechanical
test. However, the findings only provided limited information, as
only one rat per group was used for histopathological analysis at
each time-point. In further studies, analysis of changes in
structure, collagen synthesis and content of associated biological
factors will be helpful to confirm the effect of hydrogel on tendon
healing. In the present study, a rat model of tendon injury was
used, while there may be differences among various injury sites and
species. Furthermore, an animal experiment using a larger sample is
required for further comparison and confirmation of the
results.
In conclusion, the present study showed that a
tendon-derived extracellular matrix hydrogel was able to
significantly improve the ultimate failure load of injured tendons
from rats at 4 weeks after surgery. It was therefore suggested that
the hydrogel may promote tendon healing. The present study paved
the road for the development of a novel preparation for the
clinical treatment of tendon injuries.
References
|
1
|
Yu YD, Zhang YZ, Bi WD and Wu T:
Functional sensory function recovery of random-pattern abdominal
skin flap in the repair of flingertip skin defects. Exp Ther Med.
5:830–834. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Neviaser A, Andarawis-Puri N and Flatow E:
Basic mechanisms of tendon fatigue damage. J Shoulder Elbow Surg.
21:158–163. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma P and Maffulli N: Biology of tendon
injury: Healing, modeling and remodeling. J Musculoskelet Neuronal
Interact. 6:181–190. 2006.PubMed/NCBI
|
|
4
|
Longo UG, Lamberti A, Maffulli N and
Denaro V: Tissue engineered biological augmentation for tendon
healing: A systematic review. Br Med Bull. 98:31–59. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Beck J, Evans D, Tonino PM, Yong S and
Callaci JJ: The biomechanical and histologic effects of
platelet-rich plasma on rat rotator cuff repairs. Am J Sports Med.
40:2037–2044. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Virchenko O and Aspenberg P: How can one
platelet injection after tendon injury lead to a stronger tendon
after 4 weeks? Interplay between early regeneration and mechanical
stimulation. Acta Orthop. 77:806–812. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sato D, Takahara M, Narita A, Yamakawa J,
Hashimoto J, Ishikawa H and Ogino T: Effect of platelet-rich plasma
with fibrin matrix on healing of intrasynovial flexor tendons. J
Hand Surg Am. 37:1356–1363. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kampa RJ and Connell DA: Treatment of
tendinopathy: Is there a role for autologous whole blood and
platelet rich plasma injection? Int J Clin Pract. 64:1813–1823.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Andersson T, Eliasson P and Aspenberg P:
Growth hormone does not stimulate early healing in rat tendons. Int
J Sports Med. 33:240–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ngo M, Pham H, Longaker MT and Chang J:
Differential expression of transforming growth factor-beta
receptors in a rabbit zone II flexor tendon wound healing model.
Plast Reconstr Surg. 108:1260–1267. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chong AK, Ang AD, Goh JC, Hui JH, Lim AY,
Lee EH and Lim BH: Bone marrow-derived mesenchymal stem cells
influence early tendon-healing in a rabbit achilles tendon model. J
Bone Joint Surg Am. 89:74–81. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okamoto N, Kushida T, Oe K, Umeda M,
Ikehara S and Iida H: Treating Achilles tendon rupture in rats with
bone-marrow-cell transplantation therapy. J Bone Joint Surg Am.
92:2776–2784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma P and Maffulli N: Tendon injury and
tendinopathy: Healing and repair. J Bone Joint Surg Am. 87:187–202.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huss FR, Nyman E, Bolin JS and Kratz G:
Use of macroporous gelatine spheres as a biodegradable scaffold for
guided tissue regeneration of healthy dermis in humans: An in vivo
study. J Plast Reconstr Aesthet Surg. 63:848–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wolf MT, Daly KA, Brennan-Pierce EP,
Johnson SA, Carruthers CA, D'Amore A, Nagarkar SP, Velankar SS and
Badylak SF: A hydrogel derived from decellularized dermal
extracellular matrix. Biomaterials. 33:7028–7038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singelyn JM, Dequach JA, Seif-Naraghi SB,
Littlefield RB, Schup-Magoffin PJ and Christman KL: Naturally
derived myocardial matrix as an injectable scaffold for cardiac
tissue engineering. Biomaterials. 30:5409–5416. 2009. View Article : Google Scholar : PubMed/NCBI
|