Introduction
Myocardial ischemia-reperfusion injury (MIRI) refers
to the damage to the myocardium caused by both the ischemia and the
restoration of blood flow into the previously ischemic tissue.
After reperfusion, the myocardial ultrastructure, function,
metabolism and electrophysiological characteristics are further
damaged, worsening the patients' condition (1). MIRI can cause irreversible damage to
myocardial cells and reduce cell activity. Clinical manifestations
of MIRI include sudden drop in blood pressure, heart rate disorders
and even sudden death (2). The
mechanisms of MIRI include an oxygen free radical (ROS) balance
disorder, calcium overload, microvascular injury and leukocyte
activation. The prevention and treatment of these pathological
changes involve anti-oxidative therapy, calcium overload reducing
agents and ischemic preconditioning (3). Recent studies have shown that ischemic
post-conditioning has a protective effect upon MIR. Due to its
benefits, ischemic post-conditioning is now widely used in the
clinical practice (4).
Studies have shown that inhalation a certain amounts
of anesthetics before ischemia or at the beginning of reperfusion
can inhibit MIRI, and protect the myocardium (5). Sevoflurane exhibits several advantages
such as short anesthesia induction and recovery time, less
irritation, a high safety profile and adjustable depths of
anesthesia, and is therefore widely used in cardiac surgery.
Studies have shown that sevoflurane can reduce myocardial infarct
size (MIS) and mortality (6). The
mechanism of MIRI inhibition by anesthetics is similar to that of
ischemic preconditioning. Both methods protect the myocardium
through activation of multiple pathways including NF-κB, BPI3K/Akt,
ERK1/2, ROS regulation, mitoKATP channel and mPTP (5–7).
The hypoxia inducible factor 1α (HIF-1α) can induce
the expression of a series of downstream target genes under hypoxic
conditions such as necrosis, ischemia and injury, and these
downstream genes, which are involved in angiogenesis, sugar and
iron metabolism and cell proliferation, play important roles in
apoptosis (8).
In addition, HIF-1α plays a key regulatory role in
the pathogenesis of ischemic heart disease and can inhibit MIS
expansion to improve myocardial function (9). Studies have shown that HIF-1α
overexpression can significantly reduce cell apoptosis, and it
alters cysteinyl aspartate specific protease-3 (caspase-3)
expression, which in turn plays a decisive role in the process of
cell apoptosis. Therefore, IR injury can induce changes in HIF-1α
and caspase-3 expression, and it has been proposed that the
protection effect of sevoflurane on MIRI may be achieved by locally
regulating the expression of HIF-1α and caspase-3 (10). The aim of this study was to
investigate the protection effects of sevoflurane on MIRI and its
effect on the expression of HIF-1α and caspase-3 in a rat model, in
order to evaluate this hypothesis and provide new insights for the
prevention and treatment of MIRI.
Materials and methods
Experimental animals
Forty SPF grade male SD rats (weighing 250–300 g)
were purchased from Shanghai Experimental Animal Center of the
Chinese Academy of Science (Shanghai, China). The rats were
randomly divided into four groups (each with 10 rats) including a
sham operation group (Sham), an ischemia-reperfusion group (IR), a
sevoflurane preconditioning group (Sevo-Pre) and a sevoflurane
post-conditioning group (Sevo-Post). All animal experiments were
carried out strictly in accordance with the guidelines for care and
use of laboratory animals. This study was approved by the Animal
Ethics Committee of Jiangxi Provincial People's Hospital Animal
Center.
Langendorff heart perfusion model
preparation
Rats were anesthetized with 50 mg/kg nembutal sodium
(Sigma, St. Louis, MO, USA), and 1000 U/kg heparin (Aladdin,
Shanghai, China) was used for anticoagulation. After anesthesia,
the rats were fixed in a supine position, the thoracic cavity was
opened and the heart was quickly removed and placed in a pre-cooled
K-H buffer saturated with 95% O2 and 5% CO2
mixed gas. Through aorta retrograde intubation, the heart was
suspended in the Langendorff perfusion device, and perfusion was
performed using K-H buffer at 37°C with 80 mmHg constant pressure.
The left auricle was cut and a latex capsule was inserted into the
left atrium. A balloon catheter was connected to a pressure sensor,
and the MP1000 physiological signal acquisition system (Biopac
Systems, Inc., Goleta, CA, USA) was used to monitor cardiac
function parameters including heart rate (HR), left ventricular
development pressure (LVDP), left ventricular end diastolic
pressure (LVEDP) and the maximum rate of left ventricular (LV)
pressure rise (±dp/dtmax).
Experimental method for isolated heart
perfusion
The hearts in the Sham group were perfused for 150
min. Hearts in the I-R group were subjected to balanced perfusion
for 30 min, ischemia for 30 min and then reperfusion for 90 min.
The Sevo-Pre group hearts were treated with balanced perfusion for
15 min, reperfusion with K-H buffer containing 1 MAC sevoflurane
(Shanghai Hengrui Pharmaceutical, Shanghai, China) for 10 min,
washing out for 5 min, ischemia for 30 min and finally reperfusion
for 90 min. The Sevo-Post group hearts were subjected to balanced
perfusion for 30 min, ischemia for 30 min, next reperfusion with
K-H buffer containing 1 MAC sevoflurane (Shanghai Hengrui
Pharmaceutical) for 10 min, washing out for 5 min and then
reperfusion for 90 min.
MIS determination
After the completion of ischemia and perfusion
experiments, the hearts were quickly removed from the KH solution
and stored in a refrigerator at −20°C. Each heart was transversally
cut along the apex to the bottom, into 5-µm slices, the remaining
tissue was saved for other experiments (in liquid nitrogen). The
slices were then incubated in 0.1 mol/l phosphate buffer containing
1% TTC at 37°C for 20 min in the dark. With this treatment the
living myocardium can be seen to be brick red in color, while the
infarcted myocardium appears greyish-white. The excess dye was
washed out with ultrapure water and the Image-Pro Plus software
(Media Cybernetics, Rockville, MD, USA) was used to calculate the
proportion of MIS to total myocardial area.
Western blotting
After reperfusion, heart tissues were removed from
the liquid nitrogen storage and homogenized with lysing buffer
(Beyotime Biotechnology, Jiangsu, China). The homogenate was then
centrifuged at 4°C to collect the supernatant containing the
soluble fraction. The protein concentration was measured using a
Modified BCA kit (Sangon, Shanghai, China). SDS-PAGE was performed
using 50 µg protein from each sample, followed by transfer to a
PVDF membrane. After blocking with blocking solution at room
temperature for 2 h, each membrane was incubated with primary
rabbit monoclonal HIF-1α antibody (dilution, 1:500; cat. no.
ab51608); rabbit polyclonal caspase-3 antibody (dilution, 1:500;
cat. no. ab13847) and rabbit polyclonal β-actin antibody (dilution,
1:500; cat. no. ab8227) at 4°C overnight. Next day, each membrane
was washed three times with TBST followed by incubation with
secondary goat anti-rabbit (HRP) IgG antibody (dilution, 1:2,000;
cat. no. ab6721) at room temperature for 2 h. All antibodies were
all purchased from Abcam (Cambridge, MA, USA).
Bands were developed using an ECL detection system
(Thermo Fisher Scientific, Waltham, MA, USA), and ImageJ software
(National Institutes of Health, Bethesda, MD, USA) was used to
calculate the gray value of bands with β-actin as endogenous
control, to obtain relative quantities. All antibodies used were
purchased from Cell Signaling Technology, Beverly, MA, USA.
Quantitative real-time polymerase
chain reaction (qRT-PCR)
All reagents used to perform the qRT-PCR (Prime
Script® RT Reagent kit with gDNA Eraser and
SYBR® Premix Ex Taq™ II) were purchased from Takara
(Dalian, Liaoning, China) and manufacturer's instructions were
followed. Briefly, total RNA was extracted from the left
ventricular myocardium after perfusion (TRIzol reagent). cDNA was
synthesized by reverse transcription using 200 ng total RNA, and
the cDNA was used to prepare the real-time PCR reaction system. PCR
reaction was performed using CFX-96 Real-Time PCR Detection System
(Bio-Rad, New York, NY, USA). Data were processed using the
2−∆∆ct method using data from the Sham group as control
group and data from the β-actin gene to normalize the data. All
primers were synthesized by Sangon. Primer sequences are listed in
Table I.
 | Table I.Sequence of primers used in real-time
qPCR. |
Table I.
Sequence of primers used in real-time
qPCR.
| Primer | Sequence (5′-3′) |
|---|
| HIF-1α | F:
GACACCGCGGGCACCGATTC |
|
| R:
TGCTTCGCCGAGATCGTGCTG |
| Bcl-2 | F:
TGAACCGGCATCTGCACAC |
|
| R:
CGTCTTCAGAGACAGCCAGGAG |
| ACTB | F:
CAGGGCGTGATGGTGGGCA |
|
| R:
CAAACATCATCTGGGTCATCTTCTC |
Statistical analysis
The statistical analysis was performed using the
SPSS 17.0 software (SPSS, IL, USA). All experiments were repeated
three times and data were expressed as mean ± SD. Single factor
analysis of variance and two-tailed t-tests were performed for
comparisons between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of sevoflurane treatment on
cardiac function
Through the real-time monitoring of cardiac function
parameters, data were collected at the end of the balanced
perfusion and after 60 min of reperfusion. As shown in Table II, no significant differences were
found in cardiac function at the end of the balanced perfusion
between the groups (P>0.05). However, after 60 min of
reperfusion, the cardiac function was significantly reduced in the
I-R group compared with that in the Sham group (P<0.05),
indicating that MIRI can significantly reduce cardiac function.
Compared with I-R group, the values of HR, LVDP and
±dp/dtmax were significantly increased but the value of
LVEDP was significantly decreased in the Sevo-Pre and Sevo-Post
groups, suggesting that sevoflurane (1 MAC) treatment can
significantly enhance systolic and diastolic function of the
myocardium after reperfusion in rats. There were no significant
differences between Sevo-Pre and Sevo-Post group.
 | Table II.Effect of sevoflurane treatment on
hemodynamic parameters of IR treated hearts. |
Table II.
Effect of sevoflurane treatment on
hemodynamic parameters of IR treated hearts.
| Group |
HR/beats·min−1 | LVDP/kPa | LVEDP/kPa |
+dp/dtmax/kPa·s−1 |
−dp/dtmax/kPa·s−1 |
|---|
| End of balanced
perfusion |
| Sham |
307±13 |
15.7±1.4 |
0.74±0.13 |
436±17 |
338±24 |
| I-R |
310±11 |
15.9±2.0 |
0.79±0.24 |
452±13 |
324±31 |
|
Sevo-Pre |
303±18 |
16.3±1.8 |
0.82±0.11 |
446±21 |
340±17 |
|
Sevo-Post |
310±12 |
15.3±2.2 |
0.77±0.16 |
449±23 |
334±24 |
| 60 min
reperfusion |
| Sham |
298±12 |
16.3±1.5 |
0.78±0.13 |
424±16 |
328±18 |
| I-R |
173±15a |
7.8±2.6a |
4.34±1.35a |
283±12a |
189±23a |
|
Sevo-Pre |
256±13c |
10.2±1.5b |
3.26±0.45b |
326±15b |
223±13b |
|
Sevo-Post |
263±17c |
11.0±1.4b |
3.49±0.56b |
324±21b |
234±17b |
Effect of sevoflurane treatment on
MIS
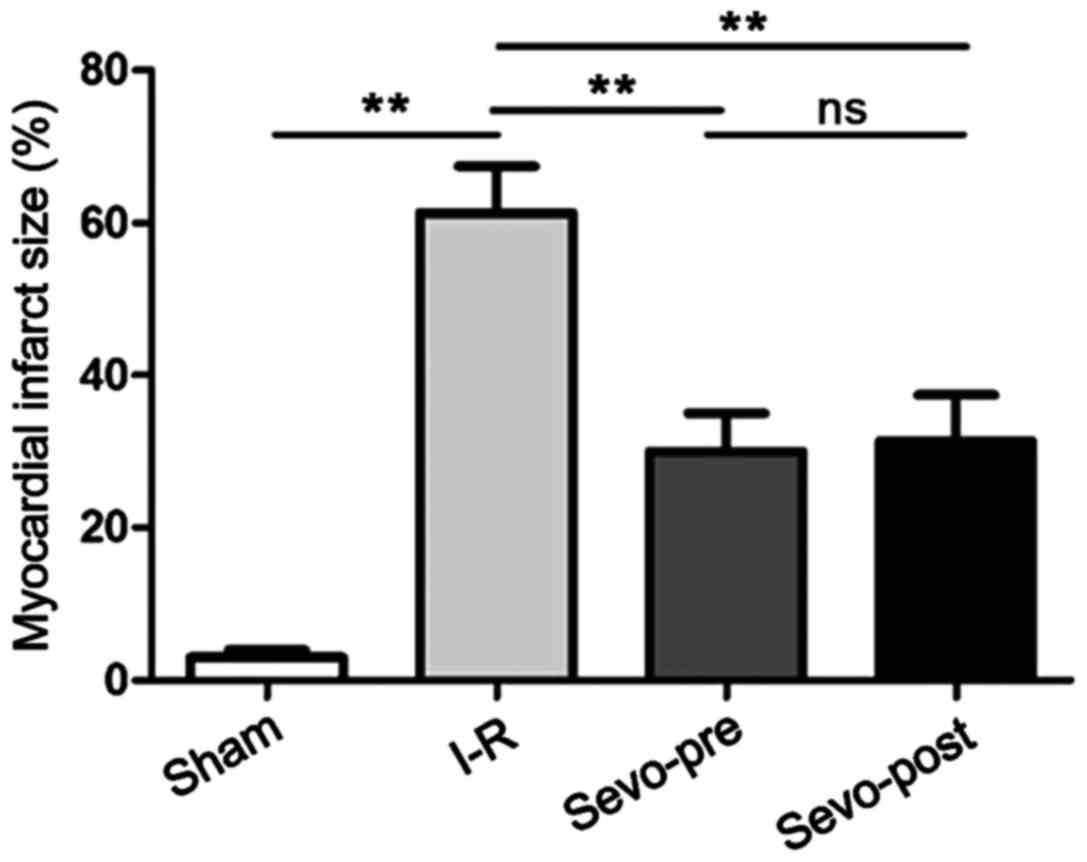
After reperfusion, the MIS of each group was
determined by TTC staining. As shown in Fig. 1, compared with the Sham group, MIS
was significantly increased in the I-R group. Compared with the I-R
group, MIS was significantly decreased in the Sevo-Pre and
Sevo-Post groups, indicating that sevoflurane treatment can
significantly reduce MIS in rats after MIRI. There were no
significant differences between Sevo-Pre and Sevo-Post groups
(P>0.05).
Effect of sevoflurane treatment on the
expression of HIF-1α and caspase-3 protein
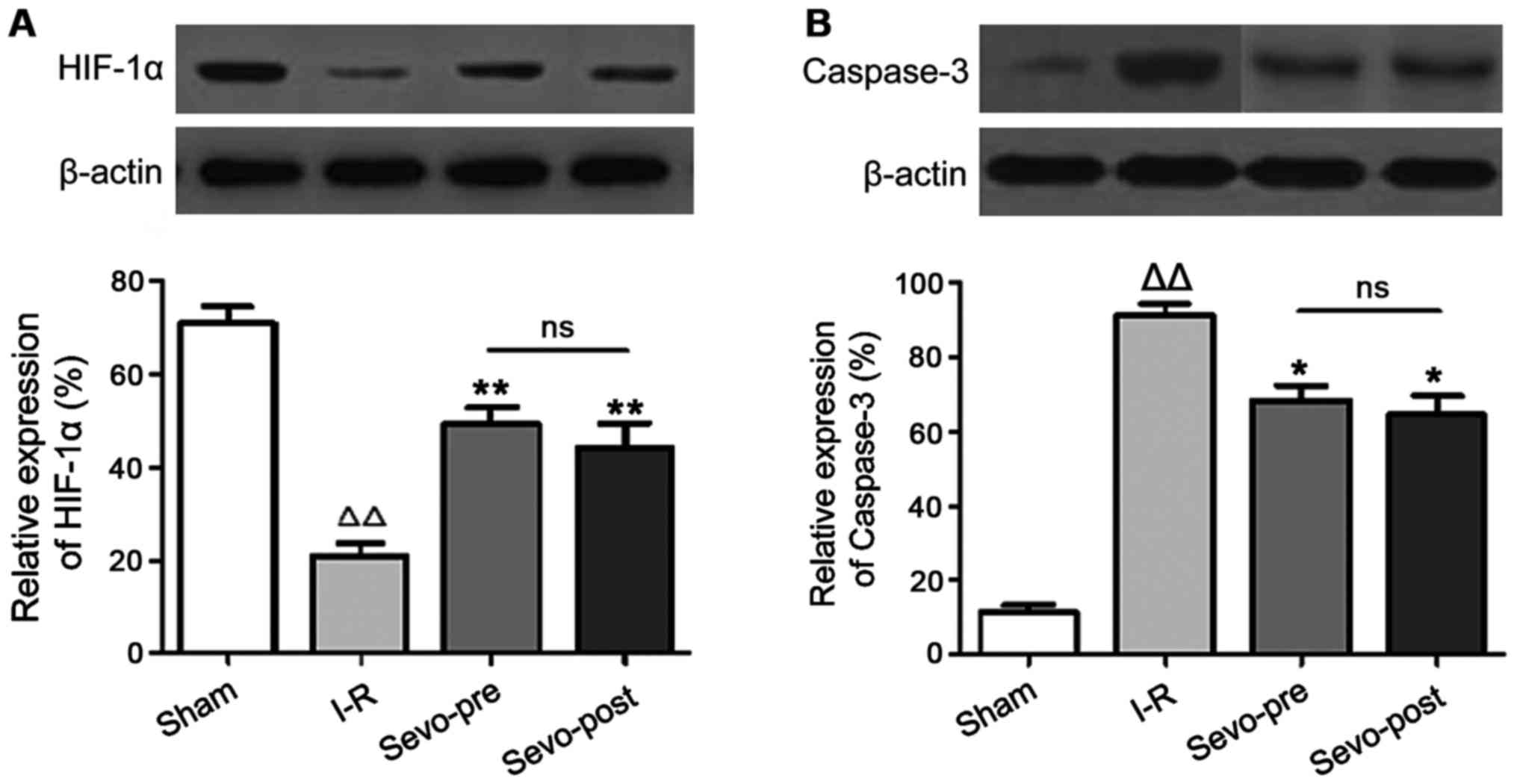
Western blotting was used to perform a
semi-quantitative analysis of the levels of HIF-1α and caspase-3
protein in cardiomyocytes after reperfusion. As shown in Fig. 2, compared with the Sham group, the
expression levels of HIF-1α were decreased and the expression
levels of caspase-3 were increased in the I-R group. In addition,
compared with the I-R group, HIF-1α was significantly increased and
caspase-3 was significantly decreased in Sevo-Pre and Sevo-Post
groups. No significant differences were found between Sevo-Pre and
Sevo-Post groups. The results suggest that sevoflurane treatment
can significantly increase the expression of HIF-1α protein and
decrease the expression of caspase-3 protein (P>0.05).
Effect of sevoflurane treatment on the
expression of HIF-1α and Bcl-2
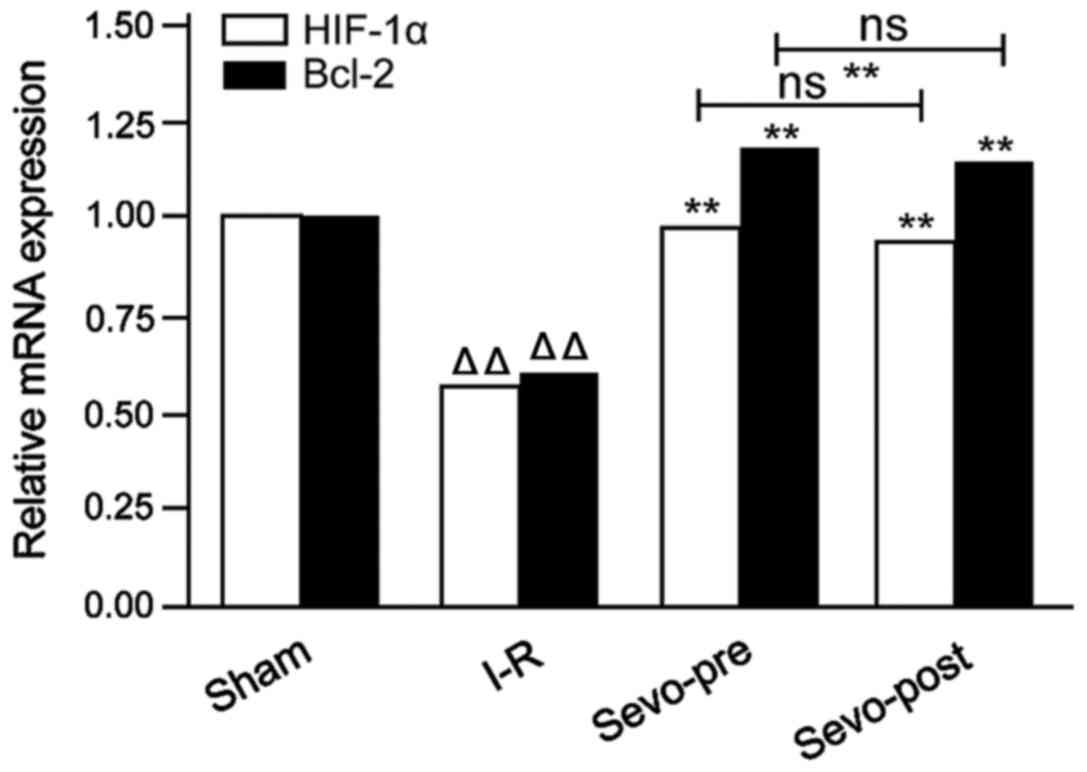
qRT-PCR was used to perform a semi-quantitative
analysis of the expression levels of HIF-1α and Bcl-2 mRNA in
cardiomyocytes after reperfusion. As shown in Fig. 3, compared with the Sham group, the
expression levels of HIF-1α and Bcl-2 mRNA were significantly
reduced in the I-R group. Moreover, compared with I-R group, the
expression levels of HIF-1α and Bcl-2 mRNA were significantly
increased in the Sevo-Pre and Sevo-Post groups. No significant
differences were found between Sevo-Pre and Sevo-Post groups. The
data indicate that sevoflurane treatment can significantly increase
the expression levels of HIF-1α and Bcl-2 mRNA.
Discussion
Inhalation of a certain dose of sevoflurane before
myocardial ischemia or during the early stages of reperfusion has
been shown to be able to protect the myocardium by inhibiting
myocardial abnormalities during reperfusion through the activation
of specific signaling pathways, and this myocardial protection is
similar to that of ischemic preconditioning (11). Studies have shown that sevoflurane
pre-conditioning and post-conditioning can enhance myocardial
contractility and diastolic ability, improve cardiac function, and
reduce myocardial infarction caused by IR, cardiovascular disorders
and other symptoms (12). In order
to confirm the cardioprotective effect of sevoflurane during MIRI,
we carried out MIRI in vitro simulations using rat hearts
for the model. Through real-time monitoring of cardiac function
parameters, we found that sevoflurane pre-/post-conditioning can
significantly improve left ventricular HR,
LVDP±dp/dtmax, and reduce left ventricular LVEDP in rat
hearts during the late stages of reperfusion, indicating that
sevoflurane treatment can enhance myocardial contractility and
diastolic ability of IR hearts. In addition, sevoflurane treatment
also reduced MIS after reperfusion compared with the control group,
indicating that sevoflurane can protect the myocardium against
MIRI.
Apoptosis, which is regulated by anti-apoptotic
factor Bcl-2 and apoptotic hydrolase caspase-3 through a series of
complex processes, is an important molecular mechanism in the
process of MIRI cell death and plays an important role in IR injury
and prognosis (13,14). Studies have shown that sevoflurane
can activate the NF-κB pathway, increase Bcl-2 expression and
reduce expression levels of intracellular adhesion factor-1 (CAM-1)
and tumor necrosis factor-α (TNF-α), so as to reduce the expression
levels of caspase-3 protein, thus inhibiting apoptosis (15). In addition, sevoflurane treatment can
reduce expression levels of Beclin-1 and LC-I/II, relieving IR
damage by inhibiting excessive autophagy (16,17).
HIF-1α has been shown to play an important role in the reduction of
MIS and the decrease of apoptosis rates after ischemic
preconditioning. HIF-1α can attenuate myocardial IR injury by
enhancing the activity of heme oxygenase-1 (18).
In order to investigate the effects of sevoflurane
on HIF-1α expression and cell apoptosis, expression levels of
HIF-1α, Bcl-2 and caspase-3 were quantitatively analyzed. Our
results comparing with those in the control group, showed that
sevoflurane treatment can significantly increase the expression of
HIF-1α mRNA and protein and Bcl-2 mRNA. Moreover, it can decrease
the expression level of caspase-3 protein. Taken together, these
facts indicate that HIF-1α can regulate the apoptosis of
cardiomyocytes by regulating the expression of caspase-3 through a
yet-undisclosed mechanism. It is known that activation of HIF-1α
can induce the expression of downstream genes involved in
cytoprotection, so as to inhibit cell apoptosis and reduce
myocardial injury caused by IR (19,20).
In conclusion, our study confirmed that sevoflurane
(1MAC) treatment can improve the function parameters of hearts
undergoing IR and reduces MIS. The protection is probably due to
sevoflurane activating the expression of HIF-1α and inhibiting the
expression of caspase-3. Our findings provide new insights for the
diagnosis and treatment of MIRI.
References
|
1
|
Goldhaber JI and Weiss JN: Oxygen free
radicals and cardiac reperfusion abnormalities. Hypertension.
20:118–127. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu Q, Chen J, Jiang C and Liu HF: Effect
of peroxisome proliferator-activated receptor gamma agonist on
heart of rabbits with acute myocardial ischemia/reperfusion injury.
Asian Pac J Trop Med. 7:271–275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bolli R: The early and late phases of
preconditioning against myocardial stunning and the essential role
of oxyradicals in the late phase: An overview. Basic Res Cardiol.
91:57–63. 1996.PubMed/NCBI
|
|
4
|
Xia A, Xue Z, Wang W, Zhang T, Wei T, Sha
X, Ding Y and Zhou W: Naloxone postconditioning alleviates rat
myocardial ischemia reperfusion injury by inhibiting JNK activity.
Korean J Physiol Pharmacol. 18:67–72. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao YY, Zhu MH, Zhang FJ, Wen CY, Ma LL,
Wang WN, Wang CC, Liu XB, Yu LN, Qian LB, et al: Activation of Akt
and cardioprotection against reperfusion injury are maximal with
only five minutes of sevoflurane postconditioning in isolated rat
hearts. J Zhejiang Univ Sci B. 14:511–517. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ceyhan D, Tanrıverdi B and Bilir A:
Comparison of the effects of sevoflurane and isoflurane on
myocardial protection in coronary bypass surgery. Anadolu Kardiyol
Derg. 11:257–262. 2011.PubMed/NCBI
|
|
7
|
Gong JS, Yao YT, Fang NX and Li LH:
Sevoflurane postconditioning attenuates reperfusion-induced
ventricular arrhythmias in isolated rat hearts exposed to
ischemia/reperfusion injury. Mol Biol Rep. 39:6417–6425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fandrey J, Gorr TA and Gassmann M:
Regulating cellular oxygen sensing by hydroxylation. Cardiovasc
Res. 71:642–651. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kido M, Du L, Sullivan CC, Li X, Deutsch
R, Jamieson SW and Thistlethwaite PA: Hypoxia-inducible factor
1-alpha reduces infarction and attenuates progression of cardiac
dysfunction after myocardial infarction in the mouse. J Am Coll
Cardiol. 46:2116–2124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng XH, Karna P, Cao Z, Jiang BH, Zhou M
and Yang L: Cross-talk between epidermal growth factor receptor and
hypoxia-inducible factor-1alpha signal pathways increases
resistance to apoptosis by up-regulating survivin gene expression.
J Biol Chem. 281:25903–25914. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kodaka M, Johansen JW and Sebel PS: The
influence of gender on loss of consciousness with sevoflurane or
propofol. Anesth Analg. 101:377–381. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kehl F, Krolikowski JG, Tessmer JP, Pagel
PS, Warltier DC and Kersten JR: Increases in coronary collateral
blood flow produced by sevoflurane are mediated by
calcium-activated potassium (BKCa) channels in vivo.
Anesthesiology. 97:725–731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meng XY, Yu HL, Zhang WC, Wang TH, Mai X,
Liu HT and Xu RC: ZFP580, a novel zinc-finger transcription factor,
is involved in cardioprotection of intermittent high-altitude
hypoxia against myocardial ischemia-reperfusion injury. PLoS One.
9:e946352014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eefting F, Rensing B, Wigman J, Pannekoek
WJ, Liu WM, Cramer MJ, Lips DJ and Doevendans PA: Role of apoptosis
in reperfusion injury. Cardiovasc Res. 61:414–426. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu G, Salem MR and Crystal GJ: Role of
adenosine receptors in volatile anesthetic preconditioning against
neutrophil-induced contractile dysfunction in isolated rat hearts.
Anesthesiology. 103:287–295. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Z, Cao J, Song D, Tian L, Chen K, Wang
Y, Gao L, Yin Z, Fan Y and Wang C: Autophagy is involved in the
cardioprotection effect of remote limb ischemic postconditioning on
myocardial ischemia/reperfusion injury in normal mice, but not
diabetic mice. PLoS One. 9:e868382014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao X, Chen A, Yang P, Song X, Liu Y, Li
Z, Wang X, Wang L and Li Y: Alpha-lipoic acid protects
cardiomyocytes against hypoxia/reoxygenation injury by inhibiting
autophagy. Biochem Biophys Res Commun. 441:935–940. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot
K, Wang L, Wei C, Trush MA and Semenza GL: Complete loss of
ischaemic preconditioning-induced cardioprotection in mice with
partial deficiency of HIF-1 alpha. Cardiovasc Res. 77:463–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu LX, Lu H, Luo Y, Date T, Belanger AJ,
Vincent KA, Akita GY, Goldberg M, Cheng SH, Gregory RJ, et al:
Stabilization of vascular endothelial growth factor mRNA by
hypoxia-inducible factor 1. Biochem Biophys Res Commun.
291:908–914. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mazure NM, Brahimi-Horn MC, Berta MA,
Benizri E, Bilton RL, Dayan F, Ginouvès A, Berra E and Pouysségur
J: HIF-1: Master and commander of the hypoxic world. A
pharmacological approach to its regulation by siRNAs. Biochem
Pharmacol. 68:971–980. 2004. View Article : Google Scholar : PubMed/NCBI
|