Introduction
Obstructive jaundice is a frequently observed
symptom in patients undergoing surgery and its major clinical
manifestation is a syndrome group, including tissue damage and a
series of pathophysiological changes in various systems of the
body. These include endotoxemia, infection, liver damage,
coagulopathy and malnutrition (1–3). Liver
damage caused by obstructive jaundice is an important cause of
other complications (4). However,
drove of end-stage severe liver injuries by obstructive jaundice
still lacks effective therapies (5).
Simvastatin (Sim) is one of the most commonly
prescribed drugs for the treatment of hypercholesterolemia since it
prevents the synthesis of cholesterol (6). In addition to its lipid-lowering
effects, Sim can also protect against cholestasis-induced liver
injury (7). However, the molecular
mechanisms underlying the hepatoprotective effects of Sim
administration on obstructive jaundice remain unknown. The
hypothesis considered in the present study was that underlying
molecular mechanisms of hepatoprotective Sim effects relate to
transforming growth factor-β1 (TGF-β1) signaling during obstructive
jaundice progression. Similarly, there is currently a lack of
knowledge regarding the effects of TGF-β1 in the early stages of
liver damage by obstructive jaundice. TGF-β1 performs a suppressor
role in hepatocytes, where it regulates growth and differentiation
and induces apoptosis (8). Moreover,
the Smads are signal transducers of TGF-β1 (9). Once the ligand binds to the cell
surface receptors, Smads are phosphorylated; they associate with
Smad4, translocate into the nucleus and regulate target gene
expression (10). Furthermore, Smad
signaling is limited by inhibitory Smad7 (11). Exogenous bone morphogenetic protein 7
(BMP-7) counteracted the effect of TGF-β1 and hepatic fibrosis was
ameliorated by the administration of BMP-7 (12).
In the present study, the process of severe liver
injury was induced in a rat model by creating obstructive jaundice
by double bile duct ligation (BDL). Moreover, the effects of Sim in
this rat model were investigated. These observations indicate that
the addition of Sim improved liver regeneration and abrogated
hepatocyte apoptosis by downregulating the hepatic TGF-β1 signaling
pathway in an experimental model of acute obstructive jaundice in
rats. However, the specific mechanism underlying this effect
remains unclear and requires further investigation.
Materials and methods
Experimental animals and groups
A total of 32 healthy male Sprague-Dawley rats (age,
6–8 weeks; weight, 250–300 g) were provided by the Experimental
Animal Center of Tongji Medical College (Wuhan, China). All the
rats were housed under habitual conditions at 21±2°C, under a 12-h
light/dark cycle and with free access to food and standard
laboratory chow. At all times the animals were bred in accordance
with the Principles and Guidelines for the Care and Use of
Laboratory Animals of Tongji Medical College, recommended by the
National Academy of Sciences and published by the National
Institute of Health (NIH publication 85–23 revised 1996). The
methodology was approved by the Ethics Committee of Tongji Medical
College.
The rats were randomly divided into four groups (n=8
per group): i) Sham group, Sham-operated; ii) BDL+NS group, animals
underwent BDL and were treated with normal saline (NS; 0.02
mg/kg/day); iii) BDL+Sim 0.02; and iv) BDL+Sim 0.2 groups underwent
BDL and were treated with 0.02 or 0.2 mg/kg/day Sim (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany), respectively.
Surgical procedure and sample
preparation
Prior to surgery the animals were anesthetized using
cotton soaked with 1.5 ml sulfuric ether (Guidechem, Hangzhou,
China). Under sterile conditions, an upper abdominal midline
incision was performed. Muscle and peritoneal structure were then
dissected to expose the common bile duct, which was carefully
isolated and double-ligated with a 5-0 silk close to the liver
hilus immediately below the bifurcation and cut between the
ligatures (BDL group). The control animals underwent a Sham
operation in which the common bile duct was not ligated but
exposed, and the abdominal incision was then closed using single
sutures (13).
In all of the groups the route of administration was
intraperitoneal via a single daily injection at 9:00 am for seven
days. This route was selected because it appeared to lead to more
stable levels of the drug in equilibrium. In total, 24 h after the
last injection each animal was weighed, anesthetized with sulfuric
ether and sacrificed by exsanguination (abdominal aorta puncture).
Blood and tissues were then collected and processed as described
below.
Hepatic function parameters
Blood samples were collected with 10 IU/ml sodium
heparin (GL Biochem, Shanghai, China) as an anticoagulant, and were
then centrifuged at 2,000 × g for 5 min at 4°C to obtain plasma.
The plasma was then used to measure the total bilirubin (TB),
alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
as parameters that indicate hepatic function. These biochemical
analyses were then performed using an autoanalyzer (7600–020;
Hitachi Ltd., Tokyo, Japan).
Western blot analysis
Liver specimens were homogenized in lysis buffer
(P0013; Beyotime Institute of Biotechnology, Haimen, China), and
the total protein concentration was determined and equalized. The
20 µg samples were then boiled in Laemmli buffer (P0015A; Beyotime
Institute of Biotechnology) and analyzed by western blotting as
previously described (14). Equal
amounts of proteins were separated by 12% SDS-PAGE (P0012A;
Beyotime Institute of Biotechnology) with 100 V for 1 h and
transferred onto polyvinylidene difluoride membranes (Merck
Millipore). The membranes were blocked with 5% bovine serum albumin
(Gibco; Thermo Fisher Scientific, Waltham, MA, USA) for 2 h and
then incubated with primary antibodies at 4°C overnight. Primary
antibodies were used at a dilution of 1:800 and horseradish
peroxidase-conjugated secondary antibodies goat anti-mouse IgG
(sc-2005) and goat anti-rabblit IgG (sc-2004; both Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at a dilution of 1:5,000.
Moreover, detection was performed using Supersignal West Pico
chemiluminescent substrate kit (Pierce Protein Biology; Thermo
Fisher Scientific, Inc., Rockford, IL, USA) and X-ray film, and was
converted to digital images. Densitometric quantification was
performed using the Quantity One version 4.62 software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). In all instances, the
membranes were stripped and reported using an antibody specific
against α-tubulin, and densitometric values were corrected to
α-tubulin expression. Furthermore, the commercial antibodies used
were: TGF-β1 (ab92486) from Abcam (Cambridge, MA, USA), Smad2
(12584) and Smad3 (9523) from Cell Signaling Technology, Inc.
(Danvers, MA, USA) and α-tubulin (sc-8035) from Santa Cruz
Biotechnology (Santa Cruz Biotechnology, Inc.).
Immunohistochemistry
Liver tissues were fixed in 10% formalin overnight
and embedded in paraffin (327204; Sigma Aldrich, Merck Millipore).
Immunohistochemical analysis of the liver tissue was performed as
described previously (15), and
TGF-β1 expression was detected. Polylysine was smeared on the
slides, 3.5-µm paraffin sections were cut and a pressure cooker
(WQC50A1P; Midea, Foshan, China) was used to prepare the hot citric
acid antigen for primary TGF-β1 antibody repair (sc-130348; 1:100;
Santa Cruz Biotechnology, Inc.). It was then incubated for 1 h with
a biotin-conjugated secondary antibody, goat anti-mouse IgG-B
(sc-2039; 1:800; Santa Cruz Biotechnology, Inc.), at room
temperature for 30 min, 3,3′-diaminobenzidine (DAB) colored for
5–10 min and stained with hematoxylin and eosin for 1–3 min. An
Olympus Corporation (Tokyo, Japan) BX51 microscope was used to
photograph the slides. Phosphate-buffered saline (PBS) instead of
the primary antibody was used as a negative control. Using a Leica
Qwin V3 image analysis system (Leica Microsystems GmbH, Wetzlar,
Germany), immunohistochemical analysis of the positive products of
the area in each section was selected at a magnification of ×200 of
the three non-overlapping representations. The percentage of each
sample was then calculated and the positive area (positive
area/measurement area) was determined. The scoring criteria used
based on a semiquantitative approach, in which the percentage of
TGF-β1-positive cells (0–100%) was determined and multiplied by the
staining intensity (0, negative; 1, weak; 2, moderate; 3,
strong).
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from liver was extracted using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) reagent according to
the manufacturer's instructions and treated with RNase-free DNaseI
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. In total, 2 µg total RNA from liver was
reverse transcribed into cDNA using the StrataScript first-strand
synthesis system (Stratagene; Agilent Technologies, Inc., Santa
Clara, CA, USA). TGF-β1 (sense: 5′-AGGGCTTTCGCTTCAGTGCT-3′ and
anti-sense: 5′-CCATGAGGAGCAGGAAGGGT-3′); Smad3 (sense:
5′-TGAACACCAAGTGCATTACCA-3′ and anti-sense:
5′-TGACTGGCTGTAGGTCCAAGT-3′), and Smad7 (sense:
5′-CGGAATTCGCCACCATGTTCAGGACCAAACGATC-3′ and anti-sense:
5′-CGGGATCCACTACCGGCTGTTGAAGATG-3′) were amplified using SYBR Green
PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and iCycler real-time PCR detection system (Bio-Rad Laboratories,
Inc.) for 40 cycles. Relative levels were then calculated using the
iCycler software and a standard equation (Applied Biosystems;
Thermo Fisher Scientific, Inc.) and normalized against
glyceraldehyde 3-phosphate dehydrogenase (sense:
5′-CCTGGTATGACAATGAATATG-3′ and anti-sense:
5′-TCTCTTGCTCTCAGTATCC-3′).
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick-end labeling
(TUNEL) assay
Hepatocyte apoptosis was quantified using the TUNEL
assay. All liver tissue specimens were fixed in freshly prepared
3.5% paraformaldehyde (158127; Sigma-Aldrich; Merck Millipore) and
sucrose (V900116 Sigma-Aldrich; Merck Millipore). Tissues were then
embedded in Thermo Shandon Cryomatrix (Thermo Fisher Scientific,
Inc.), sectioned (10 µm) using a Shandon Cryotome (M16512), and
then stored at 4°C. After treating the specimen with PBS, the
sections were processed following the instructions of an In
Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis,
IN, USA). After rinsing the specimens twice with PBS, the tissue
peroxidase activity was visualized using DAB. TUNEL-positive nuclei
were counted in five high-powered fields and expressed as a
percentage of the total nuclei counted (16). Moreover, two investigators (one of
which was blinded) scored the sections, and the mean of the two
scores is reported.
Proliferating cell nuclear antigen
(PCNA) labeling index
PCNA immunostaining was performed in order to
examine the hepatocyte proliferation with the mouse monoclonal
antibody against PCNA (clone-PC 10; 1:100; Dako Denmark A/S,
Glostrup, Denmark). Briefly, remnant liver tissue specimens were
fixed in 10% buffered formalin, embedded in paraffin and then cut
into 5-µm sections. The deparaffinized sections were then treated
by microwave heating thrice in PBS for 5 min each, then washed
three times with PBS for 5 min each. Following blocking with
endogenous peroxidase, the specimens were washed three times with
PBS for 5 min each. The sections were then incubated with the
antibody against PCNA overnight at 4°C. After washing several times
with PBS, biotin-labeled secondary antibody (sc-57636; 1:2,000;
Santa Cruz Biotechnology) was added for 1 h at room temperature,
and after washing several times with PBS the tissue peroxidase
activity was visualized using DAB (17). The PCNA labeling index was then
determined as the number of PCNA-positive cells among 1,000 counted
cells at high power (magnification, ×400).
Statistical analysis
Statistical comparison was performed using GraphPad
Prism version 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
Student's t-test, Welch's t-test or Mann-Whitney U test were
adapted for the evaluation of the significance of the differences
between groups. P<0.05 was used to indicate a statistically
significant difference. Results are expressed as the mean ±
standard error.
Results
General condition
The urine color of the rats evidently deepened 24
h-post operation. After 48 h, the tails of these rats began to turn
yellow. Following prolonged bile duct obstruction, there was
progression in jaundice, and the appetite and mental states
gradually deteriorated.
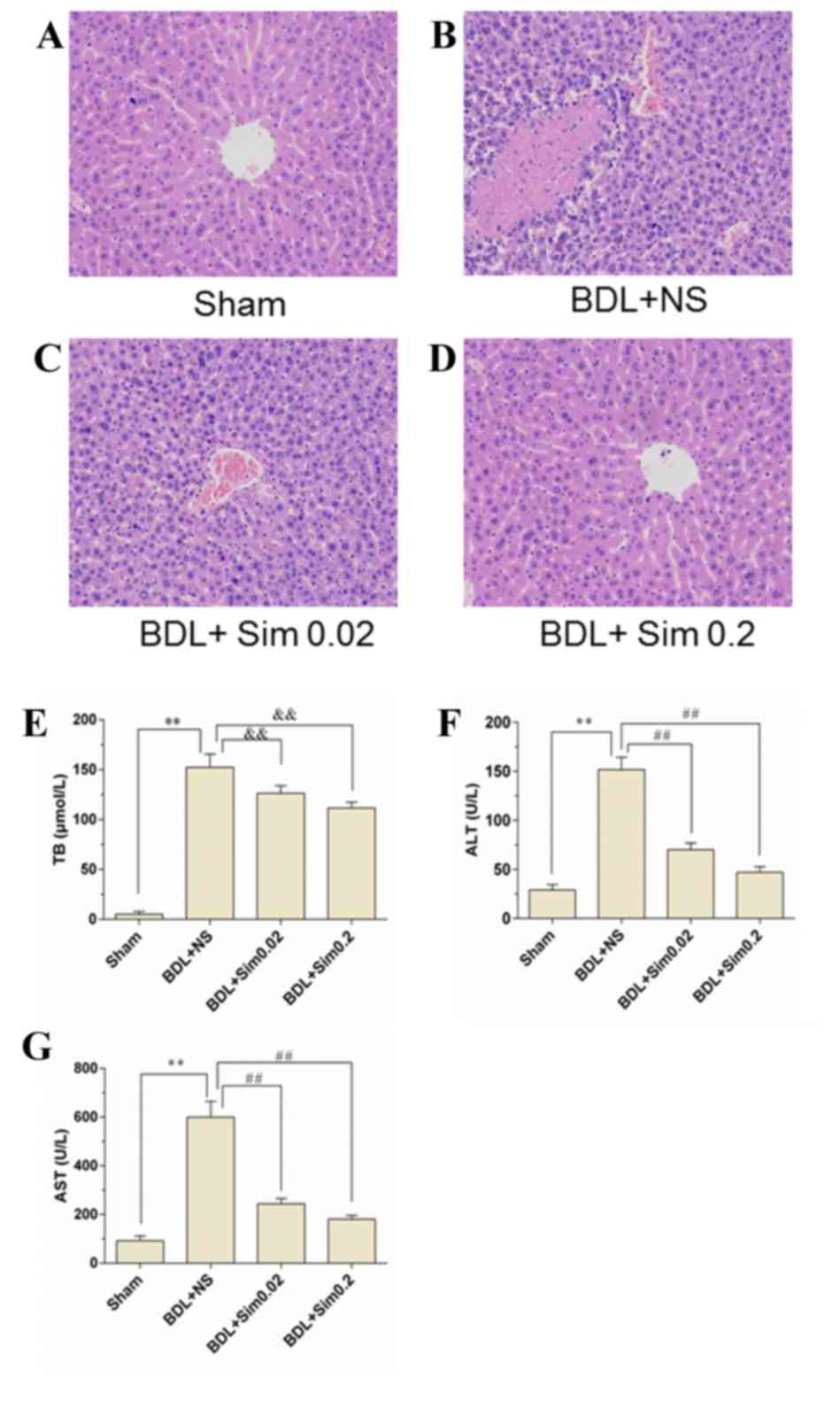
Effect of Sim on hepatic architecture
and function
Sections from hepatic tissue from the four
experimental groups were analyzed after hematoxylin and eosin
staining (Fig 1), however, no
histopathological differences were observed between the control
group (Fig. 1A) and animals
receiving Sim (Fig. 1D). By
contrast, BDL caused substantial hepatocellular injury, as
indicated by a >7.2-fold increase in liver enzymes. However, Sim
treatment significantly reduced BDL-induced liver damage (Fig. 1B and C; P<0.01 vs. BDL+NS group).
Moreover, the BDL+NS group significantly elevated the serum TB by
>17.6-fold vs. the Sham group, suggesting that an obstructive
jaundice model was successfully established (Fig. 1E). Bilirubin levels in rats treated
with Sim following BDL were not different from those in NS-treated
animals, indicating that the degree of obstructive jaundice was
similar in all of the experimental groups (Fig. 1E). Moreover, administration of 0.02
mg/kg/day Sim significantly suppressed the release of ALT and AST
from the liver by 61.02 and 58.01%, respectively, compared with the
NS-treated rats. The treatment with 0.2 mg/kg/day Sim decreased
BDL-induced ALT and AST levels by 69.14 and 65.18%, respectively
(Fig. 1F and G; P<0.01 vs. BDL+NS
group).
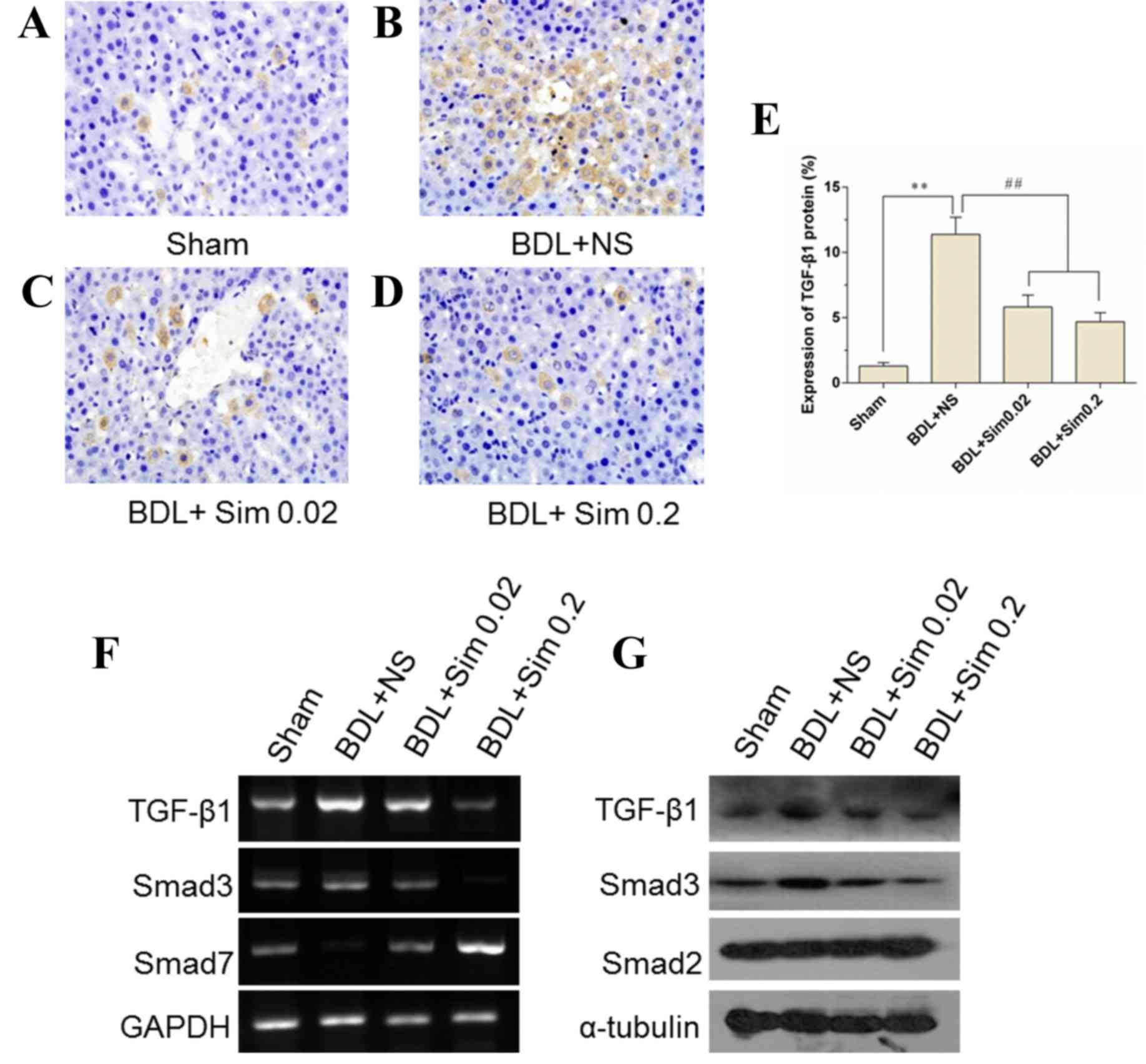
Inhibition of the TGF-β1 signaling
pathway by the addition of Sim
TGF-β1 concentrations in the liver were
significantly elevated in the livers of BDL animals compared with
those in Sham-operated animals (P<0.01) (Fig. 2A, B and E). Sim administration
markedly lowered obstructive jaundice-induced elevation of hepatic
concentration of TGF-β1 (P<0.01) (Fig. 2E). Moreover, RT-qPCR and western blot
analyses revealed that TGF-β1 was less activated in the liver
tissues obtained from Sim-treated rats compared to those from
NS-treated rats (Fig. 2F and G).
These results indicate that Sim administration blunted the TGF-β1
signaling pathway that is known to contribute to the aggression of
obstructive jaundice-induced liver injury.
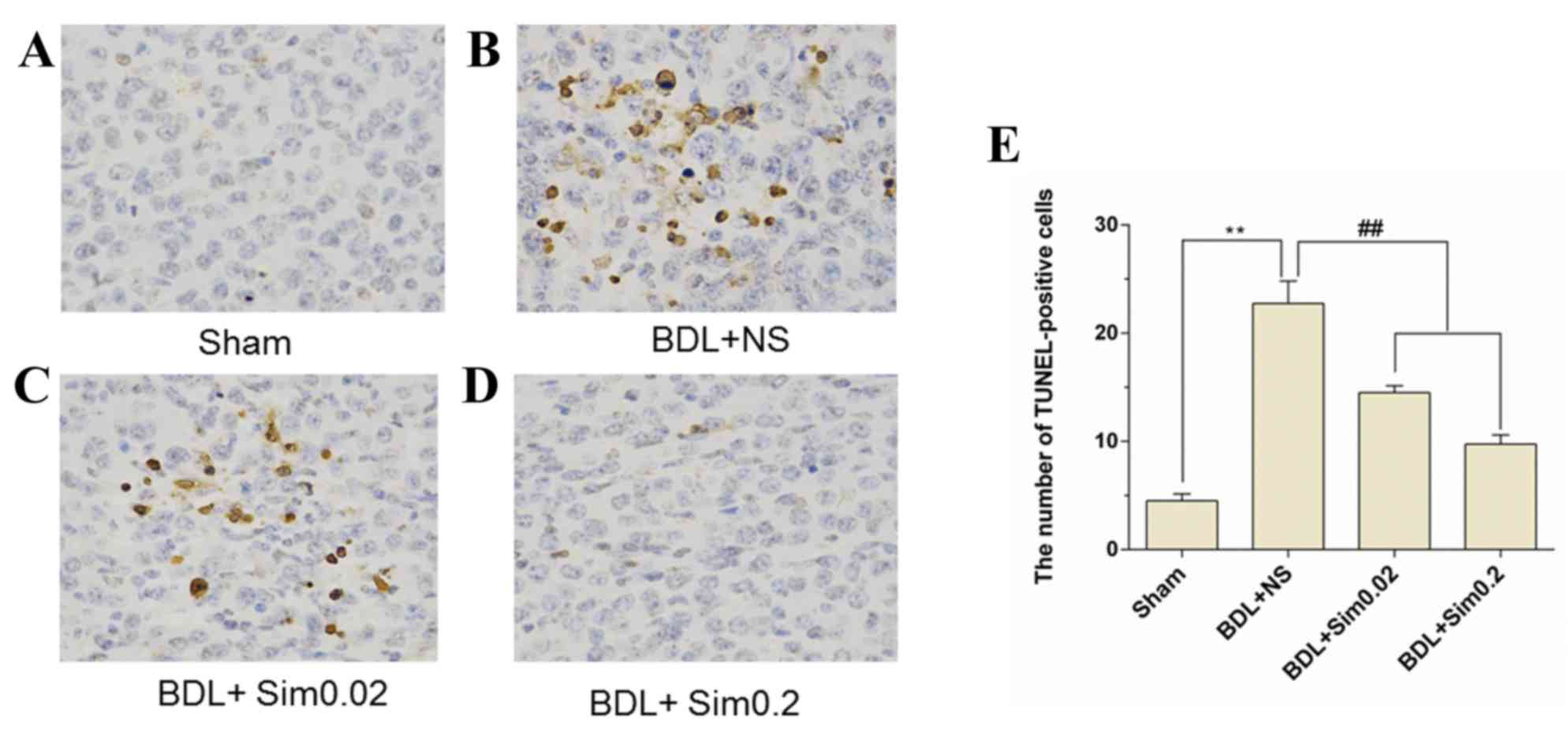
TUNEL assay
TUNEL immunohistochemistry stained liver sections
taken from Sham, BDL+NS, BDL+ Sim 0.02 and BDL+ Sim 0.2 are shown
in Fig. 3A-D. Immunohistochemistry
for TUNEL demonstrated a significant increase in TUNEL-positive
hepatocytes after BDL compared to the Sham group (P<0.01)
(Fig. 3A and B). Furthermore,
treatment of BDL animals with Sim resulted in a significant
reduction in the percentage of hepatocyte apoptosis compared to BDL
rats treated with saline (P<0.01) (Fig. 3B and D). Also, in the Sham animals no
significant change on apoptosis was noted (Fig. 3A).
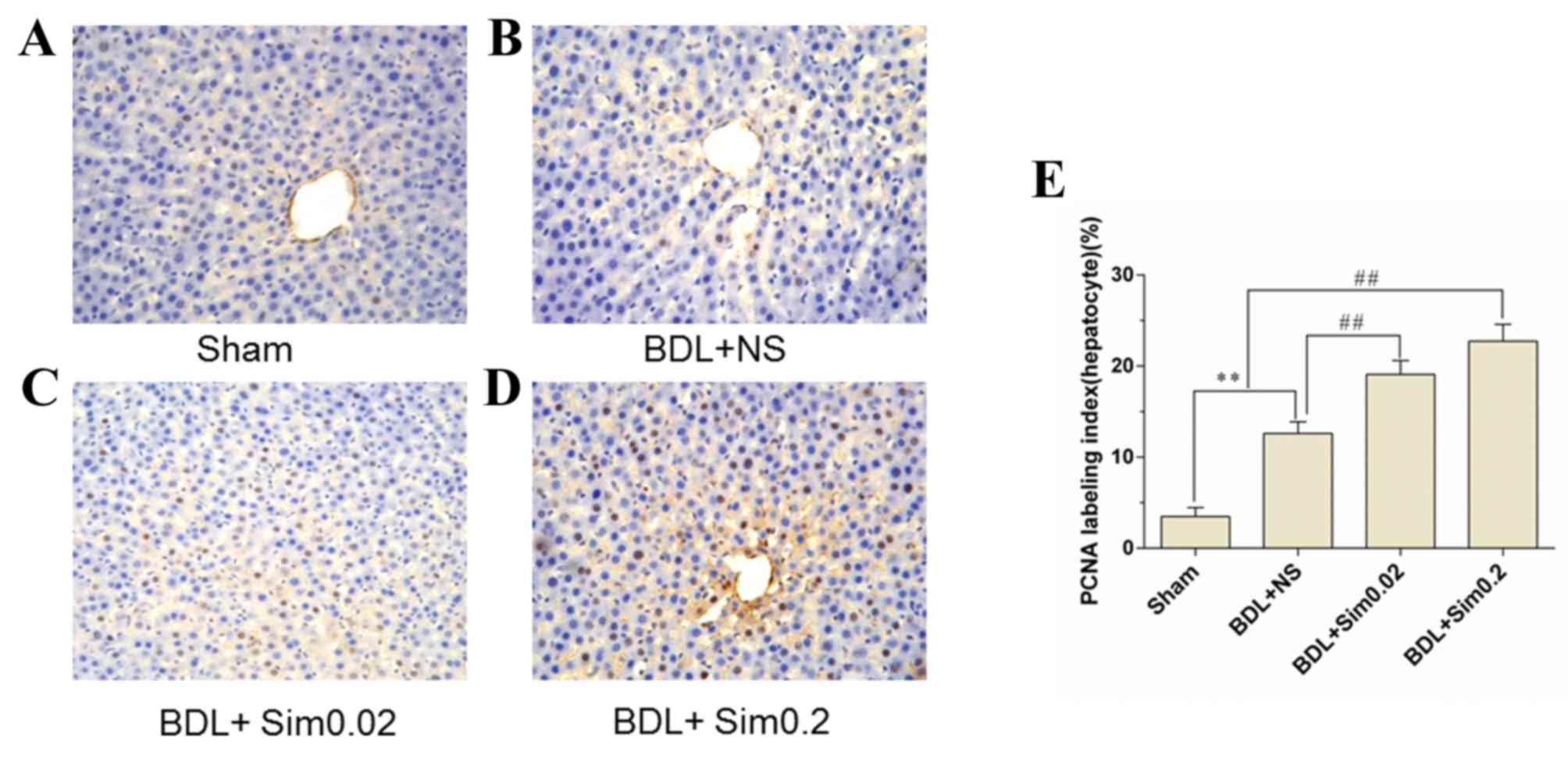
PCNA assay
PCNA immunohistochemistry stained liver sections
were collected following seven days of BDL. Sham, BDL+NS, BDL+Sim
0.02 and BDL+Sim 0.2 are depicted in Fig. 4A-D. Immunohistochemical staining for
PCNA demonstrated a significant increase in DNA synthesis in the
BDL+NS group, compared to the Sham group (Fig. 4B). Moreover, treatment of BDL rats
with Sim increased DNA synthesis in BDL animals to 27% compared to
the controls (Fig. 4C and D).
Discussion
Obstructive jaundice has been identified as a
significant risk for patients resulting from surgery, which may
result in alterations of the glycogen metabolism, decreased
cell-mediated immunity, impaired hepatic and renal functions,
increased circulating endotoxins and a depressed synthesis of
homeostasis factors (18). These
factors can decrease the tolerance of patients to anesthesia and
surgery, leading to an increasing operative risk (19).
In the present study, the addition of Sim was
demonstrated to improve liver regeneration and abrogated hepatocyte
apoptosis by downregulating the hepatic TGF-β1 signaling pathway in
an experimental model of acute obstructive jaundice in rats. These
observations demonstrate that acute obstructive jaundice by
ligation and division of the common bile duct induces liver damage.
Furthermore, the addition of Sim significantly enhances liver
regeneration and alleviates hepatic dysfunction by downregulating
the hepatic TGF-β1 signaling pathway in an experimental model of
acute obstructive jaundice in rats. The hepatic Sim content
decreases after liver injury (4),
and it is likely that Sim administration facilitates liver
regeneration. Moreover, Sim inhibits collagen processing leading to
increased ubiquitination and decreased secretion in hepatic
stellate cells (HSCs) (20).
Additionally, Sim prevents liver injury induced by alcohol in rats
by reducing liver lipid peroxidation, anti-inflammation and
antihyperplasia (21). Conversely,
during liver regeneration by partial hepatectomy, inhibition of the
liver regeneration by Sim may be mediated by liver fat accumulation
(22). Finally, prevention from the
decrease in the intracellular content of Sim, as a factor
attenuating regeneration remains unclear.
The presently investigated hypothesis was that the
underlying molecular mechanisms of hepatoprotective Sim effects
relate to TGF-β1 signaling during obstructive jaundice progression.
Obstructive jaundice alters serum TGF-β1 expression in the rat and
oral bile acid or glutamine (or both) can restore the altered serum
TGF-β1 expression in rats that have obstructive jaundice (23). In addition, an important protective
mechanism of Sim against fibrosis may be to lower TGF-β levels and
the activation of collagen I production, and particularly to
repress the activation of the collagen type I alpha 2 chain
(COL1A2) gene by preventing TGF-β effects on its responsive site in
the COL1A2 promoter (24).
In the present study Sim appeared antagonize TGF-β1
in hepatic cells though the induction of Smad7 expression.
Moreover, anti-TGF-β1 action may target collagen expression. TGF-β1
is secreted by transdifferential hepatic cells, and quiescent
hepatic cells are highly responsive to exogenous TGF-β1 (25). Smad3, an intracellular mediator of
TGF-β1 signal transduction, binds to the TbRE and stimulates COL1A2
transcription when overexpressed in HSCs, but increased COL1A2 gene
transcription of the cells is not affected by overexpression of
inhibitory Smad7 (26). In addition,
TGF-β downregulates the alcohol metabolizing enzyme alcohol
dehydrogenase 1 mRNA in cultured hepatocytes and liver tissue from
TGF-β transgenic mice via the ALK5/Smad2/3 signaling branch, with
Smad7 as a potent negative regulator (27). Conversely, TGF-β1 represses the gene
transcription of 7α-hydroxylase in human hepatocytes by a mechanism
involving Smad3-dependent inhibition of HNF4α and HDAC remodeling
of 7α-hydroxylase chromatin (28).
The inhibition of epidermal growth factor receptor
in hepatocellular carcinoma enhanced TGF-β-induced pro-apoptotic
signaling (29). TGF-β-induced
apoptosis in rat hepatocytes does not have the need for
Rac-dependent nicotinamide adenine dinucleotide phosphate oxidases,
and TGF-β upregulates the Rac-independent Nox4, which is associated
with its pro-apoptotic activity (30). Moreover, caffeine downregulates
TGF-β-induced connective tissue growth factor (CTGF), CTGF
expression in hepatocytes by the stimulation of degradation of the
TGF-β effector Smad2, inhibition of Smad3 phosphorylation and
upregulation of the peroxisome proliferator-activated receptor γ
(31).
The present study on the rat BDL model similarly
points to anti-TGF-β1 effects of Sim. These are Sim-reduced
intrinsic and TGF-β1 gene activity and phosphorylation of Smad2/3
proteins. The latter is due to the following mechanism: i)
Sim-dependent decline in the expression level of Smad2/3 was
observed in parallel with the downregulation of phosphorylation;
and ii) Smad7 expression was initiated by Sim. Among the acute
phase of liver injury, expression of the Smad7 protein is rapidly
increased by TGF-β1 signaling and fibrotic signals mediated by Smad
are inhibited (32). However, a lack
of Smad7 expression as a prerequisite for disease progression is
further suggested by the observation that tissue fibrosis in rats
is inhibited by ectopic Smad7 expression (33).
In conclusion, the data of the present study provide
evidence on the mechanism of how Sim blunts profibrotic TGF-β1
signaling. There is a causal association between Sim administration
and the TGF-β1 signaling pathway following BDL. Moreover, the
addition of Sim abrogates the harmful hepatocyte apoptosis and
beneficially augments hepatic regeneration during obstructive
jaundice progression. In addition, this mechanism may provide the
clues to improved ways that will ameliorate the complication of
liver damage and reduce the morbidity and mortality of obstructive
jaundice.
Acknowledgements
The present study was supported by a grant from the
Research Fund for the National Nature Science Funding of China (no.
81370581).
References
|
1
|
Kimmings AN, van Deventer SJH, Obertop H,
Rauws EA, Huibregtse K and Gouma DJ: Endotoxin, cytokines, and
endotoxin binding proteins in obstructive jaundice and after
preoperative biliary drainage. Gut. 46:725–731. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Addley J and Mitchell RM: Advances in the
investigation of obstructive jaundice. Curr Gastroenterol Rep.
14:511–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fang Y, Gurusamy KS, Wang Q, Davidson BR,
Lin H, Xie X and Wang C: Meta-analysis of randomized clinical
trials on safety and efficacy of biliary drainage before surgery
for obstructive jaundice. Br J Surg. 100:1589–1596. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okaya T, Nakagawa K, Kimura F, Shimizu H,
Yoshidome H, Ohtsuka M, Morita Y and Miyazaki M: Obstructive
jaundice impedes hepatic microcirculation in mice.
Hepatogastroenterology. 55:2146–2150. 2008.PubMed/NCBI
|
|
5
|
Yeki M, Koda M, Matono T, Sugihara T,
Maeda K and Murawaki Y: Preventative and therapeutic effects of
perindopril on hepatic fibrosis induced by bile duct ligation in
rats. Mol Med Report. 2:857–864. 2009.
|
|
6
|
Jadhav SB and Jain GK: Statins and
osteoporosis: New role for old drugs. J Pharm Pharmacol. 58:3–18.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dold S, Laschke MW, Lavasani S, Menger MD,
Jeppsson B and Thorlacius H: Simvastatin protects against
cholestasis-induced liver injury. Br J Pharmacol. 156:466–474.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hayashi H, Sakai K, Baba H and Sakai T:
Thrombospondin-1 is a novel negative regulator of liver
regeneration after partial hepatectomy through transforming growth
factor-beta1 activation in mice. Hepatology. 55:1562–1573. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren M, Wand B, Zhang J, Liu P, Lv Y, Liu
G, Jiang H and Liu F: Smad2 and Smad3 as mediators of the response
of adventitial fibroblasts induced by transforming growth factor
β1. Mol Med Report. 4:561–567. 2011.
|
|
10
|
Hill CS: Nucleocytoplasmic shuttling of
Smad proteins. Cell Res. 19:36–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Derynck R and Zhang YE: Smad-dependent and
Smad-independent pathways in TGF-β family signaling. Nature.
425:577–584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang T, Chen SL, Lu XJ, Shen CY, Liu Y and
Chen YP: Bone morphogenetic protein 7 suppresses the progression of
hepatic fibrosis and regulates the expression of gremlin and
transforming growth factor β1. Mol Med Rep. 6:246–252.
2012.PubMed/NCBI
|
|
13
|
Tao YY, Wang QL, Shen L, Fu WW and Liu CH:
Salvianolic acid B inhibits hepatic stellate cell activation
through transforming growth factor beta-1 signal transduction
pathway in vivo and in vitro. Exp Biol Med (Maywood).
238:1284–1296. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luan Z, He Y, Alattar M, Chen Z and He F:
Targeting the prohibitin scaffold-CRAF kinase interaction in
RAS-ERK-driven pancreatic ductal adenocarcinoma. Mol Cancer.
13:382014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu MH, Cao W, Ye D, Ren GX, Wu YN and Guo
W: Contactin 1 (CNTN1) expression associates with regional lymph
node metastasis and is a novel predictor of prognosis in patients
with oral squamous cell carcinoma. Mol Med Rep. 6:265–270.
2012.PubMed/NCBI
|
|
16
|
Canbay A, Hiquchi H, Bronk SF, Taniai M,
Sebo TJ and Gores GJ: Fas enhances fibrogenesis in the bile duct
ligated mouse: A link between apoptosis and fibrosis.
Gastroenterology. 123:1323–1330. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bird MA, Black D, Lange PA, Samson CM,
Hayden M and Behrns KE: NFkappaB inhibition decresases hepatocyte
proliferation but does not alter apoptosis in obstructive jaundice.
J Surg Res. 114:110–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsuyuguchi T, Takada T, Miyazaki M,
Miyakawa S, Tsukada K, Nagino M, Kondo S, Furuse J, Saito H, Suyama
M, et al: Stenting and interventional radiology for obstructive
jaundice in patients with unresectable biliary tract carcinomas. J
Hepatobiliary Pancreat Surg. 15:69–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lacaine F, Fourtanier G, Fingerhut A and
Hay JM: Surgical mortality and morbidity in malignant obstructive
jaundice: A prospective multivariate analysis. Eur J Surg.
161:729–734. 1995.PubMed/NCBI
|
|
20
|
Rombouts K, Kisanga E, Hellemans K,
Wielant A, Schuppan D and Geerts A: Effect of HMG-CoA reductase
inhibitors on proliferation and protein synthesis by rat hepatic
stellate cells. J Hepatol. 38:564–572. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang W, Zhao C, Zhou J, Zhen Z, Wang Y and
Shen C: Simvastatin ameliorates liver fibrosis via mediating nitric
oxide synthase in rats with non-alcoholic steatohepatitis-related
liver fibrosis. PLoS One. 8:e765382013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Slotta JE, Laschke MW, Schilling MK,
Menger MD, Jeppsson B and Thorlacius H: Simvastatin attenuates
hepatic sensitization to lipopolysaccharide after partial
hepatectomy. J Surg Res. 162:184–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheen-Chen SM, Eng HL and Hung KS: Altered
serum transforming growth factor-beta1 and monocyte chemoattractant
protein-1 levels in obstructive jaundice. World J Surg. 28:967–970.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Itoh Y, Kimoto K, Imaizumi M and Nakatsuka
K: Inhibition of RhoA/Rho-kinase pathway suppresses the expression
of type I collagen induced by TGF-beta2 in human retinal pigment
epithelial cells. Exp Eye Res. 84:464–472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv Z and Xu L: Salvianolic acid B inhibits
ERK and p38 MAPK signaling in TGF-β1-stimulated human hepatic
stellate cell line (LX-2) via distinct pathways. Evid Based
Complement Alternat Med. 2012:9601282012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang W, Ou J, Inagaki Y, Greenwel P and
Ramirez F: Synergistic cooperation between Sp1 and Smad3/Smad4
mediates transforming growth factor beta1 stimulation of alpha
2(I)-collagen (COL1A2) transcription. J Biol Chem. 275:39237–39245.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ciuclan L, Ehnert S, Ilkavets I, Weng HL,
Gaitantzi H, Tsukamoto H, Ueberham E, Meindl-Beinker NM, Singer MV,
Breitkopf K and Dooley S: TGF-beta enhances alcohol dependent
hepatocyte damage via down-regulation of alcohol dehydrogenase I. J
Hepatol. 52:407–416. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li T and Chiang JY: A novel role of
transforming growth factor beta1 in transcriptional repression of
human cholesterol 7alpha-hydroxylase gene. Gastroenterology.
133:1660–1669. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Caja L, Sancho P, Bertran E and Fabregat
I: Dissecting the effect of targeting the epidermal growth factor
receptor on TGF-β-induced-apoptosis in human hepatocellular
carcinoma cells. J Hepatol. 55:351–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carmona-Cuenca I, Roncero C, Sancho P,
Caja L, Fausto N, Fernandez M and Fabregat I: Upregulation of the
NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its
pro-apoptotic activity. J Hepatol. 49:965–976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gressner OA, Lahme B, Rehbein K, Siluschek
M, Weiskirchen R and Gressner AM: Pharmacological application of
caffeine inhibits TGF-beta-stimulated connective tissue growth
factor expression in hepatocytes via PPARgamma and
SMAD2/3-dependent pathways. J Hepatol. 49:758–767. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tahashi Y, Matsuzaki K, Date M, Yoshida K,
Furukawa F, Sugano Y, Matsusshita M, Himeno Y, Inagaki Y and Inoue
K: Differential regulation of TGF-beta signal in hepatic stellate
cells between acute and chronic rat liver injury. Hepatology.
35:49–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dooley S, Hamzavi J, Breitkopf K,
Wiercinska E, Said HM, Lorenzen J, Ten Dijke P and Gressner AM:
Smad7 prevents activation of hepatic stellate cells and liver
fibrosis in rats. Gastroenterology. 125:178–191. 2003. View Article : Google Scholar : PubMed/NCBI
|