Thalidomide was first synthesized from the glutamic
acid derivative α-phthaloyliso-glutamine in Germany in 1954 and was
mistakenly assumed to be safe due to its structural resemblance to
barbiturates (1). Thalidomide
received considerable attention due to reports of severe birth
defects in the infants of women who had been prescribed the drug as
an anti-emetic (2,3). Following these reports, it was
withdrawn from the European market for >40 years (3). Thalidomide was approved by the US Food
and Drug Administration (FDA) in 1998 due to its anti-inflammatory
effect in the treatment of erythema nodosum leprosum (ENL), a
painful inflammatory complication of leprosy (4). This led to its unrecognized
pharmacological properties being investigated as a potential
immunomodulatory, anti-inflammatory and anti-angiogenic agent for
the treatment of other serious diseases (3,4).
Currently, thalidomide and its analogues (CC5013 and
CC-4047) are being used as novel immunomodulatory drugs (IMiDs) for
the treatment of various diseases, including lupus erythematosis,
Behcet's disease, inflammatory bowel disease, solid tumors,
hematologic malignancies, and heart failure (4–7). CC5013
(lenalidomide, Revlimid) and CC4017 (ACTIMID, pomalidomide) were
initially developed as inhibitors of tumor necrosis factor-α
(TNF-α) (5). Following minor
structural modifications of thalidomide, CC5013 and CC4017 are
effective regulators of the immune reaction and cytokine response
with none of the adverse effects of thalidomide (6).
At present, the novel analogues of thalidomide are
in phase III clinical trials and are showing promising results
(7). The present review summarizes
the biological effects of thalidomide and its IMiD analogues on
cytokine elaboration, inflammation, immune cell function regulation
and angiogenesis (Table I).
Additionally, previous studies of thalidomide and its analogues
used for the treatment of fibrotic diseases such as pulmonary
fibrosis, skin fibrosis, and ophthalmopathies are presented
(Table II).
As IMiDs, thalidomide and its analogues have
clinically relevant effects on cytokine elaboration (5). In patients with ENL, a
Mycobacterial infection complication, treatment with
thalidomide reduced the lipopolysaccharide (LPS)-induced TNF-α
secretion by monocytes or macrophages through accelerating TNF-α
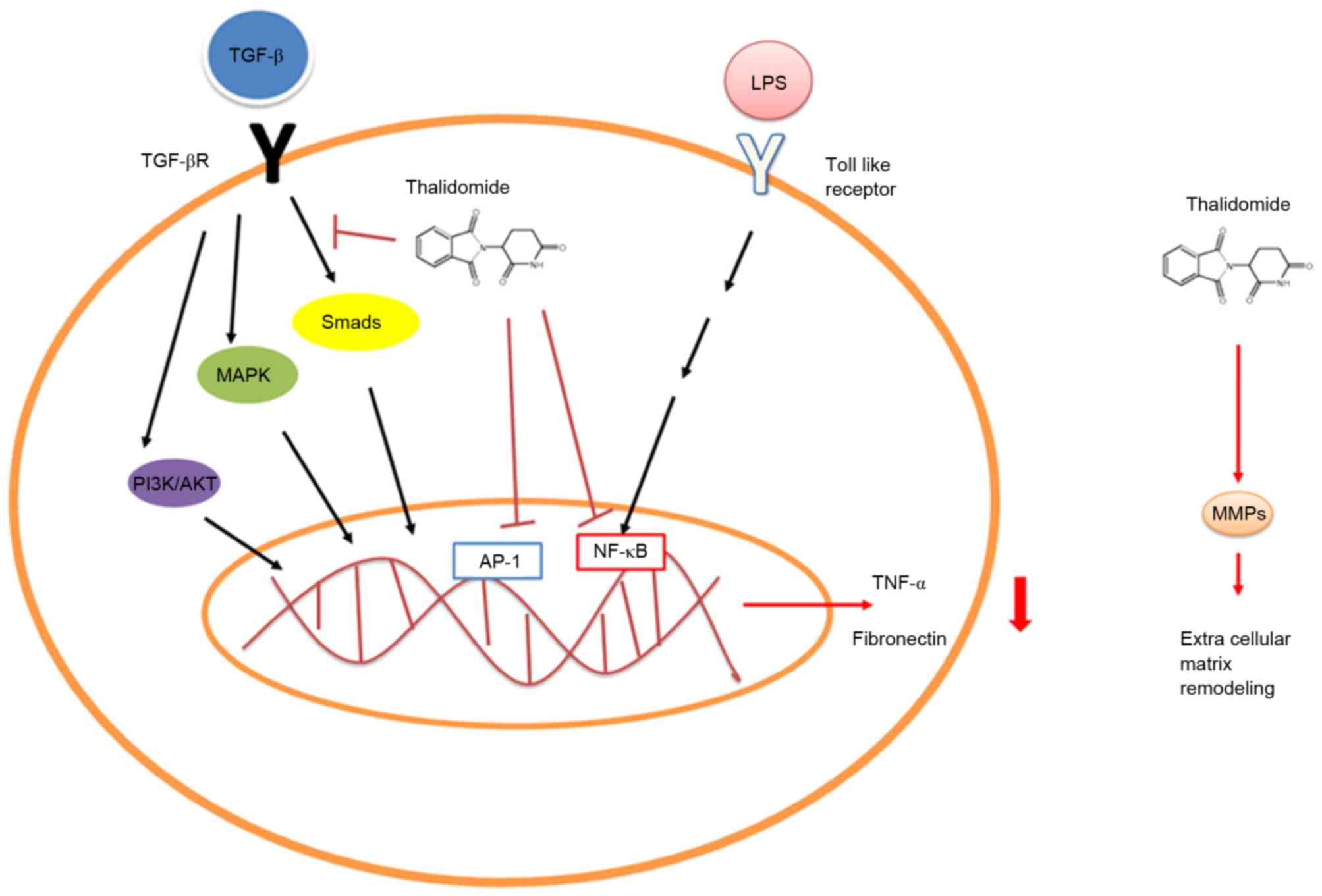
mRNA degradation and inhibiting nuclear factor κB (NF-κB)
activation (Fig. 1) (8–10).
Thalidomide has also been demonstrated to exert an effect on other
inflammatory conditions that present with TNF-α upregulation, such
as rheumatoid arthritis, Crohn's disease, tuberculosis and cancer
(11–14). Furthermore, in the setting of spinal
cord ischemia, thalidomide pretreatment has been demonstrated to
reduce early phase ischemia/reperfusion injury of the spinal cord
in rabbits via reducing TNF-α production (15). CC5013 and CC4017 are considered to be
more effective TNF-α antagonists than thalidomide, and their
inhibiting effect on TNF-α production has been reported as being as
much as 100–50,000 times higher (16,17).
The TGF-β superfamily consists of various proteins
that regulate numerous physiological processes, including
embryogenesis, immunity, carcinogenesis, cell proliferation and
migration, wound healing, inflammation and fibrosis, among others
(18). Smad and non-canonical
pathways are the two primary signaling pathways in the TGF-β system
(18). In Smad-related pathways,
complex interactions between intracellular diverse molecules
associated with the downstream TGF-β signaling cascade result in
fibrosis, such as phosphorylation or direct interaction with Smad
(19). Non-canonical signaling
pathways include the mitogen-activated protein kinase (MAPK),
Rho-like GTPase and phosphatidylinositol-3-kinase/protein kinase B
(AKT) pathways (18). Extracellular
signal-regulated kinase1/2 (ERK1/2), c-Jun-NH2-terminal
kinase, and p38 MAPK signaling pathways are well-established
non-canonical pathways of TGF-β signaling (20). In a mouse model of bleomycin-induced
lung fibrosis, Choe et al (21) demonstrated that thalidomide
administration significantly inhibited TGF-β1 mRNA expression in a
dose-dependent manner following the administration of interleukin
(IL)-6 and IL-6R, and they concluded that the anti-fibrotic effects
of thalidomide may be associated with the inhibition of
TGF-β1-mediated non-Smad ERK1/2 signaling pathways. Furthermore,
Liang et al (22) found that
thalidomide treatment inhibited fibronectin production in normal
and keloid fibroblasts stimulated by TGF-β1, and enhanced the
phosphorylation of MAPKs and Smad 2/3. They also revealed that
inhibited p38/Smad3 pathways were involved in the anti-fibrotic
mechanisms of thalidomide (Fig. 1)
(22). Other than TNF-α and TGF-β,
the expression levels of other cytokines including IL-1β, IL-6, and
granulocyte macrophage-colony stimulating factor were also
suppressed by thalidomide in LPS-induced peripheral blood
monocytes, whereas IL-10 was stimulated (23,24).
Correlative preclinical studies have suggested that
IMiDs act as co-stimulators on the release of IL-2 and interferon
(IFN)-γ from T lymphocytes undergoing CD3 ligation or mitogenic
stimulation, or in response to dendritic cells presented with
antigens (30). This may result in T
cell proliferation, T helper cells skewing and augmentation of
cytotoxic effector functions (31).
Co-stimulation of T cells by lenalidomide is only partially blocked
by the negative co-stimulator cytotoxic T lymphocyte-associated
antigen-4 (CTLA-4) in the B7/CD28 co-stimulatory axis (32). Furthermore, tyrosine phosphorylation
of CD28 induced by lenalidomide was able to facilitate the
recruitment and activation of upstream molecules in multiple
intracellular signaling pathways, including
phosphatidylinositol-3-kinase (PI3K) and NF-κB (33). As CD28 and IMiDs have no kinase
activity, other signaling proteins such as protein kinase C (PKC)
may be involved in the indirect mechanism underlying CD28
phosphorylation (34,35). Payvandi et al (35) demonstrated that IMiDs increased IL-2
production in stimulated T cells via enhancing PKC-θ activation and
DNA-binding activity of activated protein-1 (AP-1). In addition to
conventional T cells, regulatory T cells (Tregs) are a group of
immunosuppressive T cells that function in self-tolerance and the
immune response (36). Inhibition of
Tregs by lenalidomide (Revlimid; CC-5013) and pomalidomide
(CC-4047) via decreasing the expression of forkhead box P3 (FoxP3)
was observed in a preclinical study (32). Furthermore, Gandhi et al
(37) reported that the lenalidomide
(or pomalidomide)-binding protein cereblon was able to cause the
Ikaros and Aiolos to interact with the E3 ubiquitin ligase
CUL4-RBX1-DDB1-CRBN (known as CRL4CRBN), resulting in their
ubiquitination, proteasome degradation and T cell activation.
IMiDs also exert their immunomodulatory effects by
modulating the number and function of NK cells. Zhu et al
(38) reported that CC-5013 and
CC-4047 increased the population of NK and NKT cells in the setting
of hematological malignancy (38).
Furthermore, Wu et al (39)
demonstrated that lenalidomide was able to enhance the NK
cell-mediated antibody-dependent cellular cytotoxicity (ADCC)
effect on rituximab-treated CD20+ tumor cells (39). It was also revealed that enhanced NK
cell Fc-gamma receptor signaling was associated with enhanced
phosphorylation of ERK (39).
Despite the putative role of metabolism in the
IMiD-mediated inhibition of vascularization, thalidomide and its
analogues have been demonstrated to have an antagonistic effect on
several key angiogenesis regulators (31). IMiDs decrease the paracrine
production of pro-angiogenic vascular endothelial growth factor
(VEGF) and IL-6 at the bone marrow stroma and MM cell levels
(43). Furthermore, thalidomide
suppresses the expression of VEGF receptors in human umbilical vein
endothelial cells (HUVECs) via activating sphingolipid signaling
(44). Reduced cell migration
responses to basic fibroblast growth factor (bFGF) and VEGF due to
downregulation of AKT phosphorylation was observed in lenalidomide
treated HUVEC cells (45,46). The suppression of pro-angiogenic
factors such as IL-6, TNF-α, VEGF, NF-κB and prostaglandin
synthesis by thalidomide and its analogues may indirectly
downregulate angiogenesis in vivo.
Skin fibrosis, characterized by physiological tissue
architecture damage, is a major hallmark of systemic sclerosis
(SSc) and causes high morbidity among patients (47). As efficient immunomodulators,
thalidomide and its analogues have been suggested as a treatment
for treat skin fibrosis. Oliver et al (48) reported that thalidomide improved the
clinical manifestations of patients with SSc by stimulating the
immune response. In a preclinical study by Weingärtner et al
(49), the newest second generation
IMiD, pomalidomide, was demonstrated to prevent spontaneous
hypodermal fibrosis and induce its regression in bleomycin-induced
dermal fibrosis mice and tight-skin mice. An international,
multicenter, controlled phase II clinical trial (gov ID:
NCT01559129) was initiated to evaluate pomalidomide as a treatment
for patients with SSc (49). Most
recently, Ingen-Housz-Oro et al (50) reported that two cases of Ig4-related
skin diseases were successfully treated with thalidomide, and they
concluded that interactions between Tregs and mast cells may result
in IL-6 production and fibrosis via the TGF-β/phospho-Smad 2/3
pathway.
Idiopathic pulmonary fibrosis (IPF), characterized
by epithelial injury and fibroblast proliferation in the lungs, is
a chronic progressive lung disease with a prevalence of 16–18 in
10,000 and a five-year mortality rate over 50% (51). Inflammatory cytokines and
angiogenesis serve critical roles in the pathological progression
of IPF. It has been reported that thalidomide exerts an inhibitory
effect on the cytokine profile in patients with IPF (52,53).
Tabata et al (54) reported
that thalidomide pretreatment prevented the development of
bleomycin-induced pulmonary fibrosis (PF) in a mouse model. Choe
et al (21) further confirmed
that the TGF-β1-induced activation of the ERK1/2 signaling pathway
accounted for the anti-fibrotic effect of thalidomide in a
bleomycin-induced PF model. Knobloch et al (55) reported that the underlying mechanisms
of this effect included downregulation of bone morphogenetic
protein signaling, an increase in the activity of Wnt and Akt and
enhanced apoptosis resistance. In a clinical trial conducted by
Horton et al (56),
thalidomide treatment was demonstrated to improve coughing and
respiratory symptoms, as well as quality of life, in patients with
IPF.
D'Amato, an ophthalmologist, first considered
thalidomide when searching for treatments for two diseases that
cause blindness, macular degeneration and diabetic retinopathy
(57). Both diseases result in
excessive blood vessel growth, which burst in the eye and destroy
the patient's vision (57). It was
reported that thalidomide was an effective anti-angiogenesis
inhibitor in the setting of bFGF-induced angiogenesis in a rabbit
cornea micropocket assay (58), and
Kruse et al (59) suggested
that thalidomide inhibited VEGF-induced corneal angiogenesis in New
Zealand white rabbits. Ribeiro et al (60) used a novel thalidomide hybrid
(LASSBio-596) to treat inflammatory corneal angiogenesis in
rabbits, and reported that it had an inhibitory effect. In an
alkali burn model of corneal angiogenesis, Abbas et al
(61) demonstrated that thalidomide
was able to prevent corneal angiogenesis and prolong graft survival
(as measured by graft clarity in donor corneas in eyes).
Furthermore, Lee and Chung (62) has
reported a similar inhibitory effect of thalidomide on corneal
neovascularization in a silk suture-induced rabbit model, though to
be achieved via regulating VEGF and TNF-α expression levels.
However, Srinivasan et al (63) reported the case of a patient who
presented with bilateral symmetrical corneal endothelial changes,
which was found to be associated with thalidomide toxicity. Huang
et al (64) reported a
45-year woman with gradual visual decline due to crystalline
materials in the cornea. The patient was treated with thalidomide
and, at a seven-year follow up, her vision was stable and the
corneal crystalline deposits had decreased and migrated.
In addition to functioning as an anti-angiogenesis
inhibitor, thalidomide may also used as anti-inflammatory agent in
ophthalmopathy. In a rat model of endotoxin-induced uveitis (EIU),
Guex-Crosier et al (65)
revealed that high-dose thalidomide had an effective
anti-inflammatory effect, whereas lower doses were insufficient to
reduce inflammation. Rodrigues et al (66) further investigated the preventive and
therapeutic anti-inflammatory effects of systemic and topical
thalidomide in EIU rats. Parentin et al (67) reported that thalidomide was an
effective treatment for bilateral chronic idiopathic anterior
uveitis in a three-year-old child; however, Ip et al
(68) reported a case in which
thalidomide was not able to prevent the recurrence of a choroidal
neovascular membrane (CNM). Using a mouse model, Rabinowitz et
al (69) also demonstrated that
thalidomide had no significant effect on neovascularization in
oxygen-induced retinopathy.
It has been reported that exposure to thalidomide
has negative side effects on the eyes. For example, anophthalmia
and microphthalmia (the absence of an eye and the presence of a
small eye within the orbit, respectively) are both disease caused
by exposure to thalidomide (70).
There have been no large sample clinical trials investigating the
effect of thalidomide on ophthalmopathies, and so the application
of thalidomide in the patients with certain types of eye diseases
remains controversial.
Birth defects such as phocomelia and deformities of
the ears, eyes, and gastrointestinal tract caused by thalidomide
have been widely reported (3). A
novel generation IMiD, slenalidomide, caused thalidomide-like fetal
malformations in monkeys (71), but
not in rabbits (72). Pomalidomide,
another thalidomide analogue approved by the FDA, was teratogenic
in both rats and rabbits when administered during the period of
organogenesis (73). In rabbits,
pomalidomide has been found to cause cardiac malformations and
anomalies in the limbs and digits. Fetal abnormalities observed in
rats included fetal visceral defects and abnormalities in vertebral
elements (74).
Pomalidomide has been evaluated in a number of
clinical trials involving patients with relapsed MM and
myelofibrosis (75). The primary
complication reported previously was neutropenia, followed by
anemia and thrombocytopenia (76,77).
Although thrombo-prophylaxis (daily low-dose aspirin in the
majority of cases) was applied, the frequency of venous
thromboembolism was similar to that observed with other IMiDs. In a
Mayo Clinic phase II trial (NCT00558896) (78), peripheral neuropathy was reported in
60 (17.3%) patients during the treatment period. In a phase II
trial of patients with myelofibrosis, the major side effects
included fatigue/asthenia, thrombocytopenia, pneumonia/sepsis and
anemia (79).
Lenalidomide has also been assessed in two phase III
registration clinical trials (MM-009 and MM-010) (80,81).
Despite being an immunomodulatory agent, the toxicity profile of
lenalidomide is different from that of thalidomide. In clinical
practice, lenalidomide is typically used in combination with
dexamethasone and the most frequent toxic events of this
combination therapy include myelosuppression (neutropenia,
thrombocytopenia, and less typically, anemia), infections, and
thrombosis (particularly when combined with high dose of
dexamethasone and with antithrombotic prophylaxis) (82).
Thalidomide and its analogues have been shown to
have pleotropic immunomodulatory and anti-angiogenesis effects. In
addition to its conventional use to treat patients with solid
tumors or hematological malignancies, thalidomide also exhibits
therapeutic effects as a treatment for skin fibrosis, idiopathic
pulmonary fibrosis and ophthalmopathies. The molecular mechanisms
underlying IMiD activity are likely to be both divergent and
complementary across these distinct diseases. The complexity of
IMiD biology is highlighted by the combined ability to modulate
multiple targets from the epigenetic to post-translational protein
modulations, systemic cytokine networks and immune cell function
regulation. However, the adverse effects of thalidomide treatment,
including birth deformity, neutropenia, anemia, thrombocytopenia,
and even DVT reduces the safety of thalidomide treatment. It is
hoped that identification of novel drug targets may allow for
therapeutic manipulation of novel non-IMiD pharmaceuticals to
elicit the clinical benefits without the adverse side effects in
the future.
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81170852 and
81470629) and the Natural Science Foundation of Chongqing (grant
no. CSTC2013jjB10030).
|
1
|
Schulz M: Dark remedy: the impact of
thalidomide and its revival as a vital medicine. BMJ. 322:16082001.
View Article : Google Scholar
|
|
2
|
Han ZX, Xu J, Wang HM, Ma J, Sun X and Du
XP: Antiemetic role of thalidomide in a rat model of
cisplatin-induced emesis. Cell Biochem Biophys. 70:361–365. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vargesson N: Thalidomide-induced
teratogenesis: History and mechanisms. Birth Defects Res C Embryo
Today. 105:140–156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Teo S, Resztak KE, Scheffler MA, Kook KA,
Zeldis JB, Stirling DI and Thomas SD: Thalidomide in the treatment
of leprosy. Microbes Infect. 4:1193–1202. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopez-Millan B, de la Guardia Diaz R,
Roca-Ho H, García-Herrero CM, Lavoie JR, Rosu-Myles M, Gonzalez-Rey
E, O'Valle F, Criado G, Delgado M and Menendez P: Therapeutic
effect of the immunomodulatory drug lenalidomide, but not
pomalidomide, in experimental models of rheumatoid arthritis and
inflammatory bowel disease. Exp Mol Med. 49:e2902017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartlett JB, Dredge K and Dalgleish AG:
The evolution of thalidomide and its IMiD derivatives as anticancer
agents. Nat Rev Cancer. 4:314–322. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galustian C, Meyer B, Labarthe MC, Dredge
K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, et
al: The anti-cancer agents lenalidomide and pomalidomide inhibit
the proliferation and function of T regulatory cells. Cancer
Immunol Immunother. 58:1033–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreira AL, Sampaio EP, Zmuidzinas A,
Frindt P, Smith KA and Kaplan G: Thalidomide exerts its inhibitory
action on tumor necrosis factor alpha by enhancing mRNA
degradation. J Exp Med. 177:1675–1680. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Keifer JA, Guttridge DC, Ashburner BP and
Baldwin AS Jr: Inhibition of NF-kappa B activity by thalidomide
through suppression of Ikappa B kinase activity. J Biol Chem.
276:22382–22387. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sampaio EP, Kaplan G, Miranda A, Nery JA,
Miguel CP, Viana SM and Sarno EN: The influence of thalidomide on
the clinical and immunologic manifestation of erythema nodosum
leprosum. J Infect Dis. 168:408–414. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zuo XX, Gong YH, Zhou YO, Luo H and Xiao
XZ: The plasmic translocation and release of high mobility group
box chromosomal protein 1 in peripheral blood monocytes of patients
with rheumatoid arthritis and the effect of thalidomide. Zhonghua
Nei Ke Za Zhi. 47:374–377. 2008.PubMed/NCBI
|
|
12
|
Barkin JA, Schonfeld WB and Deshpande AR:
Successful use of thalidomide for refractory esophageal Crohn's
disease. Am J Gastroenterol. 108:855–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fourcade C, Mauboussin JM, Lechiche C,
Lavigne JP and Sotto A: Thalidomide in the treatment of immune
reconstitution inflammatory syndrome in HIV patients with
neurological tuberculosis. AIDS Patient Care STDS. 28:567–569.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beedie SL, Peer CJ, Pisle S, Gardner ER,
Mahony C, Barnett S, Ambrozak A, Gütschow M, Chau CH, Vargesson N
and Figg WD: Anti-Cancer properties of a novel class of
tetrafluorinated thalidomide analogs. Mol Cancer Ther.
14:2228–2237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee CJ, Kim KW, Lee HM, Nahm FS, Lim YJ,
Park JH and Kim CS: The effect of thalidomide on spinal cord
ischemia/reperfusion injury in a rabbit model. Spinal Cord.
45:149–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Muller GW, Corral LG, Shire MG, Wang H,
Moreira A, Kaplan G and Stirling DI: Structural modifications of
thalidomide produce analogs with enhanced tumor necrosis factor
inhibitory activity. J Med Chem. 39:3238–3240. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muller GW, Chen R, Huang SY, Corral LG,
Wong LM, Patterson RT, Chen Y, Kaplan G and Stirling DI:
Amino-substituted thalidomide analogs: Potent inhibitors of
TNF-alpha production. Bioorg Med Chem Lett. 9:1625–1630. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leask A and Abraham DJ: TGF-beta signaling
and the fibrotic response. FASEB J. 18:816–827. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Javelaud D and Mauviel A: Crosstalk
mechanisms between the mitogen-activated protein kinase pathways
and Smad signaling downstream of TGF-beta: Implications for
carcinogenesis. Oncogene. 24:5742–5750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YE: Non-Smad pathways in TGF-beta
signaling. Cell Res. 19:128–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choe JY, Jung HJ, Park KY, Kum YS, Song
GG, Hyun DS, Park SH and Kim SK: Anti-fibrotic effect of
thalidomide through inhibiting TGF-beta-induced ERK1/2 pathways in
bleomycin-induced lung fibrosis in mice. Inflamm Res. 59:177–188.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang CJ, Yen YH, Hung LY, Wang SH, Pu CM,
Chien HF, Tsai JS, Lee CW, Yen FL and Chen YL: Thalidomide inhibits
fibronectin production in TGF-β1-treated normal and keloid
fibroblasts via inhibition of the p38/Smad3 pathway. Biochem
Pharmacol. 85:1594–1602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
George A, Marziniak M, Schäfers M, Toyka
KV and Sommer C: Thalidomide treatment in chronic constrictive
neuropathy decreases endoneurial tumor necrosis factor-alpha,
increases interleukin-10 and has long-term effects on spinal cord
dorsal horn met-enkephalin. Pain. 88:267–275. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shannon E, Noveck R, Sandoval F and Kamath
B: Thalidomide suppressed IL-1beta while enhancing TNF-alpha and
IL-10, when cells in whole blood were stimulated with
lipopolysaccharide. Immunopharmacol Immunotoxicol. 30:447–457.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li S, Pal R, Monaghan SA, Schafer P,
Ouyang H, Mapara M, Galson DL and Lentzsch S: IMiD immunomodulatory
compounds block C/EBP-beta translation through eIF4E
down-regulation resulting in inhibition of MM. Blood.
117:5157–5165. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Yoon JH, Won HJ, Ji HS, Yuk HJ, Park
KH, Park HY and Jeong TS: Isotrifoliol inhibits pro-inflammatory
mediators by suppression of TLR/NF-κB and TLR/MAPK signaling in
LPS-induced RAW264.7 cells. Int Immunopharmacol. 110–119. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lai CS, Lee JH, Ho CT, Liu CB, Wang JM,
Wang YJ and Pan MH: Rosmanol potently inhibits
lipopolysaccharide-induced iNOS and COX-2 expression through
downregulating MAPK, NF-kappaB, STAT3 and C/EBP signaling pathways.
J Agric Food Chem. 57:10990–10998. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hoang B, Zhu L, Shi Y, Frost P, Yan H,
Sharma S, Sharma S, Goodglick L, Dubinett S and Lichtenstein A:
Oncogenic RAS mutations in myeloma cells selectively induce cox-2
expression, which participates in enhanced adhesion to fibronectin
and chemoresistance. Blood. 107:4484–4490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prince HM, Mileshkin L, Roberts A, Ganju
V, Underhill C, Catalano J, Bell R, Seymour JF, Westerman D,
Simmons PJ, et al: A multicenter phase II trial of thalidomide and
celecoxib for patients with relapsed and refractory multiple
myeloma. Clin Cancer Res. 11:5504–5514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Knight R: IMiDs: A novel class of
immunomodulators. Semin Oncol. 32 4 Suppl 5:S24–S30. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Teo SK: Properties of thalidomide and its
analogues: Implications for anticancer therapy. AAPS J. 7:E14–E19.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Galustian C, Meyer B, Labarthe MC, Dredge
K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, et
al: The anti-cancer agents lenalidomide and pomalidomide inhibit
the proliferation and function of T regulatory cells. Cancer
Immunol Immunother. 58:1033–1045. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Luptakova K, Rosenblatt J, Glotzbecker B,
Mills H, Stroopinsky D, Kufe T, Vasir B, Arnason J, Tzachanis D,
Zwicker JI, et al: Lenalidomide enhances anti-myeloma cellular
immunity. Cancer Immunol Immunother. 62:39–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Altman A and Villalba M: Protein kinase
C-theta (PKC theta): A key enzyme in T cell life and death. J
Biochem. 132:841–846. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Payvandi F, Wu L, Naziruddin SD, Haley M,
Parton A, Schafer PH, Chen RS, Muller GW, Hughes CC and Stirling
DI: Immunomodulatory drugs (IMiDs) increase the production of IL-2
from stimulated T cells by increasing PKC-theta activation and
enhancing the DNA-binding activity of AP-1 but not NF-kappaB,
OCT-1, or NF-AT. J Interferon Cytokine Res. 25:604–616. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim BS, Kim JY, Lee JG, Cho Y, Huh KH, Kim
MS and Kim YS: Immune modulatory effect of thalidomide on T cells.
Transplant Proc. 47:787–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gandhi AK, Kang J, Havens CG, Conklin T,
Ning Y, Wu L, Ito T, Ando H, Waldman MF, Thakurta A, et al:
Immunomodulatory agents lenalidomide and pomalidomide co-stimulate
T cells by inducing degradation of T cell repressors Ikaros and
Aiolos via modulation of the E3 ubiquitin ligase complex
CRL4(CRBN). Br J Haematol1. 64:811–821. 2014. View Article : Google Scholar
|
|
38
|
Zhu D, Corral LG, Fleming YW and Stein B:
Immunomodulatory drugs Revlimid (lenalidomide) and CC-4047 induce
apoptosis of both hematological and solid tumor cells through NK
cell activation. Cancer Immunol Immunother. 57:1849–1859. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu L, Adams M, Carter T, Chen R, Muller G,
Stirling D, Schafer P and Bartlett JB: lenalidomide enhances
natural killer cell and monocyte-mediated antibody-dependent
cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clin
Cancer Res. 14:4650–4657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yaccoby S, Johnson CL, Mahaffey SC,
Wezeman MJ, Barlogie B and Epstein J: Antimyeloma efficacy of
thalidomide in the SCID-hu model. Blood. 100:4162–4168. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lv P, Luo HS, Zhou XP, Xiao YJ, Paul SC,
Si XM and Zhou YH: Reversal effect of thalidomide on established
hepatic cirrhosis in rats via inhibition of nuclear
factor-kappaB/inhibitor of nuclear factor-kappaB pathway. Arch Med
Res. 38:15–27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lebrin F, Srun S, Raymond K, Martin S, van
den Brink S, Freitas C, Bréant C, Mathivet T, Larrivée B, Thomas
JL, et al: Thalidomide stimulates vessel maturation and reduces
epistaxis in individuals with hereditary hemorrhagic
telangiectasia. Nat Med. 16:420–428. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gupta D, Treon SP, Shima Y, Hideshima T,
Podar K, Tai YT, Lin B, Lentzsch S, Davies FE, Chauhan D, et al:
Adherence of multiple myeloma cells to bone marrow stromal cells
upregulates vascular endothelial growth factor secretion:
Therapeutic applications. Leukemia. 15:1950–1961. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yabu T, Tomimoto H, Taguchi Y, Yamaoka S,
Igarashi Y and Okazaki T: Thalidomide-induced antiangiogenic action
is mediated by ceramide through depletion of VEGF receptors and is
antagonized by sphingosine-1-phosphate. Blood. 106:125–134. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dredge K, Horsfall R, Robinson SP, Zhang
LH, Lu L, Tang Y, Shirley MA, Muller G, Schafer P, Stirling D, et
al: Orally administered lenalidomide (CC-5013) is anti-angiogenic
in vivo and inhibits endothelial cell migration and Akt
phosphorylation in vitro. Microvasc Res. 69:56–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Komorowski J, Jerczyńska H, Siejka A,
Barańska P, Ławnicka H, Pawłowska Z and Stepień H: Effect of
thalidomide affecting VEGF secretion, cell migration, adhesion and
capillary tube formation of human endothelial EA. hy 926 cells.
Life sciences. 78:2558–2563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jinnin M: Mechanisms of skin fibrosis in
systemic sclerosis. J Dermatol. 37:11–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Oliver SJ, Moreira A and Kaplan G: Immune
stimulation in scleroderma patients treated with thalidomide. Clin
Immunol. 97:109–120. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Weingärtner S, Zerr P, Tomcik M,
Palumbo-Zerr K, Distler A, Dees C, Beyer C, Shankar SL, Cedzik D,
Schafer PH, et al: Pomalidomide is effective for prevention and
treatment of experimental skin fibrosis. Ann Rheum Dis.
71:1895–1899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ingen-Housz-Oro S, Ortonne N, Elhai M,
Allanore Y, Aucouturier P and Chosidow O: IgG4-related skin disease
successfully treated by thalidomide: A report of 2 cases with
emphasis on pathological aspects. JAMA Dermatol. 149:742–187. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Selman M, King TE and Pardo A: American
Thoracic Society; European Respiratory Society; American College of
Chest Physicians: Idiopathic pulmonary fibrosis: Prevailing and
evolving hypotheses about its pathogenesis and implications for
therapy. Ann Intern Med. 134:136–151. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
SHI Jie, Qiang N-X and YU Min: Clinical
research and correlated cytokine study of thalidomide combined with
prednisone on idiopathic pulmonary fibrosis. Practical Pharmacy and
Clinical Remedies. 10:52012.
|
|
53
|
Zhang L and Yang WL: Effect of thalidomide
on the expressions of IL-6, TNF-α and TGF-β1 in BALF of elder
patients with idiopathic pulmonary fibrosis. Journal of Xian
Jiaotong University (Medical Sciences). 5:622–625. 2012.
|
|
54
|
Tabata C, Tabata R, Kadokawa Y, Hisamori
S, Takahashi M, Mishima M, Nakano T and Kubo H: Thalidomide
prevents bleomycin-induced pulmonary fibrosis in mice. J Immunol.
179:708–714. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Knobloch J, Jungck D and Koch A: Apoptosis
induction by thalidomide: Critical for limb teratogenicity but
therapeutic potential in idiopathic pulmonary fibrosis? Curr Mol
Pharmacol. 4:26–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Horton MR, Santopietro V, Mathew L, Horton
KM, Polito AJ, Liu MC, Danoff SK and Lechtzin N: Thalidomide for
the treatment of cough in idiopathic pulmonary fibrosis: A
randomized trial. Ann Intern Med. 157:398–406. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rethinking thalidomide: Environ Health
Perspect. 103:1321995.
|
|
58
|
Mall JW, Schwenk W, Philipp AW, Büttemeyer
R and Pollmann C: Intraperitoneal administration of the
angiogenesis inhibitor thalidomide does not impair anastomotic
healing following large bowel resection in a rabbit model. World J
Surg. 27:1119–1123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kruse FE, Joussen AM, Rohrschneider K,
Becker MD and Völcker HE: Thalidomide inhibits corneal angiogenesis
induced by vascular endothelial growth factor. Graefes Arch Clin
Exp Ophthalmol. 236:461–466. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ribeiro JC, Vagnaldo Fechine F, Ribeiro
MZ, Barreiro EJ, Lima LM, Ricardo NM, de Moraes Amaral ME and de
Moraes Odorico M: Potential inhibitory effect of LASSBio-596, a new
thalidomide hybrid, on inflammatory corneal angiogenesis in
rabbits. Ophthalmic Res. 48:177–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Abbas A, Khan B, Feroze AH and Hyman GF:
Thalidomide prevents donor corneal graft neovascularization in an
alkali burn model of corneal angiogenesis. J Pak Med Assoc.
52:476–482. 2002.PubMed/NCBI
|
|
62
|
Lee YK and Chung SK: The inhibitory effect
of thalidomide analogue on corneal neovascularization in rabbits.
Cornea. 32:1142–1148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Srinivasan S, Perez-Gomez I, O'Donnell C
and Batterbury M: Corneal endothelial abnormalities associated with
thalidomide toxicity. Cornea. 24:103–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Huang YH and Tseng SH: Corneal snowflakes.
Lancet. 380:5062012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Guex-Crosier Y, Pittet N and Herbort CP:
The effect of thalidomide and supidimide on endotoxin-induced
uveitis in rats. Graefes Arch Clin Exp Ophthalmol. 233:90–93. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Rodrigues GB, Passos GF, Di Giunta G,
Figueiredo CP, Rodrigues EB, Grumman A Jr, Medeiros R and Calixto
JB: Preventive and therapeutic anti-inflammatory effects of
systemic and topical thalidomide on endotoxin-induced uveitis in
rats. Exp Eye Res. 84:553–560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Parentin F, Da Pozzo S, Lepore L and
Perissutti P: Thalidomide effectiveness for bilateral chronic
idiopathic anterior uveitis in a three-year-old child.
Ophthalmologica. 215:70–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ip M and Gorin MB: Recurrence of a
choroidal neovascular membrane in a patient with punctate inner
choroidopathy treated with daily doses of thalidomide. Am J
Ophthalmol. 122:594–595. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rabinowitz R, Katz G, Rosner M, Pri-Chen S
and Spierer A: The effect of thalidomide on neovascularization in a
mouse model of retinopathy of prematurity. Graefes Arch Clin Exp
Ophthalmol. 246:843–848. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Verma AS and Fitzpatrick DR: Anophthalmia
and microphthalmia. Orphanet J Rare Dis. 2:472007. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ema M, Ise R, Kato H, Oneda S, Hirose A,
Hirata-Koizumi M, Singh AV, Knudsen TB and Ihara T: Fetal
malformations and early embryonic gene expression response in
cynomolgus monkeys maternally exposed to thalidomide. Reprod
Toxicol. 29:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Christian MS, Laskin OL, Sharper V,
Hoberman A, Stirling DI and Latriano L: Evaluation of the
developmental toxicity of lenalidomide in rabbits. Birth Defects
Res B Dev Reprod Toxicol. 80:188–207. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zeldis JB, Carter TL, Knight RD and Hui J:
Pomalidomide is teratogenic in rats and rabbits and can be
neurotoxic in humans. Proc Natl Acad Sci USA. 110:pp. E48192013,
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Narita N, Kato M, Tazoe M, Miyazaki K,
Narita M and Okado N: Increased monoamine concentration in the
brain and blood of fetal thalidomide- and valproic acid-exposed
rat: Putative animal models for autism. Pediatr Res. 52:576–579.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Elkinson S and McCormack PL: Pomalidomide:
First global approval. Drugs. 73:595–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lacy MQ: New immunomodulatory drugs in
myeloma. Curr Hematol Malig Rep. 6:120–125. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Streetly MJ, Gyertson K, Daniel Y, Zeldis
JB, Kazmi M and Schey SA: Alternate day pomalidomide retains
anti-myeloma effect with reduced adverse events and evidence of in
vivo immunomodulation. Br J Haematol. 141:41–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Lacy MQ, Kumar SK, LaPlant BR, Laumann K,
Gertz MA, Hayman SR, Buadi FK, Dispenzieri A, Lust JA, Russell R,
et al: Pomalidomide plus low-dose dexamethasone (Pom/Dex) in
relapsed myeloma: Long term follow up and factors predicing outcome
in 345 patients. Blood. 120:2012012.
|
|
79
|
Daver N, Shastri A, Kadia T, Newberry K,
Pemmaraju N, Jabbour E, Zhou L, Pierce S, Cortes J, Kantarjian H
and Verstovsek S: Phase II study of pomalidomide in combination
with prednisone in patients with myelofibrosis and significant
anemia. Leuk Res. 38:1126–1129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Weber DM, Chen C, Niesvizky R, Wang M,
Belch A, Stadtmauer EA, Siegel D, Borrello I, Rajkumar SV,
Chanan-Khan AA, et al: Lenalidomide plus dexamethasone for relapsed
multiple myeloma in North America. N Engl J Med. 357:2133–2142.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Dimopoulos M, Spencer A, Attal M, Prince
HM, Harousseau JL, Dmoszynska A, San Miguel J, Hellmann A, Facon T,
Foà R, et al: Lenalidomide plus dexamethasone for relapsed or
refractory multiple myeloma. N Engl J Med. 357:2123–2132. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Chen C, Reece DE, Siegel D, Niesvizky R,
Boccia RV, Stadtmauer EA, Abonour R, Richardson P, Matous J, Kumar
S, et al: Expanded safety experience with lenalidomide plus
dexamethasone in relapsed or refractory multiple myeloma. Br J
Haematol. 146:164–170. 2009. View Article : Google Scholar : PubMed/NCBI
|