Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancers types, which is the third leading cause of
cancer-associated death worldwide (1). Surgery is the primary therapy for
early-stage HCC, but metastasis and recurrence rates remain high at
five years post-surgery. In spite of the progress regarding the
therapeutic options for advanced HCC, the prognosis of HCC patients
remains poor (2). Research on drugs
with antitumor activity may lead to the development of novel
chemotherapeutic agents.
Niu-Huang-Shen (NHS), a modern Chinese medicine, is
prepared from cholic acid, hyodeoxycholic acid, baicalin and five
other medicinal materials (3,4). A
previous study revealed that NHS has a wide pharmacological
spectrum, exerting antipyretic, anti-inflammatory and vasodilation
effects (5). However, whether NHS
has any inhibitory effect on HCC cell phenotypes has remained
elusive.
As indicated by several studies, dysregulation of
the Hippo signaling pathway may lead to the formation of HCC
(6,7). The Hippo signaling cascade regulates
the expression of genes associated with cell cycle progression,
proliferation, differentiation and survival. Yes-associated protein
(YAP) is the effect or of the Hippo pathway, which is markedly
elevated in samples of hepatitis B virus-induced HCC (8). Overexpression of YAP was significantly
associated with a higher tumor grade and higher serum α-fetoprotein
levels (P=0.021) (9). YAP
coordinates interactions with these signaling pathways by the
induction of the expression of various genes, including those
involved in the transforming growth factor-β/SMAD, WNT/β-catenin,
phosphoinositide-3 kinase/AKT, c-Jun N-terminal kinase, Hedgehog,
Janus kinase/signal transducer and activator of transcription,
Notch and apoptotic pathways (10).
The present study assessed the effect of NHS on malignant
phenotypes of HCC. It was revealed that NHS modulated cell
phenotypes by regulating YAP expression. It was concluded that NHS
downregulated YAP expression, inhibited the Hippo signaling
pathway, and suppressed HCC cell growth and invasion. NHS may
potentially be a novel therapeutic for HCC.
Materials and methods
Cell lines
The SMMC-7721 and MHCC-97H HCC cell lines were
obtained from the Cell Bank of the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China). Cells were
maintained at 37°C in a humidified incubator containing 5%
CO2 in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.).
Preparation and treatment of NHS
solution
To produce stock solutions, NHS (supplied by Hubei
Chinese Medical University, Wuhan, China) was dissolved in
different volumes of 50% dimethylsulfoxide (DMSO) solution (10%, 1
g NHS in 10 ml; 20%, 1 g NHS in 5 ml; 40%, 1 g NHS in 2.5 ml 50%
DMSO). After mixing, the NHS solution was filtered (0.45 pm pore
filter). A total of 2 µl of NHS solution at different
concentrations of (0, 10, 20 and 40%) was incubated with DMEM with
different concentration for 48 h at 37°C and used for following
experiments.
Cell Counting Kit-8 (CCK-8) assay
HCC cells were seeded in 96-well plates at
1×103 cells with 100 µl NHS solution per well. Cells
were treated with different concentration of NHS (0, 10, 20 and
40%) as described above. The viability of the cells was assessed
from three replicates in three independent experiments by the CCK-8
assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
Colony formation assay
Cells were seeded at a density of 1,000 cells per
well in 6-well plates and cultured with different concentrations of
NHS (0, 10, 20 and 40%) for 2 weeks at 37°C; the colonies were
stained with 1% crystal violet (Beijing Solarbio Science &
Technology Co., Ltd., Beijing, China) and counted.
Western blot analysis
HCC cells were lysed with radio immune precipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
supplemented with protease inhibitors (Roche Diagnostics, Basel,
Switzerland). Concentration of protein samples was determined by
Bicinchoninic acid (BCA) method (Invitrogen; Thermo Fisher
Scientific, Inc.). Protein samples (30 µg) were separated by 10%
SDS-PAGE and transfer redonto polyvinylidene difluoride membranes
(EMD Millipore, Billerica, MA, USA), blocked with 5% bovine serum
albumin (Beijing Solarbio Science & Technology Co., Ltd.) for 1
h at room temperature and immunoblotted with rabbit anti-YAP
(sc-15407; 1:500) and mouse anti-GAPDH (sc-47724; 1:1,000; both
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C.
After the incubation with the corresponding secondary antibodies
conjugated to horseradish peroxidase (HRP-conjugated Affinipure
Goat Anti-Mouse or rabbit IgG (H+L); cat. no. SA00001-1/2;
Proteintech, Wuhan, China; 1:5,000) for 1 h at room temperature,
the signals of the membranes were detected by an Immobilon Western
Chemiluminescent HRP Substrate (EMD Millipore). Bands were
visualized using the ECL detection system (GE Amersham ECL Prime;
GE Healthcare Life Sciences, Little Chalfont, UK).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) following the
standard procedure. cDNA was synthesized using a Prime Script 1st
Strand cDNA Synthesis kit (Takara Bio Inc., Otsu, Japan). The
thermo cycling conditions were as follows: 42°C for 10 min followed
by 37°C for 30 min. qPCR was performed using SYBR Premix Ex Taq
(Takara Bio Inc.). The thermo cycling conditions were as follows:
initial denaturation at 95°C for 10 min followed by 40 cycles of
95°C for 10 sec and 60°C for 30 sec. The following primers were
used: YAP forward, 5′-CAGAACCGTTTCCCAGACTAC-3′ and reverse,
5′-ATCAGCTCCTCTCCTTCTATGT-3′; GAPDH forward,
5′-GGTGTGAACCATGAGAAGTATGA-3′ and reverse,
5′-GAGTCCTTCCACGATACCAAAG-3′; CTGF forward,
5′-GCTGACCTGGAAGAGAACATTA-3′ and reverse, 5′-TGCAGCCAGAAAGCTCAA-3′;
cyclin E forward, 5′-GTATCAGTGGTGCGACATAGAG-3′ and reverse,
5′-GTATGTTGTGTGCATCTTCATCAG-3′; c-myc forward,
5′-CATACATCCTGTCCGTCCAAG-3′ and reverse,
5′-GAGTTCCGTAGCTGTTCAAGT-3′. Values were normalized to the control
using the 2−∆∆Cq method (11).
Fluorescence-activated cell sorting
(FACS) assay
Cells (5×105) were trypsinized and
re-suspended to generate single-cell suspensions. For cell cycle
analysis, cells were fixed in 70% ethanol, stained with propidium
iodideand analyzed with a FACS can flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA). For apoptosis analysis, cells were
stained with fluorescein isothiocyanate-conjugated Annexin V and
7-aminoactinomycin D (Keygen Biotech, Nanjing, China) according to
the manufacturer's protocol. Cells were then analyzed with a FACS
can flow cytometer. All data were analyzed by FlowJo software
(FlowJo LLC, Ashland, OR, USA).
Invasion assay
Cells (1×105/well) were seeded in
serum-free DMEM in the upper chambers of a 24-well Transwell
invasion insert (BD Biosciences) whose membranes were coated with
Matrigel. The lower chamber was filled with DMEM containing 10%
FBS. After 24 h, cells on the upper side of the membrane were
removed and the cells that had transgressed to the membrane were
fixed in 4% paraformaldehyde and then stained with crystal violet
for 1 h at room temperature.
Statistical analysis
All statistical analyses were performed using SPSS
software 18.0 (SPSS, Inc., Chicago, IL, USA). Data were analyzed
using Student's t-test or one-way analysis of variance with
Dunnett's post hoc test. Values are expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference between groups.
Results
NHS suppresses the growth of HCC
cells
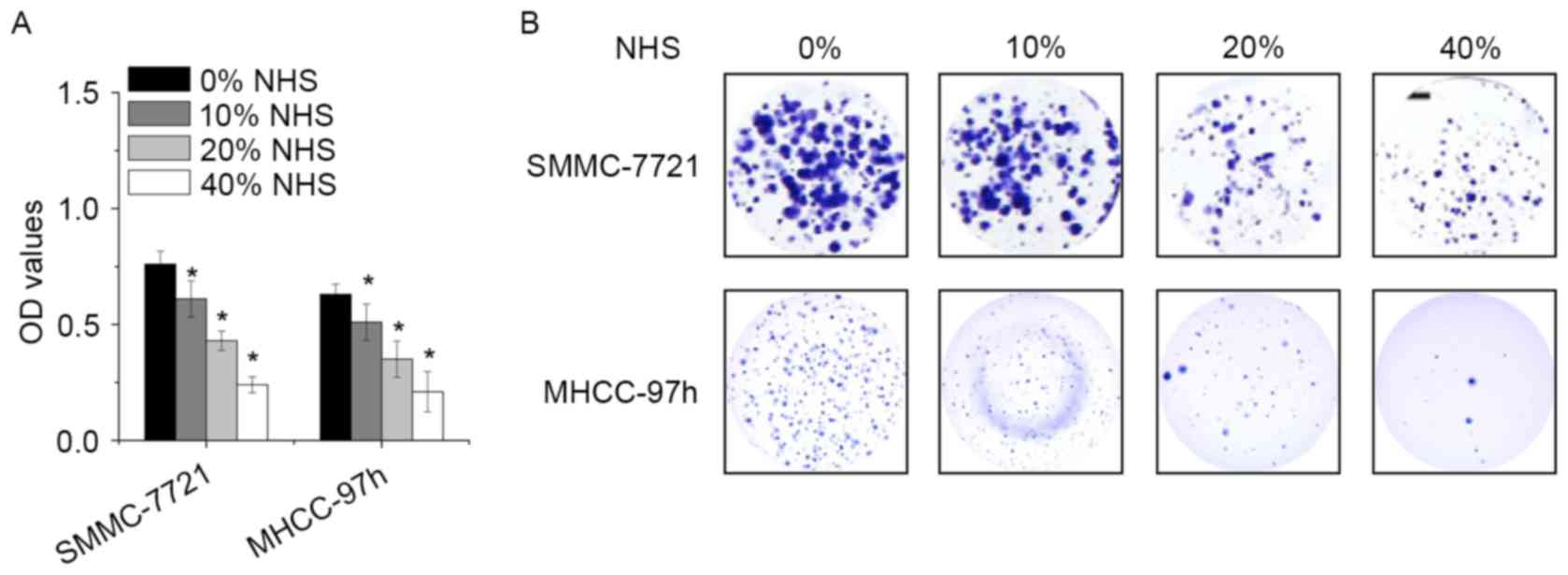
To examine the biological effect of NHS, SMMC-7721
and MHCC-97h cells were treated with different concentration of NHS
for 48 h, and the cell viability was assessed by a CCK-8 assay. NHS
decreased the proliferation of SMMC-7721 and MHCC-97h cells in a
dose-dependent manner (Fig. 1A). In
addition, high-dose NHS decreased the number of colonies. Colony
formation was robustly suppressed by NHS at 0.4% (Fig. 1B). These results demonstrated that
NHS exhibits potent anti-proliferative effects in HCC cells.
NHS induces S-phase arrest and
apoptosis in HCC cells
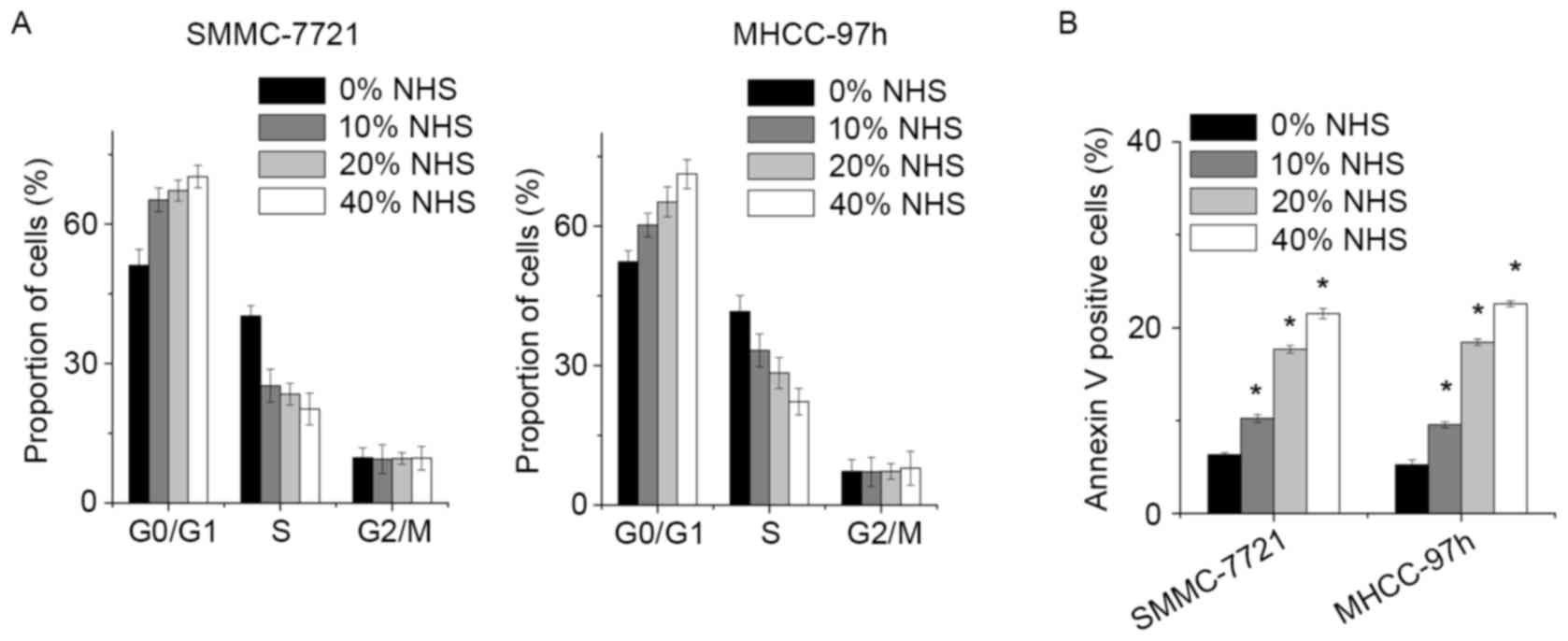
To gain insight into the mechanism by which NHS
inhibited the growth of HCC cells, the cell cycle distribution of
SMMC-7721 and MHCC-97h cells following NHS treatment was determined
by FACS. In comparison with the control group, NHS induced a
significant decrease in the S-phase population and increased the
percentage of G1-phase cells in a dose-dependent manner (Fig. 2A).
Next, a FACS assay was used to assess whether NHS
induced cell apoptosis. Increased amounts of apoptotic cells were
detected among the SMMC-7721 and MHCC-97h cells treated with NHS.
Of note, NHS potently induced SMMC-7721 cell apoptosis in a
dose-dependent manner (Fig. 2B).
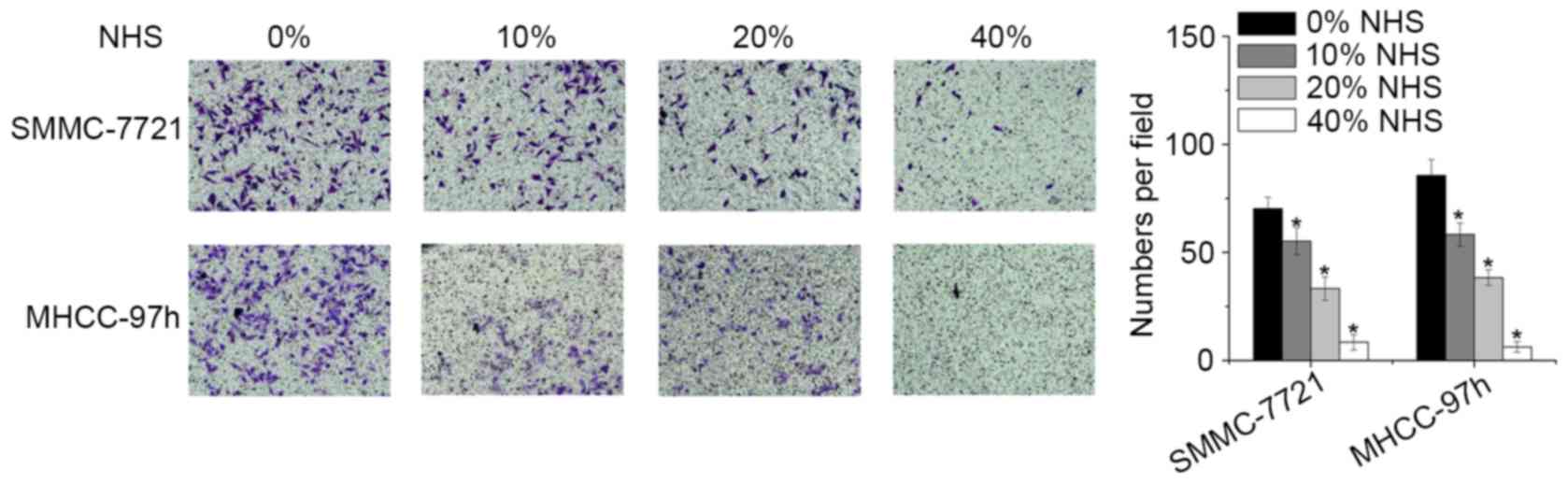
NHS suppresses HCC cell invasion
The present study also investigated alterations in
the invasive capacity of HCC cells treated with NHS using a
Matrigel Transwell assay. Compared with the control and low-dose (0
and 0.01%) groups, cells treated with NHS at high doses (0.02 and
0.040%) exhibited a significantly decreased invasive capacity
(Fig. 3).
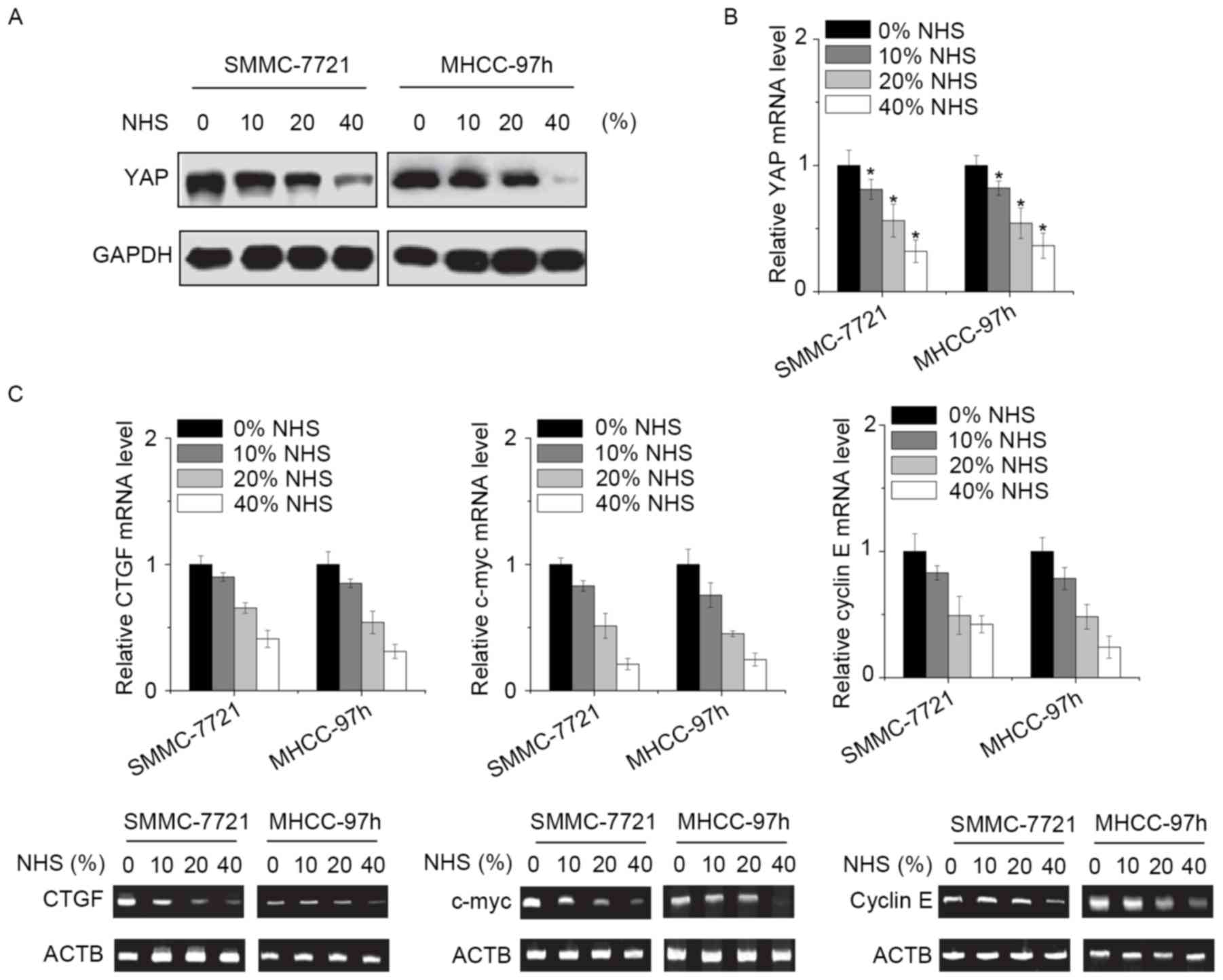
NHS regulates YAP expression
Finally, the present study explored the potential
molecular mechanisms by which NHS suppressed the proliferation and
invasion of HCC cells. YAP is the effect or of the Hippo pathway,
which is involved in the growth and metastasis of HCC cells
(7). As YAP was likely to be
involved in NHS-induced inhibitory effect, its expression was
assessed. Of note, the mRNA and protein levels of YAP were
significantly downregulated by NHS in a dose-dependent manner
(Fig. 4A and B). In addition,
certain target genes of YAP, including connective tissue growth
factor, c-myc and cyclin E were assessed. The results indicated
that all of these genes were suppressed by NHS treatment in HCC
cells (Fig. 4C). It was therefore
suggested that NHS inhibits HCC cells through suppression of YAP
expression.
Discussion
To the best of our knowledge, the effect of NHS in
cancer therapy has not previously been investigated. The present
study demonstrated that NHS is capable of inhibiting the
proliferation and invasion of HCC cells, and to induce cell cycle
arrest and apoptosis. Furthermore, the anti-cancer effects of NHS
in HCC cells were assessed. The possible underlying mechanisms via
which NHS inhibits HCC cells were also investigated. To the best of
our knowledge, the present study was the first to assess the effect
and mechanism of action of NHS in HCC cells.
The antitumor activity of NHS on HCC cells was
assessed using CCK-8 and colony forming assays. The results
demonstrated that NHS inhibited cell growth in a dose-dependent
manner. FACS analysis revealed that NHS induced cell cycle arrest
at the G1 phase in a dose-dependent manner. FACS analysis also
demonstrated that NHS produced a dose-dependent increase in the
apoptotic cell population, suggesting that apoptosis may be a
sequential event of cell cycle arrest induced by NHS.
YAP acts as an oncogene in several tissue types if
its activity is aberrantly increased. Increased YAP activity has a
potent pro-metastatic effect in cancer cells. YAP is able to
interact with the TEA domain family/transcriptional enhancer factor
transcription factors, which is essential for YAP-mediated tumor
growth and metastasis. YAP enhances multiple processes known to be
important for tumor progression and metastasis, including cellular
proliferation, transformation, migration and invasion (12–14). The
present study revealed a dose-dependent suppression of YAP
expression by NHS. A decrease of YAP was accompanied with
suppression of cell growth and invasion. Therefore, identifying the
exact component of NHS that selectively suppresses YAP1 will be
helpful for treating HCC more effectively.
In conclusion, the present study found that NHS
inhibited HCC cell growth and invasion in vitro. NHS was
also identified to decrease the expression of YAP and several of
its downstream signaling molecules. NHS led to G1 phase arrest of
the HCC cells as well as apoptosis. This dual effect of NHS may
have led to its marked inhibitory effect on HCC cells in
vitro. The present study revealed the ability of NHS to inhibit
HCC in vitro. These results suggested that NHS may represent
an effective drug for use in HCC cancer therapy.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chow PK, Choo SP, Ng DC, Lo RH, Wang ML,
Toh HC, Tai DW, Goh BK, Wong JS, Tay KH, et al: National cancer
centre Singapore consensus guidelines for hepatocellular carcinoma.
Liver Cancer. 5:97–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang GH, Lan R, Zhen XD, Zhang W, Xiang J
and Cai DF: An-Gong-Niu-Huang Wan protects against cerebral
ischemia induced apoptosis in rats: Up-regulation of Bcl-2 and
down-regulation of Bax and caspase-3. J Ethnopharmacol.
154:156–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu YF, Wu Q, Liang SX, Miao JW, Shi JS and
Liu J: Evaluation of hepatotoxicity potential of
cinnabar-containing An-Gong-Niu-Huang Wan, a patent traditional
Chinese medicine. Regul Toxicol Pharmacol. 60:206–211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yan SK, Xin WF, Luo GA, Wang YM and Cheng
YY: An approach to develop two-dimensional fingerprint for the
quality control of Qingkailing injection by high-performance liquid
chromatography with diode array detection. J Chromatogr A.
1090:90–97. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zender L, Spector MS, Xue W, Flemming P,
Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et
al: Identification and validation of oncogenes in liver cancer
using an integrative oncogenomic approach. Cell. 125:1253–1267.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kowalik MA, Saliba C, Pibiri M, Perra A,
Ledda-Columbano GM, Sarotto I, Ghiso E, Giordano S and Columbano A:
Yes-associated protein regulation of adaptive liver enlargement and
hepatocellular carcinoma development in mice. Hepatology.
53:2086–2096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang T, Zhang J, You X, Liu Q, Du Y, Gao
Y, Shan C, Kong G, Wang Y, Yang X, et al: Hepatitis B virus X
protein modulates oncogene Yes-associated protein by CREB to
promote growth of hepatoma cells. Hepatology. 56:2051–2059. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li H, Wang S, Wang G, Zhang Z, Wu X, Zhang
T, Fu B and Chen G: Yes-associated protein expression is a
predictive marker for recurrence of hepatocellular carcinoma after
liver transplantation. Dig Surg. 31:468–478. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mauviel A, Nallet-Staub F and Varelas X:
Integrating developmental signals: A Hippo in the (path)way.
Oncogene. 31:1743–1756. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamar JM, Stern P, Liu H, Schindler JW,
Jiang ZG and Hynes RO: The Hippo pathway target, YAP, promotes
metastasis through its TEAD-interaction domain. Proc Natl Acad Sci
USA. 109:pp. E2441–E2450. 2012, View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chan SW, Lim CJ, Chong YF, Pobbati AV,
Huang C and Hong W: Hippo pathway-independent restriction of TAZ
and YAP by angiomotin. J Biol Chem. 286:7018–7026. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee KP, Lee JH, Kim TS, Kim TH, Park HD,
Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, et al: The
Hippo-Salvador pathway restrains hepatic oval cell proliferation,
liver size, and liver tumorigenesis. Proc Natl Acad Sci USA.
107:pp. 8248–8253. 2010, View Article : Google Scholar : PubMed/NCBI
|