Introduction
Normocytic normochromic anemia is one of the
hallmarks of progressive chronic kidney disease (CKD). Normocytic
normochromic anemia is defined by a decrease in hemoglobin (Hb) to
<130 g/l in men and <120 g/l in women (1). Relatively little is known about the
development and progression of anemia in patients with CKD. As
kidney function declines in patients with advanced CKD, the
incidence of anemia increases (2).
The occurrence of anemia in patients with CKD (renal anemia) is
primarily due to an absolute or relative decrease in erythropoietin
(EPO) production by the failing kidney (1). However, other factors, including iron
and vitamin deficiency, in addition to inflammation, contribute to
the development of anemia and reduced response to treatment in
patients with CKD (2).
Erythropoiesis-stimulating agents (ESAs) and
adjuvant iron therapy are the primary methods for treating anemia
associated with CKD. ESAs potent and can increase HGB levels
significantly. However, recombinant ESAs are expensive, require
cold storage and are administered by the parenteral route. In
addition, with frequent subcutaneous administrations, ESA therapy
is cumbersome for the long-term treatment of dialysis-independent
patients with CKD. Furthermore, in patients with CKD undergoing
hemodialysis, intravenous administration of ESAs increases the
hospital's workload. Recent clinical trials have demonstrated that
higher Hb targets (≥11.3 g/dl) and/or the application of high doses
of ESAs may increase cardiovascular risk (3–5).
Therefore, the identification of novel drugs to treat
CKD-associated anemia is an important issue.
Klotho (KL) was originally identified as an
anti-aging gene, which when overexpressed extended the lifespan of
mice (6). The KL gene encodes
a single-pass transmembrane protein, and is expressed in the kidney
and parathyroid gland (7).
Furthermore, the KL protein functions as an obligate subunit of the
receptor for fibroblast growth factor 23 (FGF23) (8). FGF23 is a hormone secreted by
osteocytes and osteoblasts, which acts on renal tubular cells to
promote phosphate excretion into the urine and suppress the
synthesis of the active form of vitamin D [1,25-dihydroxyvitamin
D3; 1,25(OH)2D3] (9). Growing
evidence suggests that α-KL is a significant marker for CKD
(10,11) and a pathogenic factor in the
progression of CKD progression (12,13). A
recent study has reported that decreasing α-KL levels are
associated with the occurrence of anemia in patients with CKD
(14). The present study reviews the
recent advances in understanding of the role served by α-KL in the
development of anemia in patients with CKD, and summarizes novel
approaches for the clinical treatment of anemia in CKD.
Generation and function of KL
The α-KL protein is encoded by the KL gene,
which was identified in 1997 (7). To
date, three types of α-KL protein have been identified: Full-length
transmembrane, soluble and secreted α-KL (15). The full-length α-KL protein is a
transmembrane protein that contains two separate glycosyl hydrolase
domains, KL1 and KL2. The truncated α-KL protein, also known as
soluble α-KL, is released from the cell membrane and may contain
KL1 or KL1 and KL2 (16). Soluble
α-KL is generated from the cleavage of the membrane form of KL by
α- and β-secretases (α-cut, KL1 and KL2 domains, ~130 kDa; β-cut,
KL1 domain only, ~65 kDa) and by insulin stimulation (16). The cleaving process is inhibited by a
phosphoinositide 3-kinase (PI3K) inhibitor, which suggests that
PI3K serves a role in the cleavage process (17). Secreted α-KL (~65 kDa), generated by
alternative RNA splicing, is the primary form of circulating KL and
contains only the KL1 domain, in which the acid/base site is
mutated and the nucleophile site is conserved (16). Total circulating α-KL may include
soluble and secreted α-KL. Circulating α-KL is able to function as
a hormone to regulate the functions of cells or tissues that do not
express α-KL (16). Secreted α-KL
and the shorter form of soluble α-KL contain the KL1 domain and
have approximately the same molecular weight (~65 kDa) (16).
α-KL is a multifunctional protein that regulates
essential cellular processes and is predominantly expressed in the
kidneys (18). In addition to its
anti-aging function, α-KL serves an essential role in the
phosphatonin FGF23 signaling pathway and secreted KL functions as
an endocrine hormone (19). Previous
studies have identified that α-KL is associated with a range of
diseases, including periodontitis (20), cardiovascular disease (21) and kidney disease (18). However, the role of α-KL in
hemopathies, including anemia, remains unclear.
Association between α-KL and anemia in
CKD
Erythropoietin (EPO) and α-KL
The release of EPO by the kidneys, a hormone that is
important in the regulation of erythropoiesis, is triggered by
tissue hypoxia (22). Under hypoxic
conditions, EPO stimulates the differentiation of erythroid
progenitor cells and normoblasts to increase the amount of red
blood cell (RBCs) (22).
Hypoxia-inducible factors (HIFs) are heterodimeric proteins that
regulate the physiological response to hypoxia by altering the
expression of downstream genes, including EPO, in interstitial
cells in the renal cortex near the proximal tubule (23).
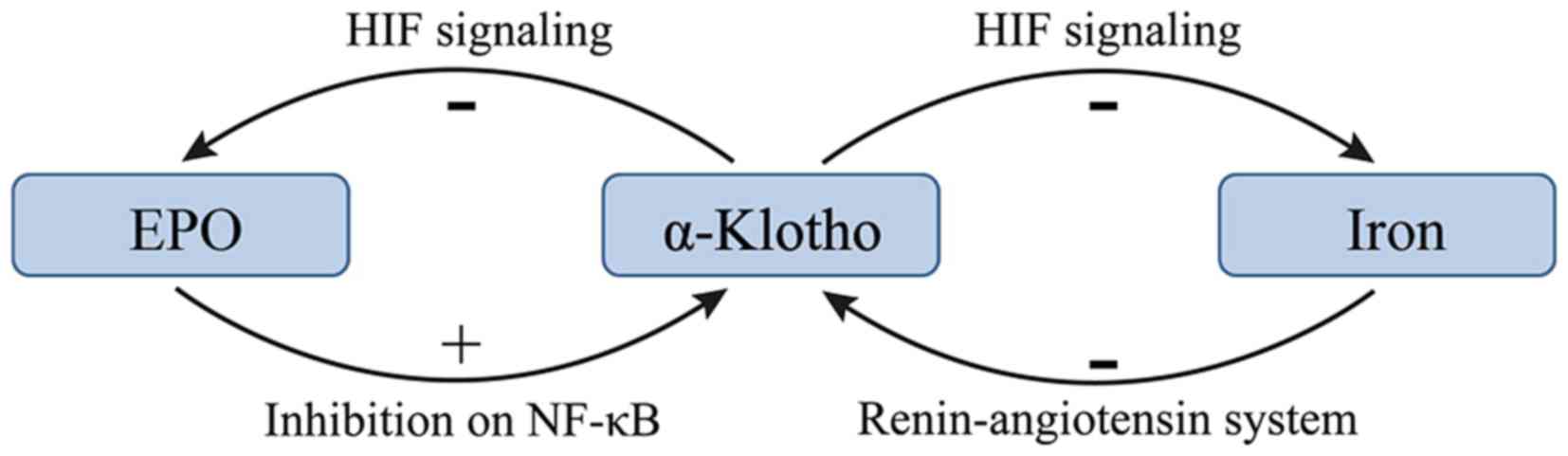
There may be a negative feedback between EPO and
α-KL such that EPO promotes the expression of α-KL and increasing
α-KL suppresses the production of EPO in CKD (22,24). KL
deficiency is a characteristic feature of CKD with anemia (22). Hu et al (10) revealed that KL expression was reduced
during the progression of kidney disease. In addition, the
treatment of anemia with EPO has been demonstrated to enhance renal
and extrarenal production of α-KL in patients with CKD (14,24).
Furthermore, EPO has been demonstrated to inhibit uremia-induced
nuclear factor-κB (NF-κB) production in endothelial cells and
prevented a decrease in intracellular KL (24). However, a previous study reported
that KL directly regulates hematopoietic stem cell differentiation,
and erythroid cell generation and maturation (22). KL deficiency in mice resulted in
increased erythropoiesis through activation of the HIF signaling
pathway, and subsequent upregulation of renal EPO synthesis and
secretion (22). Additionally, the
expression of HIF-1α and HIF-2α was significantly upregulated in
KL−/− bone tissue, resulting in localized
overexpression of EPO (22). The
molecular mechanism responsible for the KL-induced inhibition of
the HIF signaling pathway and EPO expression may be associated with
reduced osteoblast numbers and osteopenia (22). The effects of α-KL on EPO and the HIF
signaling pathway are summarized in Fig.
1.
Kempe et al (25) demonstrated that a lack of KL
expression leads to increased cytosolic Ca2+ activity,
leading to enhanced scrambling of cell membrane phospholipids and
cell shrinkage in erythrocytes. This suggests that KL deficiency
accelerates eryptosis, the suicidal death of erythrocytes. Thus,
the role of KL in hemopoiesis remains unclear and further studies
into this area are warranted.
Iron metabolism and α-KL
Iron-deficiency anemia (IDA) is responsible for ~50%
of all anemia cases (26). It has
been reported that 273,000 individuals succumb to IDA each year
worldwide (26). ‘Absolute’ iron
deficiency is common in patients with CKD and anemia (27). Different conditions, including
inflammation, liver disease and pregnancy, may induce
iron-restricted erythropoiesis and aggravate renal anemia (1). The distinction between these conditions
is clinically relevant; however, peripheral iron indices are of
little help in the differential diagnosis (28).
Iron deficiency may lead to high expression of KL in
patients with CKD. A study of 70 patients with stages I–V CKD
demonstrated that the FGF23 levels were elevated and KL levels were
decreased, and the magnitude of these changes increased as the
tumor stage increased (29). In
addition, significant correlations were identified between serum KL
levels and ferritin levels, and serum KL levels and transferrin
saturation percentage, which suggest that KL serves a negative role
in iron regulation (29). However,
little is known about the underlying molecular mechanisms of these
effects. A previous study demonstrated that serum iron overload
decreased renal expression of KL at the mRNA and protein levels,
and that iron chelation suppressed angiotensin II-induced
downregulation of KL (30). These
results indicate that the underlying mechanism of the angiotensin
II-induced downregulation of KL is the alteration of iron
metabolism in the kidney. Therefore, the renin-angiotensin system
may serve a role in the regulation of iron and α-KL.
HIFs affect the majority of aspects of iron
metabolism (23). HIF-2 enhances
iron absorption by small bowel enterocytes and augments iron export
from duodenal cells (23).
Additionally, HIFs serve a role in the utilization of iron from
senescent RBCs via reticuloendothelial system macrophages (31). In this manner HIFs increases iron
stores through absorption from the duodenum and the recycling of
senescent RBCs (31). The increased
efflux of iron to the circulation is achieved via HIF-mediated
increased expression of ferroportin in duodenal enterocytes and
macrophages (31). KL deficiency in
mice results in the activation of the HIF signaling pathway
(22). The effects the HIF signaling
pathway on iron supplies are summarized in Fig. 1.
Vitamin D and α-KL
Accumulating evidence suggests that vitamin D
deficiency increased the progression of CKD (32–34).
Vitamin D is important in anemia, with low vitamin D levels being
associated with an increased risk of anemia (35,36).
Thus, vitamin D deficiency may be a factor in the development of
anemia in patients with CKD.
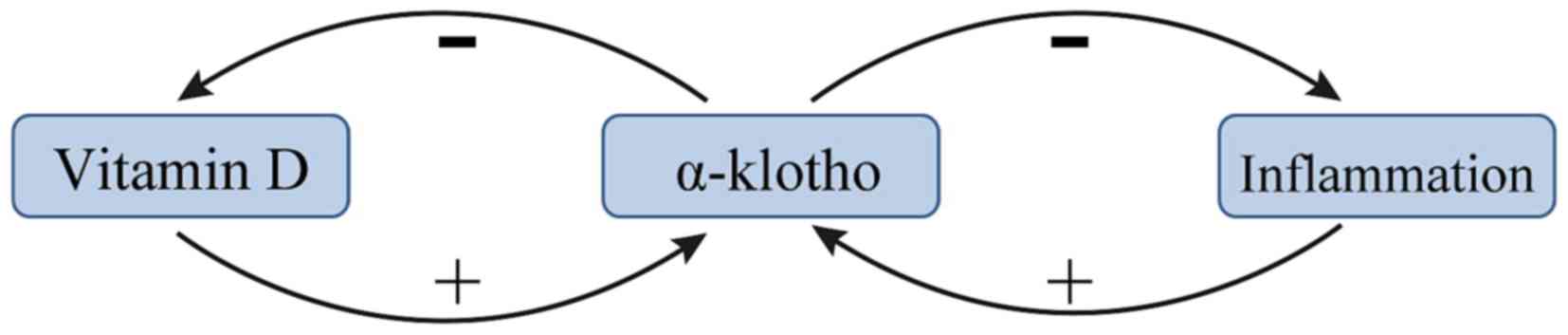
Vitamin D and KL exert negative feedback towards one
another in patients with renal anemia. A previous study suggested
that the vitamin D/parathyroid hormone (PTH) signaling pathway
regulates the KL/FGF23 signaling pathway, and vice versa (37). 1,25-dihydroxyvitamin D [1,25(OH)D2],
the metabolically active form of vitamin D, was demonstrated to
upregulate KL expression (37). PTH
may indirectly upregulate KL via upregulating 1,25(OH)D2 (38). In addition, several recent studies
have demonstrated that vitamin D stimulates the expression of FGF23
and KL, and that vitamin D formation is limited by a negative
feedback regulation (39–41). This negative feedback is lost in
kl−/kl− mice and in these mice
the formation of 1,25(OH)2D3 is a function of dietary vitamin D
even at excessive 1,25(OH)2D3 concentrations (42). The mechanism by which KL inhibits the
production of 1,25(OH)2D3 may be via inhibiting 25-hydroxyvitamin D
1α-hydroxylase (39). A summary of
the current knowledge of the association between vitamin D and α-KL
is presented in Fig. 2.
Inflammation and α-KL
Inflammation is an essential component to the body's
defense system; however, excessive inflammation is considered to be
the cause of anemia in patients with CKD (43). Inflammatory cells release large
amounts of chemokines and vasoactive factors, including monocyte
chemotactic protein-1 (MCP-1) and NF-κB, which induce the
production of profibrotic cytokines following kidney injury
(44). At the site of injury,
proinflammatory factors, including interleukin (IL)-12, MCP-1 and
NF-κB, stimulate the generation of myofibroblasts and the
deposition of extracellular matrix, eventually leading to renal
dysfunction and potentially to the indirect development of anemia
(45). Notably, anemia is a common
complication in patients with infections, autoimmune disorders,
malignancies, CKD and other inflammation-associated disorders
(46). Inflammatory cytokines impair
erythropoiesis by inhibiting the production and function of EPO,
and inhibiting erythroid progenitor cell proliferation and
differentiation. Notably, inflammation induces the expression of
iron regulatory hormone hepcidin and suppresses the iron exporter
ferroportin, restricting the supply of iron for erythropoiesis
(46).
KL is known to be an inhibitor of several
inflammatory cytokines (47). The
transcription factor NF-κB is an important stimulator of
inflammation in CKD (48). A recent
study reported that intracellular KL diminished the DNA binding
ability of NF-κB and stabilized the NF-κB/NF-κB inhibitor α
complex, thus preventing uremia-induced NF-κB expression (24). KL serves as an anti-inflammatory
modulator through negatively regulating the production of
NF-κB-associated inflammatory proteins (47). A potential mechanism for this effect
is that KL inhibits the Ser536 phosphorylation of the
NF-κB p65 subunit and its subsequent recruitment to the promoter
sites of multiple cytokines (49).
Additionally, KL may attenuate the glucose-stimulated activation of
the NF-κB by downregulating the expression of toll-like receptor 4
(50), which is associated with CKD
(51). Conversely, NF-κB regulates
the activity of exogenous and intracellular KL in endothelial cells
(52). NF-κB also suppresses the
activity of KL, potentially by promoting the production of reactive
oxygen species (52). Finally, NF-κB
also inhibits the proinflammatory effect of IL-12 (20). These results demonstrate the
anti-inflammatory effects of KL (Fig.
2).
Conclusions
Although several studies have reported that there is
reduction of α-KL in patients with CKD with anemia, the mechanisms
are complicated. Based on previous studies, it appears that low
levels of EPO in patients with CKD with anemia downregulates the
expression of α-KL, which results in high serum levels of iron and
vitamin D, mitigating anemia. This suggests that low expression of
α-KL may be a compensatory mechanism to attenuate the effects of
anemia. Meanwhile, the expression of α-KL declines when serum
levels of iron increase, and the expression of α-KL increases with
vitamin D. Overall, further understanding of the role of α-KL in
renal anemia is needed, which may offer novel insights into the
treatment of patients with CKD complicated with anemia.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460142).
References
|
1
|
Zadrazil J and Horak P: Pathophysiology of
anemia in chronic kidney diseases: A review. Biomed Pap Med Fac
Univ Palacky Olomouc Czech Repub. 159:197–202. 2015.PubMed/NCBI
|
|
2
|
Collister D, Ferguson T, Komenda P and
Tangri N: The patterns, risk factors and prediction of progression
in chronic kidney disease: A narrative review. Semin Nephrol.
36:273–282. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh AK, Szczech L, Tang KL, Barnhart H,
Sapp S, Wolfson M and Reddan D: CHOIR Investigators: Correction of
anemia with epoetin alfa in chronic kidney disease. N Engl J Med.
355:2085–2098. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drüeke TB, Locatelli F, Clyne N, Eckardt
KU, Macdougall IC, Tsakiris D, Burger HU and Scherhag A: CREATE
Investigators: Normalization of hemoglobin level in patients with
chronic kidney disease and anemia. N Engl J Med. 355:2071–2084.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pfeffer MA, Burdmann EA, Chen CY, Cooper
ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R,
Levey AS, et al: A trial of darbepoetin alfa in type 2 diabetes and
chronic kidney disease. N Engl J Med. 361:2019–2032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akasaka-Manya K, Manya H and Endo T:
Function and change with aging of alpha-klotho in the kidney. Vitam
and horm. 101:239–256. 2016. View Article : Google Scholar
|
|
7
|
Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi
H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et
al: Mutation of the mouse klotho gene leads to a syndrome
resembling ageing. Nature. 390:45–51. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kurosu H and Kuro OM: The Klotho gene
family as a regulator of endocrine fibroblast growth factors. Mol
Cell Endocrinol. 299:72–78. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ide N, Olauson H, Sato T, Densmore MJ,
Wang H, Hanai JI, Larsson TE and Lanske B: In vivo evidence for a
limited role of proximal tubular klotho in renal phosphate
handling. Kidney Int. 99:348–362:. 2016. View Article : Google Scholar
|
|
10
|
Hu MC, Shi M, Zhang J, Addo T, Cho HJ,
Barker SL, Ravikumar P, Gillings N, Bian A, Sidhu SS, et al: Renal
production, uptake and handling of circulating αKlotho. J Am Soc
Nephrol. 27:79–90. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou L, Mo H, Miao J, Zhou D, Tan RJ, Hou
FF and Liu Y: Klotho ameliorates kidney injury and fibrosis and
normalizes blood pressure by targeting the renin-angiotensin
system. Am J Pathol. 185:3211–3223. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kadoya H, Satoh M, Haruna Y, Sasaki T and
Kashihara N: Klotho attenuates renal hypertrophy and glomerular
injury in Ins2Akita diabetic mice. Clin Exp Nephrol. 20:671–678.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie J, Yoon J, An SW, Kuro-o M and Huang
CL: Soluble klotho protects against uremic cardiomyopathy
independently of fibroblast growth factor 23 and phosphate. J Am
Soc Nephrol. 26:1150–1160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milovanov YS, Mukhin NA, Kozlovskaya LV,
Milovanova SY and Markina MM: Impact of anemia correction on the
production of the circulating morphogenetic protein alpha-Klotho in
patients with stages 3B-4 chronic kidney disease: A new direction
of cardionephroprotection. Ter Arkh. 88:21–25. 2016.(In Russian;
Abstract available in Russian from the publisher). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y and Sun Z: Current understanding of
klotho. Ageing Res Rev. 8:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y and Sun Z: Molecular basis of Klotho:
from gene to function in aging. Endocr Rev. 36:174–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen CD, Podvin S, Gillespie E, Leeman SE
and Abraham CR: Insulin stimulates the cleavage and release of the
extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad
Sci USA. 104:pp. 19796–19801. 2007, View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Olauson H, Lindberg K, Amin R, Jia T,
Wernerson A, Andersson G and Larsson TE: Targeted deletion of
Klotho in kidney distal tubule disrupts mineral metabolism. J Am
Soc Nephrol. 23:1641–1651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martin A, David V and Quarles LD:
Regulation and function of the FGF23/klotho endocrine pathways.
Physiol Rev. 92:131–155. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y and Zhang Q: Periodontitis
aggravated pancreatic beta-cell dysfunction in diabetic mice
through interleukin-12 regulation on Klotho. J Diabetes Investig.
7:303–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J, Lin Y and Sun Z: Deficiency in the
anti-aging gene Klotho promotes aortic valve fibrosis through
AMPKα-mediated activation of RUNX2. Aging cell. 15:853–860:. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Madathil Vadakke S, Coe LM, Casu C and
Sitara D: Klotho deficiency disrupts hematopoietic stem cell
development and erythropoiesis. Am J Pathol. 184:827–841. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Solak Y, Cetiner M, Siriopol D, Tarim K,
Afsar B, Covic A and Kanbay M: Novel masters of erythropoiesis:
hypoxia inducible factors and recent advances in anemia of renal
disease. Blood purif. 42:160–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Buendia P, Carracedo J, Soriano S, Madueño
JA, Ortiz A, Martín-Malo A, Aljama P and Ramírez R: Klotho prevents
NFκB translocation and protects endothelial cell from
senescence induced by uremia. J Gerontol A Biol Sci Med Sci.
70:1198–1209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kempe DS, Ackermann TF, Fischer SS, Koka
S, Boini KM, Mahmud H, Föller M, Rosenblatt KP, Kuro-O M and Lang
F: Accelerated suicidal erythrocyte death in Klotho-deficient mice.
Pflugers Arch. 458:503–512. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pasricha SR, Drakesmith H, Black J,
Hipgrave D and Biggs BA: Control of iron deficiency anemia in low-
and middle-income countries. Blood. 121:2607–2617. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stancu S, Stanciu A, Zugravu A, Bârsan L,
Dumitru D, Lipan M and Mircescu G: Bone marrow iron, iron indices
and the response to intravenous iron in patients with
non-dialysis-dependent CKD. Am J Kidney Dis. 55:639–647. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barsan L, Stanciu A, Stancu S, Căpuşă C,
Brătescu L, Mandache E, Radu E and Mircescu G: Bone marrow iron
distribution, hepcidin and ferroportin expression in renal anemia.
Hematology. 20:543–552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Milovanova L, Iu Milovanov S, Kozlovskaia
LV and Mukhin NA: Significance of the morphogenetic proteins FGF-23
and Klotho as predictors of prognosis of chronic kidney disease.
Ter Arkh. 86:36–44. 2014.(In Russian). PubMed/NCBI
|
|
30
|
Saito K, Ishizaka N, Mitani H, Ohno M and
Nagai R: Iron chelation and a free radical scavenger suppress
angiotensin II-induced downregulation of klotho, an anti-aging
gene, in rat. FEBS Lett. 551:58–62. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taylor M, Qu A, Anderson ER, Matsubara T,
Martin A, Gonzalez FJ and Shah YM: Hypoxia-inducible factor-2α
mediates the adaptive increase of intestinal ferroportin during
iron deficiency in mice. Gastroenterology. 140:2044–2055. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Goncalves JG, de Braganca AC, Canale D,
Shimizu MH, Sanches TR, Moysés RM, Andrade L, Seguro AC and Volpini
RA: Vitamin D deficiency aggravates chronic kidney disease
progression after ischemic acute kidney injury. PLoS One.
9:e1072282014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goldsmith DJ: Pro: Should we correct
vitamin D deficiency/insufficiency in chronic kidney disease
patients with inactive forms of vitamin D or just treat them with
active vitamin D forms? Nephrol Dial Transplant. 31:698–705. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Agarwal R and Georgianos PI: Con:
Nutritional vitamin D replacement in chronic kidney disease and
end-stage renal disease. Nephrol Dial Transplant. 31:706–713. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Monlezun DJ, Camargo CA Jr, Mullen JT and
Quraishi SA: Vitamin d status and the risk of anemia in
community-dwelling adults: Results from the national health and
nutrition examination survey 2001–2006. Medicine (Baltimore).
94:e17992015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Suh YJ, Lee JE, Lee DH, Yi HG, Lee MH, Kim
CS, Nah JW and Kim SK: Prevalence and relationships of iron
deficiency anemia with blood cadmium and vitamin D levels in Korean
women. J Korean Med Sci. 31:25–32. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Forster RE, Jurutka PW, Hsieh JC, Haussler
CA, Lowmiller CL, Kaneko I, Haussler MR and Whitfield Kerr G:
Vitamin D receptor controls expression of the anti-aging klotho
gene in mouse and human renal cells. Biochem Biophys Res Commun.
414:557–562. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lips P: Vitamin D physiology. Prog Biophys
Mol Biol. 92:4–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuro-o M: Klotho, phosphate and FGF-23 in
ageing and disturbed mineral metabolism. Nat Rev Nephrol.
9:650–660. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Alesutan I, Feger M, Pakladok T, Mia S,
Ahmed MS, Voelkl J and Lang F: 25-Hydroxyvitamin D3
1-α-hydroxylase-dependent stimulation of renal klotho expression by
spironolactone. Kidney Blood Press Res. 37:475–487. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takenaka T, Inoue T, Ohno Y, Miyazaki T,
Nishiyama A, Ishii N and Suzuki H: Calcitriol supplementation
improves endothelium-dependent vasodilation in rat hypertensive
renal injury. Kidney Blood Press Res. 39:17–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leibrock CB, Voelkl J, Kuro-O M, Lang F
and Lang UE: 1,25(OH)2D3 dependent overt hyperactivity phenotype in
klotho-hypomorphic mice. Sci Rep. 6:248792016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ichii O, Nakamura T, Irie T, Kouguchi H,
Nakamura D, Nakamura S, Sato S, Yokoyama K, Horino T, Sunden Y, et
al: Female cotton rats (Sigmodon hispidus) develop chronic
anemia with renal inflammation and cystic changes. Histochem Cell
Biol. 146:351–362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chung ACK and Lan HY: Chemokines in renal
injury. J Am Soc Nephrol. 22:802–809. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mack M and Yanagita M: Origin of
myofibroblasts and cellular events triggering fibrosis. Kidney Int.
87:297–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang CY and Babitt JL: Hepcidin regulation
in the anemia of inflammation. Curr Opin Hematol. 23:189–197. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kurosu H, Yamamoto M, Clark JD, Pastor JV,
Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M,
Kawaguchi H, et al: Suppression of aging in mice by the hormone
Klotho. Science. 309:1829–1833. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wada J and Makino H: Inflammation and the
pathogenesis of diabetic nephropathy. Clin Sci (Lond). 124:139–152.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhao Y, Banerjee S, Dey N, LeJeune WS,
Sarkar PS, Brobey R, Rosenblatt KP, Tilton RG and Choudhary S:
Klotho depletion contributes to increased inflammation in kidney of
the db/db mouse model of diabetes via RelA (serine)536
phosphorylation. Diabetes. 60:1907–1916. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wu C, Lv C, Chen F, Ma X, Shao Y and Wang
Q: The function of miR-199a-5p/Klotho regulating TLR4/NF-kappaB
p65/NGAL pathways in rat mesangial cells cultured with high glucose
and the mechanism. Mol Cell Endocrinol. 417:84–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shao Y, Sha M, Chen L, Li D, Lu J and Xia
S: HMGB1/TLR4 signaling induces an inflammatory response following
high-pressure renal pelvic perfusion in a porcine model. Am J
Physiol Renal Physiol. 311:F915–F925. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang K, Nie L, Huang Y, Zhang J, Xiao T,
Guan X and Zhao J: Amelioration of uremic toxin indoxyl
sulfate-induced endothelial cell dysfunction by Klotho protein.
Toxicol Lett. 215:77–83. 2012. View Article : Google Scholar : PubMed/NCBI
|