Introduction
Bambusa tulda Roxburgh, also known as Indian
timber bamboo, is an evergreen gregarious bamboo with grey or
greyish-green culms that is native to the Indian subcontinent,
Indochina, Tibet and Yunnan, and naturalized in Puerto Rico and
parts of South America (1).
Bambusa tulda has been used for various purposes and has
been widely considered as one of the most useful bamboo species
(2). Bambusa tulda is widely
used in paper pulp industry in Asia (3). Bambusa tulda produces nutritive
shoots and there is high demand for edible bamboo shoots in many
Asian ethnic groups (4).
The leaves of Bambusa tulda are 3–4 cm broad
and 20–35 cm in length with an oblong-lanceolate shape (5). Medicinal uses of the leaves of
Bambusa tulda have not yet been widely studied, and the
effects of Bambusa tulda leaf extract on stem cells remain
to be thoroughly assessed. The present study aimed to evaluate the
effects of Bambusa tulda methanolic extract (BBT) on the
morphology and proliferative potential of human mesenchymal stem
cells.
Materials and methods
Preparation of plant materials
The Bambusa tulda Roxburgh was collected in
the village of Amki, Sonaimuri Upazilla, Noakhali District,
Bangladesh and a voucher specimen (no. PB022073) was deposited in
the herbarium of the Korea Research Institute of Bioscience and
Biotechnology. After drying and grinding the leaves of Bambusa
tulda, the powder (63 g) was added to 500 ml methanol. The
extraction was performed using the method of repercolation at room
temperature. The extract was filtered and concentrated by a
rotavapor under reduced pressure to obtain 2.75 g BBT.
Stem cells isolated from human
gingiva
A healthy, 63-year-old, female patient visiting the
Department of Periodontics of Seoul St. Mary's Hospital (College of
Medicine, the Catholic University of Korea, Seoul, Korea) provided
the gingivae for the study. The Institutional Review Board at Seoul
St. Mary's Hospital reviewed and gave approval for the study (no.
KC11SISI0348), and written informed consent was obtained from the
patient. All the methods used in this study were performed in
accordance with the ethical standards of the Institutional Research
Committee and with the 1964 Helsinki Declaration and its later
amendments. The epithelium of the obtained gingiva was removed and
the tissue was cut into 1–2 mm fragments. The gingival tissues were
digested with media containing dispase (1 mg/ml; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) and collagenase IV (2 mg/ml;
Sigma-Aldrich; Merck KGaA) (6).
Cells that were not attached to the culture dish were removed. The
media was changed every 2–3 days and cells were cultured in an
incubator with a humidified atmosphere of 5% CO2 and 95%
air at 37°C.
Evaluation of cellular morphology
The cells were seeded in 96-well plates at a density
of 2.0×103 cells/well and incubated in control medium
[α-minimal essential medium (α-MEM); Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA] supplemented with 15% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 200 mM
L-glutamine, 10 mM ascorbic acid 2-phosphate, 100 U/ml penicillin
and 100 µg/ml streptomycin (all from Sigma-Aldrich; Merck KGaA) in
the presence of BBT at final concentrations of 0 (control), 0.001,
0.01, 0.1 and 1%. BBT was dissolved in dimethyl sulfoxide (DMSO)
and equal amounts of DMSO (0.1%) were added to each culture sample
to offset the influence of this dissolving vehicle. The morphology
of the stem cells was evaluated using an inverted microscope
(CKX41SF; Olympus Corp., Tokyo, Japan) on days 1, 3, 5 and 7.
Cellular proliferation
Evaluation of the proliferation of the cells grown
in medium containing BBT was performed on days 1, 3, 5 and 7 using
the Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan) assay.
Tetrazolium monosodium salt was added to the culture, followed by
incubation at 37°C for 2 h. The spectrophotometric absorbance at
450 nm was determined using a microplate reader (BioTek Instruments
Inc., Winooski, VT, USA).
Immunofluorescence
Collagen I antibody (cat. no. ab76956; Abcam,
Cambridge, UK) was used for the immunofluorescent assay performed
on days 1, 3, 5 and 7. Following the specific incubation with BBT,
the cells were fixed, permeabilized, blocked and incubated with
primary antibodies to collagen I (1:67) overnight at 4°C. The
cultures were incubated for two h at room temperature with
fluorescein isothiocyanate-conjugated secondary antibody (cat. no.
F2761; 1:100; Thermo Fisher Scientific, Inc.) and then stained with
DAPI. A fluorescence microscope (Axiovert 200; Zeiss, Jena,
Germany) was used for the analyses.
Statistical analysis
Values are expressed as the mean ± standard
deviation of the experiments. A test of normality was performed and
one-way analysis of variance with Scheffe's test post hoc test was
performed to determine differences between the groups with
commercially available software (SPSS 12 for Windows; SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
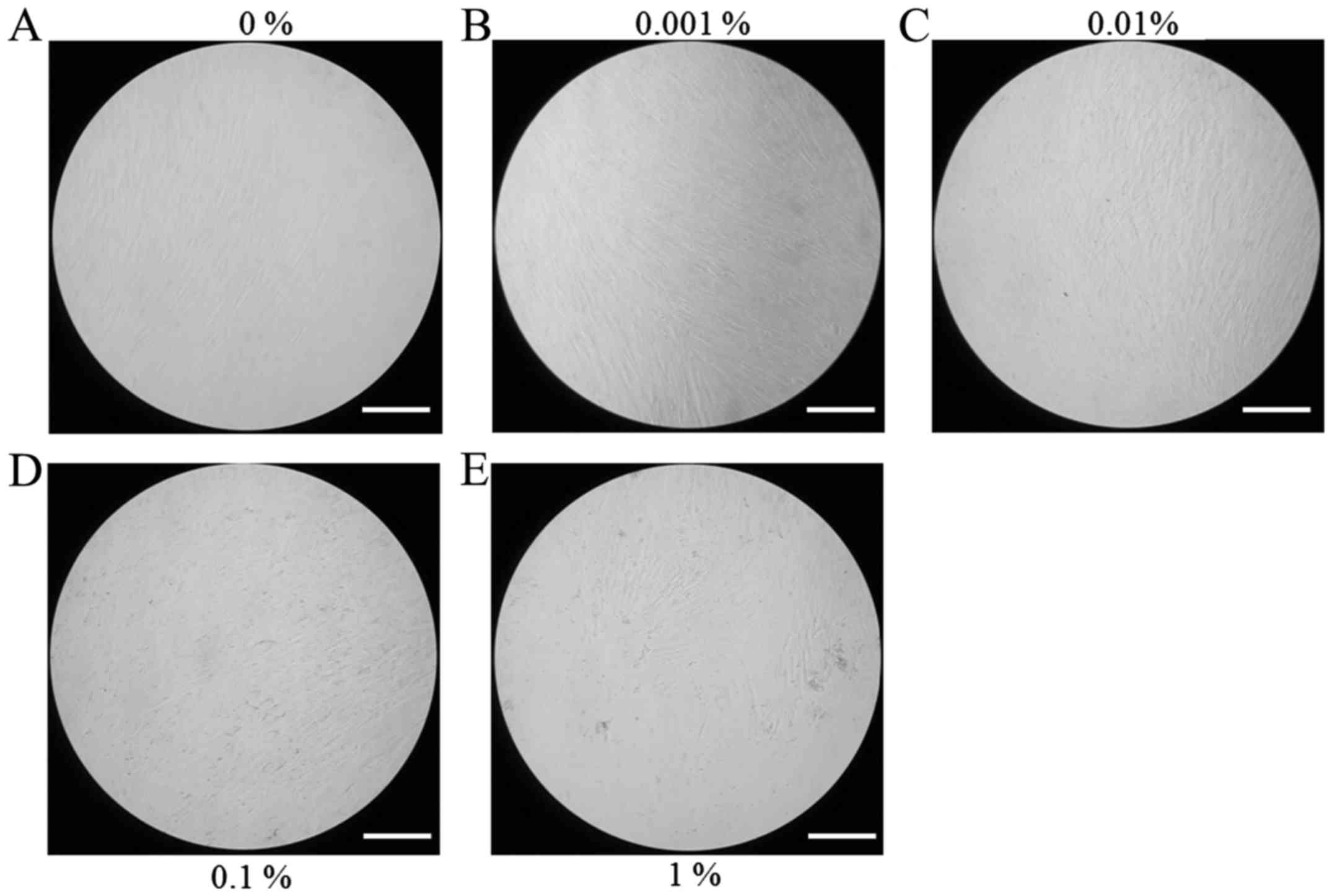
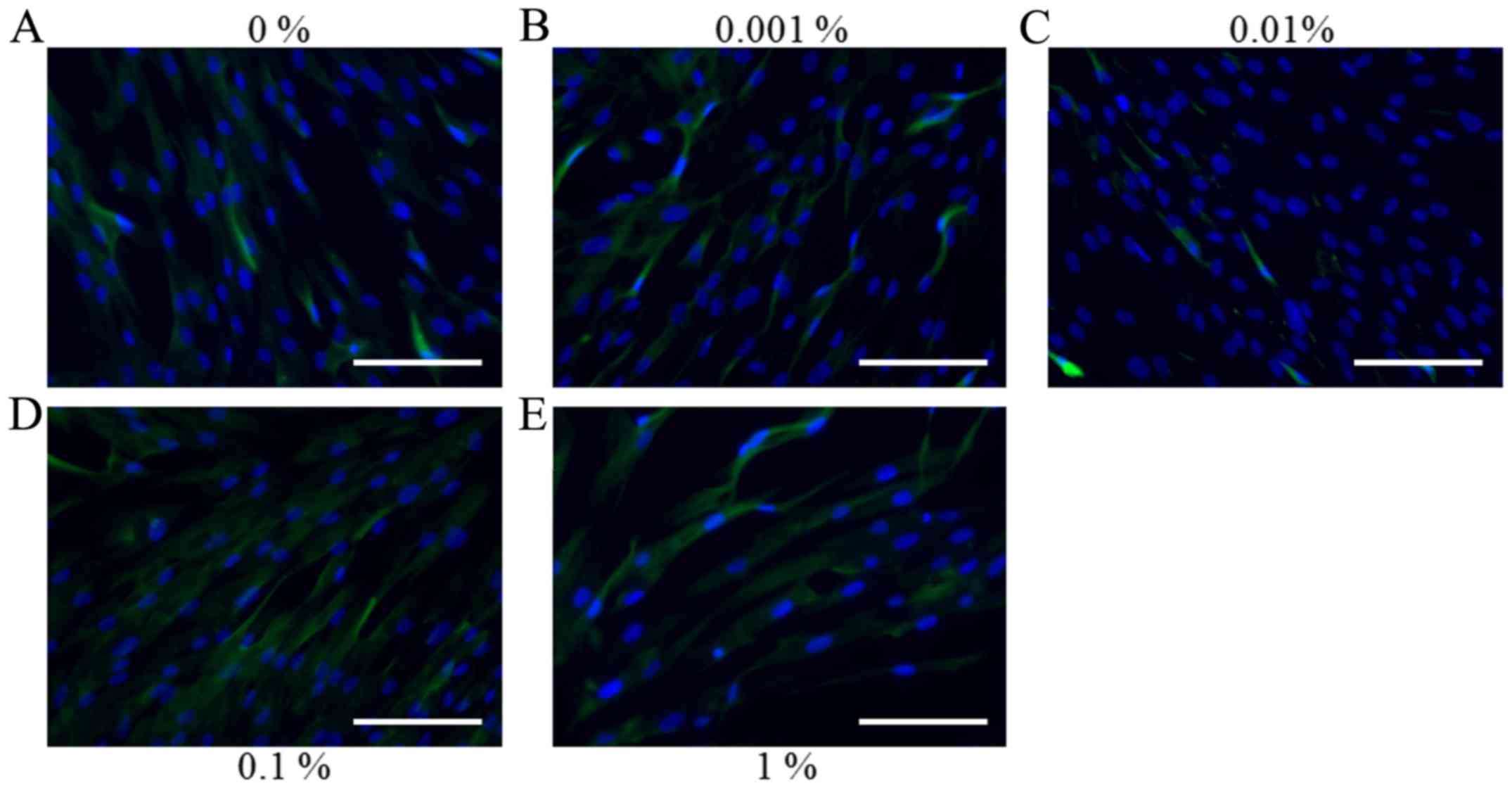
Evaluation of cell morphology
The morphology of stem cells treated with BBT at
final concentrations of 0, 0.001, 0.01, 0.1 and 1% on day 1 is
presented in Fig. 1. Stem cells in
the control group had a fibroblast-like morphology on day 1
(Fig. 1A). No noticeable differences
in the morphology of stem cells treated with BBT were observed when
compared with that in the untreated control group (Fig. 1B-E). The results on the cell
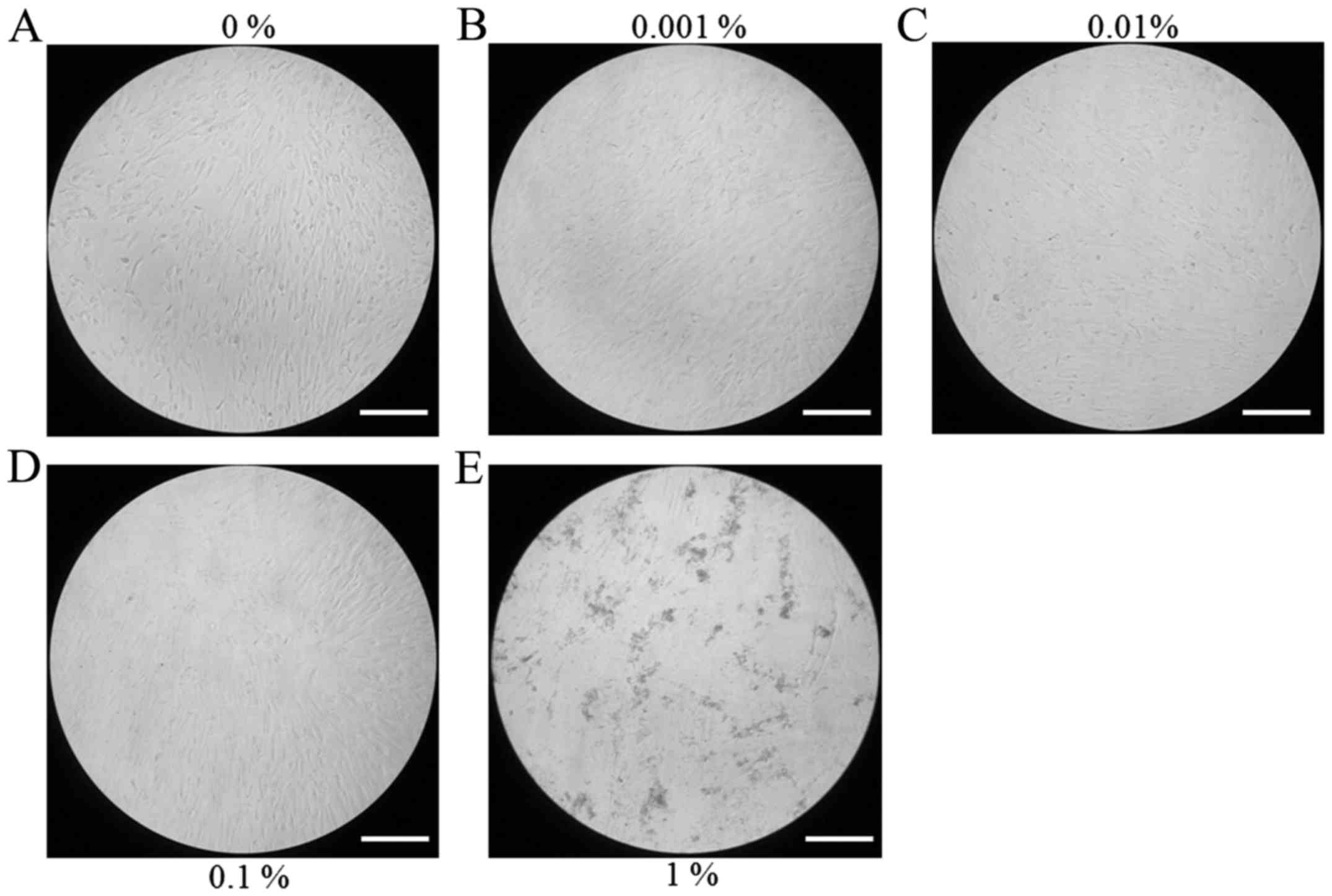
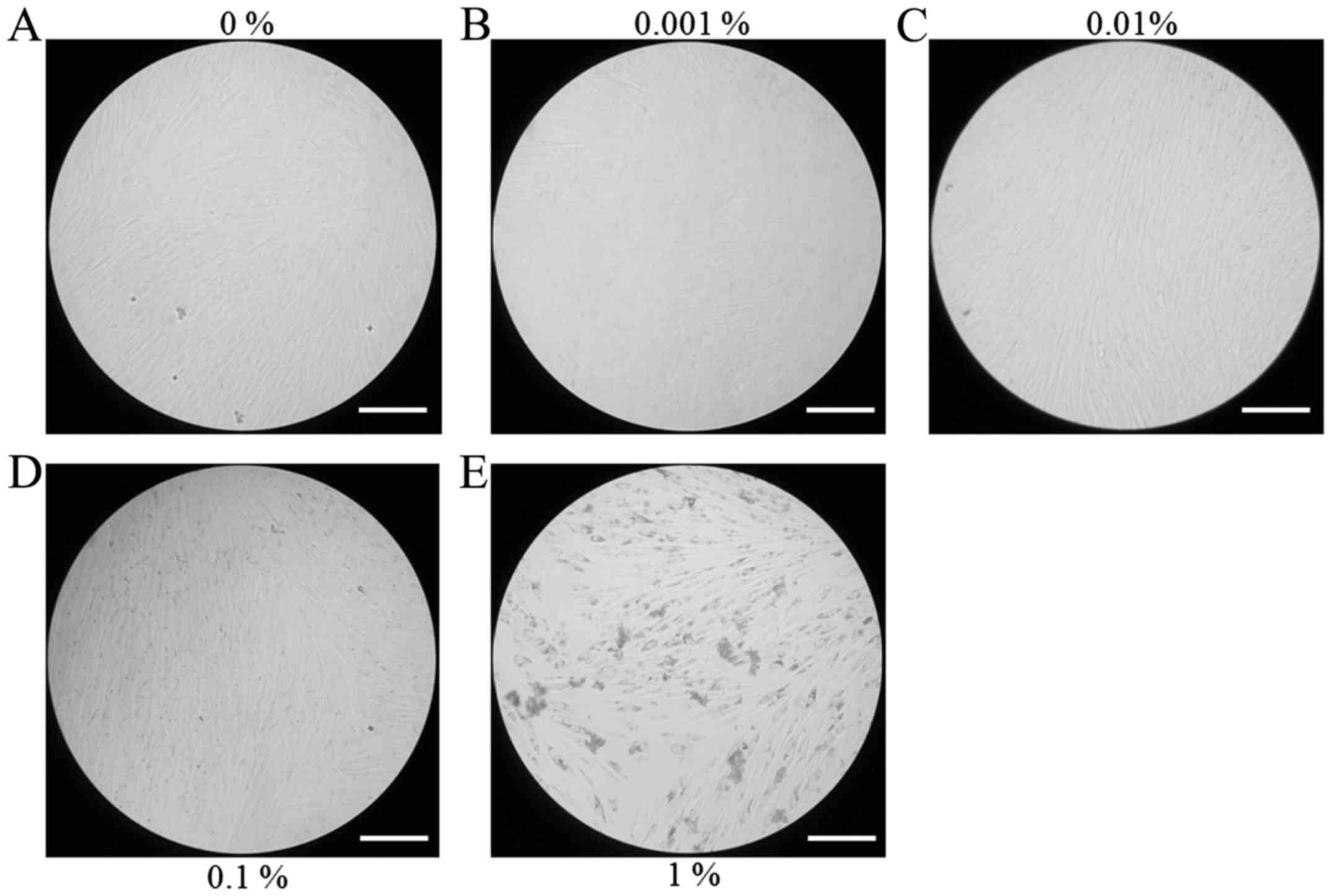
morphology at days 3, 5 and 7 are presented in Figs. 2–4,
respectively. Cells appeared to be more densely gathered in the 1%
group at day 5 and 7 compared with other groups at the same time
points.
Cellular proliferation
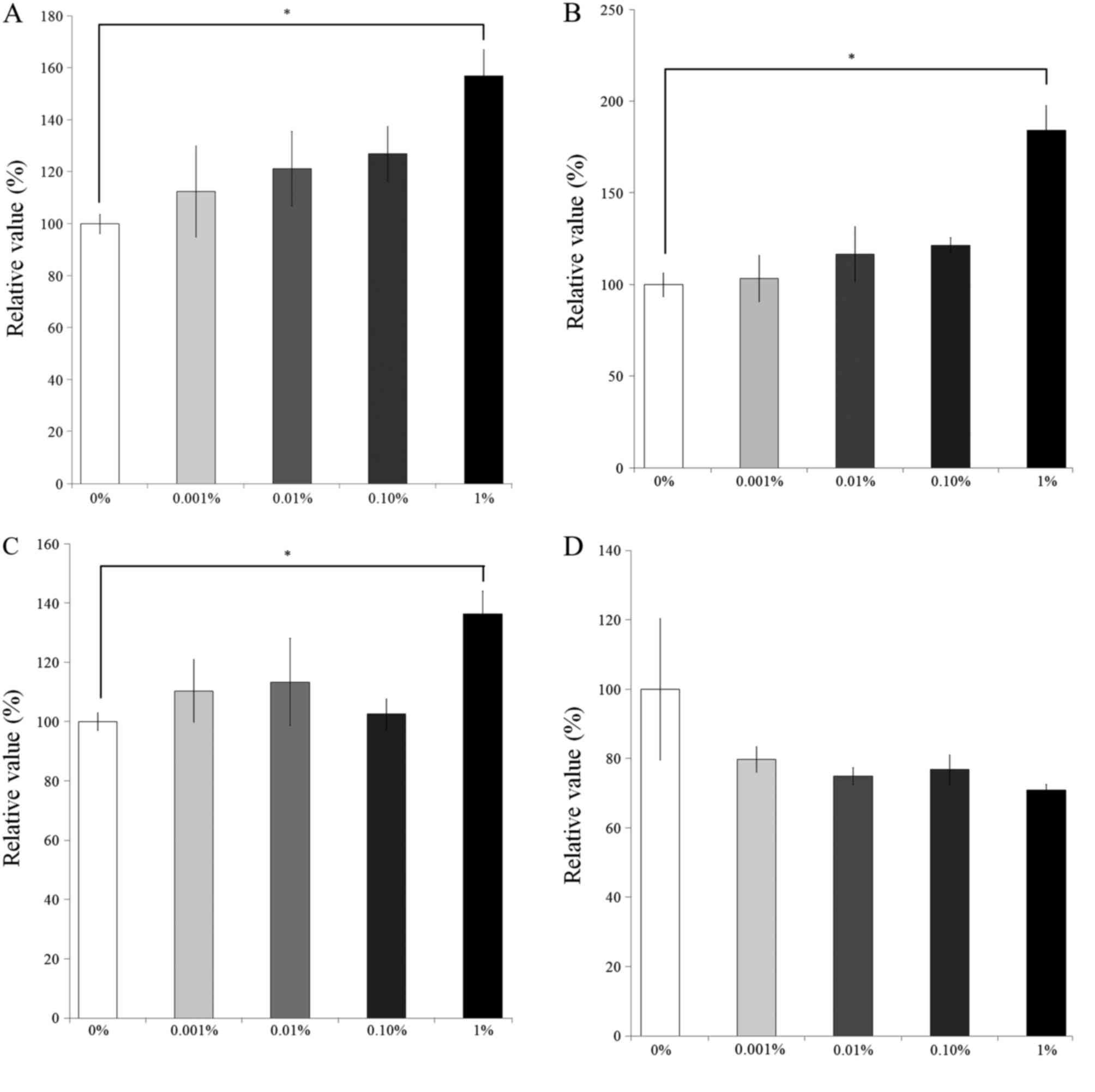
The results of the CCK-8 assay revealing the effects
of BBT on cellular viability on days 1, 3, 5 and 7 are presented in
Fig. 5. After 1 day of culture, the
number of viable cells in the 0.001, 0.01, 0.1 and 1% BBT groups
was 112.5±17.5, 121.2±14.3, 126.9±10.5 and 157.0±13.1% of that of
the control group, respectively (P<0.05). On day 3, the relative
number of viable cells in the 0.001, 0.01, 0.1 and 1% BBT groups
was 103.4±12.5, 116.7±15.1, 121.6±4.2 and 184.4±13.3%,
respectively, of that in the control group (P<0.05). The
relative number of viable cells in the 0.001, 0.01, 0.1 and 1%
groups on day 5 was 110.4±10.6, 113.4±14.8, 102.6±5.2 and
136.4±7.7% of the control group, respectively (P<0.05). On day
7, the relative number of viable cells in the 0.001, 0.01, 0.1 and
1% groups was 79.8±3.7, 75.0±2.4, 76.8±4.3 and 70.9±1.6%,
respectively, compared with that in the control group.
Immunofluorescence
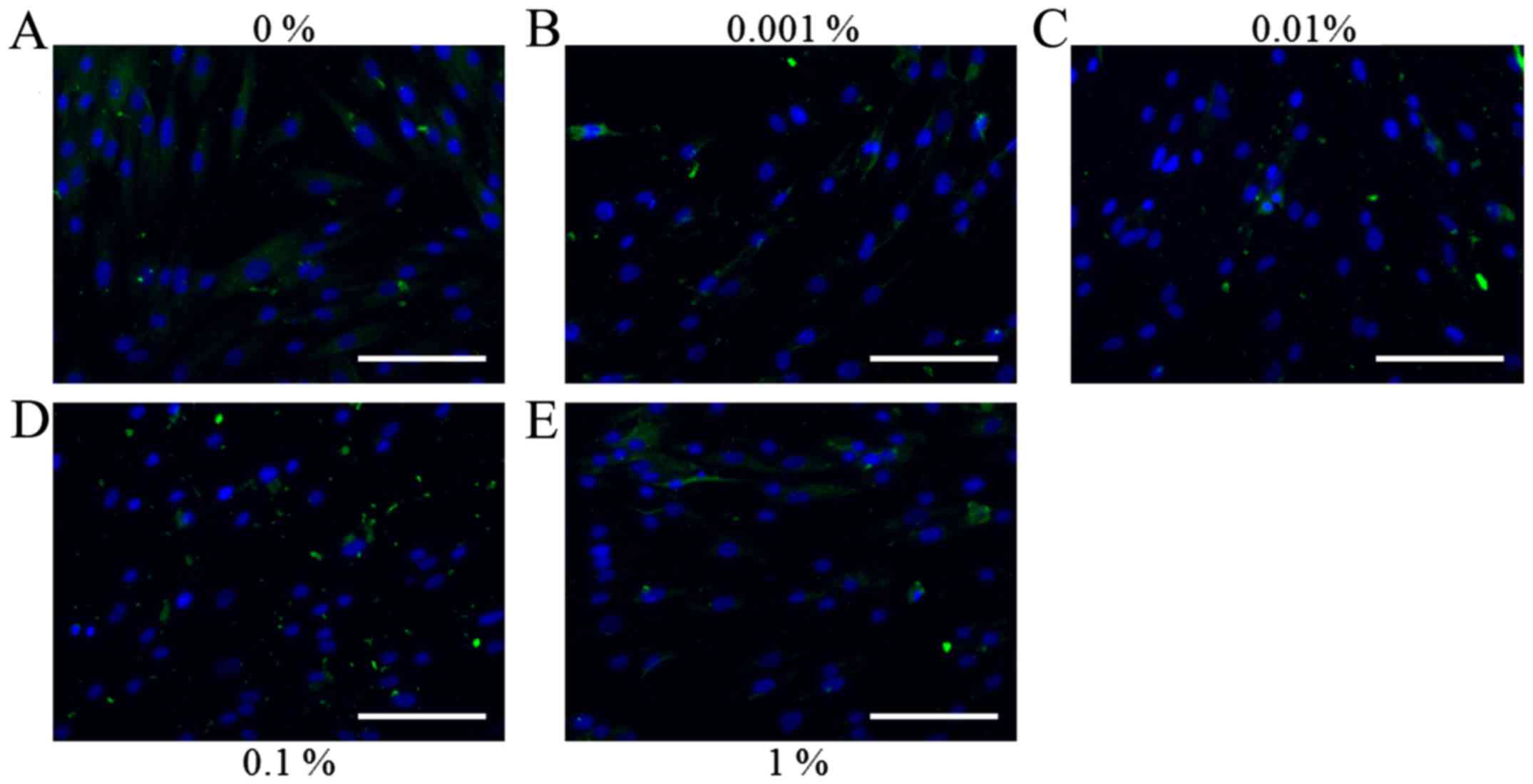
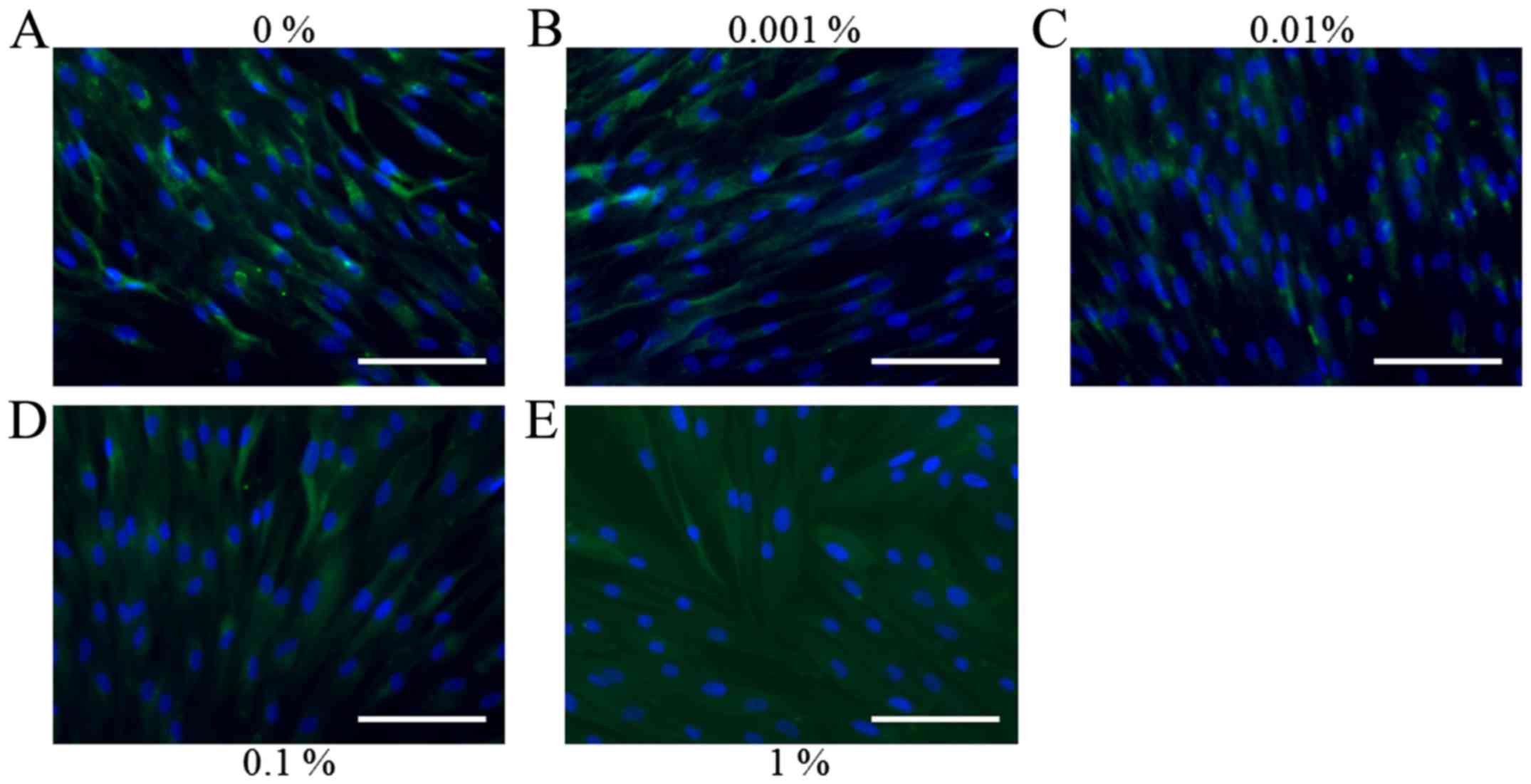
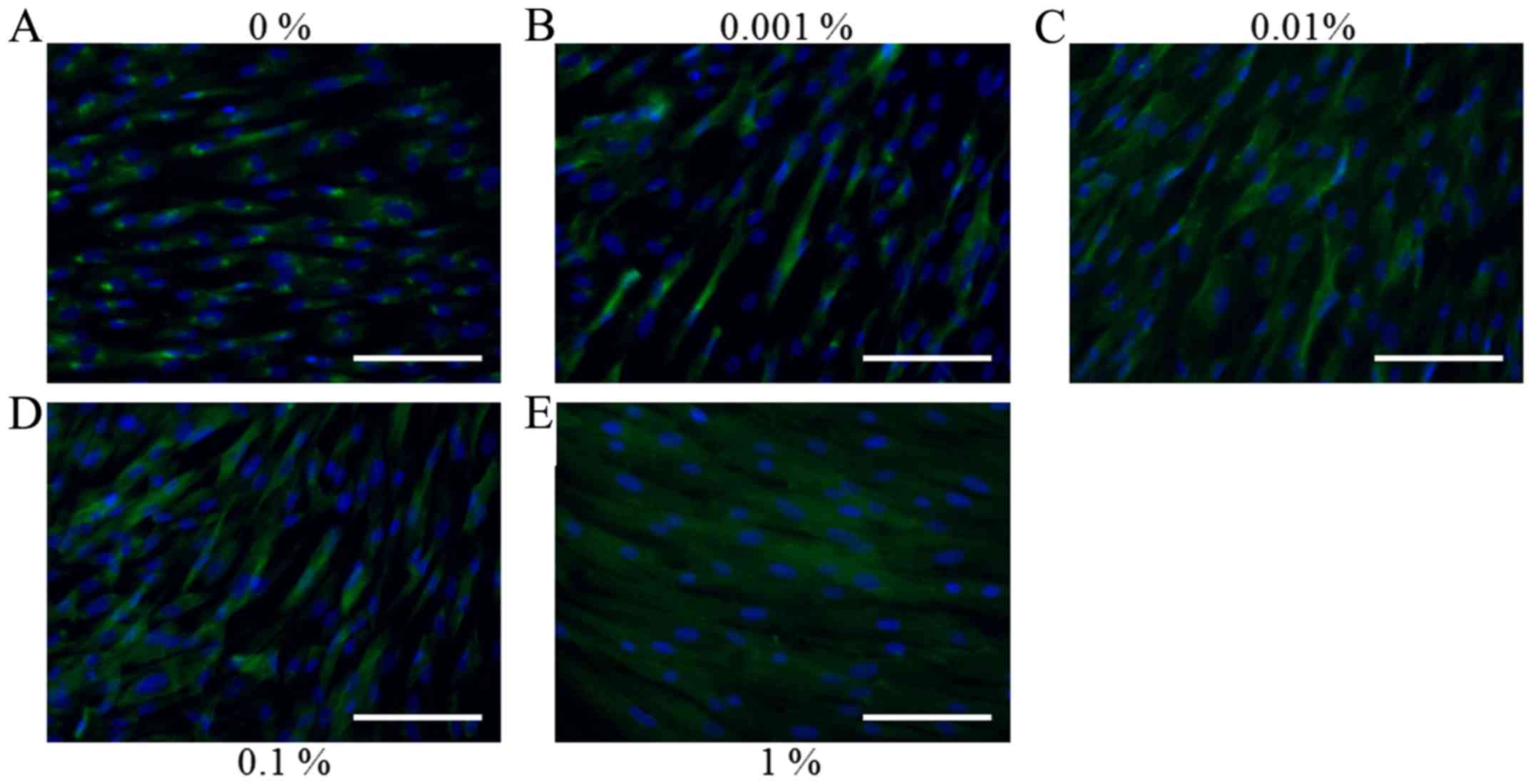
Representative immunofluorescence images for
collagen I staining on days 1, 3, 5 and 7 for the cells treated
with various concentrations of BBT are presented in Figs. 6–9. No
marked changes in the expression of collagen I were observed on day
1 (Fig. 6). On day 3, a noticeable
increase of collagen I expression was observed following the
application of BBT, particularly at a concentration of 1% (Fig. 7). However, the results on day 5
indicated that a gradual reduction of collagen I expression
occurred when higher doses of BBT were applied (Fig. 8). Similar trends for collagen I
expression were observed in the presence of BBT on day 7 (Fig. 9).
Discussion
The present study investigated the effects of
various concentrations of BBT on the morphology and cellular
proliferation of stem cells derived from human gingival tissues. It
was clearly demonstrated that the short-term application of
Bambusa tulda enhanced the proliferation of mesenchymal stem
cells with enhanced collagen I expression. Certain herbal extracts,
which have long been used in traditional Asian medicine, are used
as sources of novel therapeutics (7). These extracts may be considered
prevalidated for effectiveness and are expected have fewer safety
issues than chemically synthesized drugs (7,8). In
previous studies, various herbal extracts have been applied to
mesenchymal stem cells for the enhancement of cell proliferation
(9). Extracts of another herb,
Polygala tenuifolia, promoted the proliferation of neural
stem cells (10). Similarly,
Ginkgo biloba herbal extract has been demonstrated to
promote osteogenic differentiation of human bone marrow mesenchymal
stem cells (11). Promotion of the
neuronal differentiation of bone marrow stem cells was achieved by
application of Salvia miltiorrhiza extract (12). In the present study, application of
BBT enhanced the cell proliferation by up to 50% depending on the
concentration and incubation time. With this regard, BBT may be
applied to overcome the limited number of available cells or
improve the survival of implanted stem cells. Herbal extracts may
be combined with growth factors for synergistic effects (13).
There has been a great interest in using stem cells
in cell therapy due to their promising characteristics that are
beneficial for tissue regeneration and treatment of diseases
(7). Stem cells have a self-renewal
capacity and pluripotency with the ability to differentiate into
various cell types, including bone, adipose tissue and cartilage
(14,15). Stem cells may be obtained from
various sources, including the intraoral area (16,17).
Dental pulp and periodontal ligaments are considered preferred
sources; however, due to the limited amount of tissue, an
additional procedure of tooth extraction is required (6,18).
Gingiva may be a more favorable source of stem cells (19). Previous studies by our group
indicated that gingiva-derived stem cells possessed colony-forming
abilities, plastic adherence and a multilineage differentiation
potency with the capacity to undergo osteogensis, adipogenesis and
chondrogenesis, and expressed stem cell markers, including CD44,
CD73, CD90 and CD105 (6,20). In addition, harvesting stem cells
from gingiva may be easier than from bone marrow of the maxilla and
mandible due to the lesser invasiveness, pain and paresthesia
(21–23).
Various methods have been applied for the extraction
of herbs (24). Cold pressing and
expeller pressing use a heat-controlled environment, with a higher
temperature being applied for expeller pressing (25). Various solvents, including ethanol,
methanol, butanol, hexane, methylene chloride, ethyl acetate and
water may be used for solvent extraction (7,24,26,27).
A novel method of microwave-assisted extraction was also developed
for the extraction of Bambusa bambos (28). Different solubility and boiling
points are noted between different types of solvents (24,29). In
a previous study, the dried powdered leaves were extracted with a
hydroalcoholic mixture (30). It
should be noted that different effects may be achieved depending on
the type of solvent and the concentration (24).
Based on these findings, it was concluded that BBT
produced beneficial effects on the proliferation of mesenchymal
stem cells with enhanced collagen I expression at early time
points.
Acknowledgements
This study was funded by the Ministry of Science,
Information and Communication Technology (ICT) and Future Planning,
Republic of Korea government [National Research Foundation of Korea
(NRF) grant no. NRF-2016K1A1A8A01939075], the Catholic Institute of
Cell Therapy (Seoul, Korea) and the Basic Science Research Program
through the NRF funded by the Ministry of Science, ICT and Future
Planning (grant no. NRF-2017R1A1A1A05001307).
References
|
1
|
Saxena S: In vitro propagation of the
bamboo (Bambusa tulda Roxb.) through shoot proliferation.
Plant Cell Rep. 9:431–434. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sahariah B, Sinha I, Sharma P, Goswami L,
Bhattacharyya P, Gogoi N and Bhattacharyya SS: Efficacy of
bioconversion of paper mill bamboo sludge and lime waste by
composting and vermiconversion technologies. Chemosphere.
109:77–83. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Larpkern P, Moe SR and Totland Ø: Bamboo
dominance reduces tree regeneration in a disturbed tropical forest.
Oecologia. 165:161–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waikhom SD and Louis B: An effective
protocol for micropropagation of edible bamboo species (Bambusa
tulda and Melocanna baccifera) through nodal culture.
ScientificWorldJournal. 2014:3457942014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhattacharya S, Das M, Bar R and Pal A:
Morphological and molecular characterization of Bambusa
tulda with a note on flowering. Ann Bot. 98:529–535. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jin SH, Lee JE, Yun JH, Kim I, Ko Y and
Park JB: Isolation and characterization of human mesenchymal stem
cells from gingival connective tissue. J Periodontal Res.
50:461–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jeong SH, Lee JE, Jin SH, Ko Y and Park
JB: Effects of Asiasari radix on the morphology and viability of
mesenchymal stem cells derived from the gingiva. Mol Med Rep.
10:3315–3319. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oh SM, Kim J, Lee J, Yi JM, Oh DS, Bang OS
and Kim NS: Anticancer potential of an ethanol extract of
Asiasari radix against HCT-116 human colon cancer cells
in vitro. Oncol Lett. 5:305–310. 2013.PubMed/NCBI
|
|
9
|
Cai B, Zhang AG, Zhang X, Ge WJ, Dai GD,
Tan XL, Roodrajeetsing G and Cai JP: Promoting effects on
proliferation and chondrogenic differentiation of bone
marrow-derived mesenchymal stem cells by four ‘Kidney-Tonifying’
traditional Chinese herbs. Biomed Res Int. 2015:7921612015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park HJ, Lee K, Heo H, Lee M, Kim JW,
Whang WW, Kwon YK and Kwon H: Effects of Polygala tenuifolia
root extract on proliferation of neural stem cells in the
hippocampal CA1 region. Phytother Res. 22:1324–1329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gu Q, Chen C, Zhang Z, Wu Z, Fan X, Zhang
Z, Di W and Shi L: Ginkgo biloba extract promotes osteogenic
differentiation of human bone marrow mesenchymal stem cells in a
pathway involving Wnt/β-catenin signaling. Pharmacol Res. 97:70–78.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang N, Kang T, Xia Y, Wen Q, Zhang X, Li
H, Hu Y, Hao H, Zhao D, Sun D, et al: Effects of salvianolic acid B
on survival, self-renewal and neuronal differentiation of bone
marrow derived neural stem cells. Eur J Pharmacol. 697:32–39. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park JB: Combination of simvastatin and
bone morphogenetic protein-2 enhances the differentiation of
osteoblasts by regulating the expression of phospho-Smad1/5/8. Exp
Ther Med. 4:303–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ji M, Bai C, Li L, Fan Y, Ma C, Li X and
Guan W: Biological characterization of sheep kidney-derived
mesenchymal stem cells. Exp Ther Med. 12:3963–3971. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ha DH, Yong CS, Kim JO, Jeong JH and Park
JB: Effects of tacrolimus on morphology, proliferation and
differentiation of mesenchymal stem cells derived from gingiva
tissue. Mol Med Rep. 14:69–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ballini A, De Frenza G, Cantore S, Papa F,
Grano M, Mastrangelo F, Tetè S and Grassi FR: In vitro stem cell
cultures from human dental pulp and periodontal ligament: New
prospects in dentistry. Int J Immunopathol Pharmacol. 20:9–16.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagatomo K, Komaki M, Sekiya I, Ballini A,
De Frenza G, Cantore S, Papa F, Grano M, Mastrangelo F, Tetè S and
Grassi FR: Stem cell properties of human periodontal ligament
cells. J Periodontal Res. 41:303–310. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JB, Lee G, Yun BG, Kim CH and Ko Y:
Comparative effects of chlorhexidine and essential oils containing
mouth rinse on stem cells cultured on a titanium surface. Mol Med
Rep. 9:1249–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y and
Le AD: Mesenchymal stem cells derived from human gingiva are
capable of immunomodulatory functions and ameliorate
inflammation-related tissue destruction in experimental colitis. J
Immunol. 183:7787–7798. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jin SH, Kweon H, Park JB and Kim CH: The
effects of tetracycline-loaded silk fibroin membrane on
proliferation and osteogenic potential of mesenchymal stem cells. J
Surg Res. 192:e1–e9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JB, Kim YS, Lee G, Yun BG and Kim CH:
The effect of surface treatment of titanium with
sand-blasting/acid-etching or hydroxyapaptite-coating and
application of bone morphogenetic protein-2 on attachment,
proliferation and differentiation of stem cells derived from buccal
fat pad. Tissue Eng Regen Med. 10:115–121. 2013. View Article : Google Scholar
|
|
22
|
Fournier BP, Larjava H and Häkkinen L:
Gingiva as a source of stem cells with therapeutic potential. Stem
Cells Dev. 22:3157–3177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou
J, Jin Y and Chen FM: Comparison of mesenchymal stem cells derived
from gingival tissue and periodontal ligament in different
incubation conditions. Biomaterials. 34:7033–7047. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang MH, Jeong SH, Guo H and Park JB:
Anti-inflammatory and cytotoxic effects of methanol, ethanol, and
water extracts of Angelicae Dahuricae Radix. J Oral Sci.
58:125–131. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SY, An SY, Lee JS and Heo JS:
Zanthoxylum schinifolium enhances the osteogenic potential of
periodontal ligament stem cells. In Vitro Cell Dev Biol Anim.
51:165–173. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong SH, Kim BB, Lee JE, Ko Y and Park
JB: Evaluation of the effects of Angelicae dahuricae radix
on the morphology and viability of mesenchymal stem cells. Mol Med
Rep. 12:1556–1560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeong SH, Lee JE, Kim BB, Ko Y and Park
JB: Evaluation of the effects of Cimicifugae Rhizoma on the
morphology and viability of mesenchymal stem cells. Exp Ther Med.
10:629–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soumya V, Muzib YI and Venkatesh P: A
novel method of extraction of bamboo seed oil (Bambusa
bambos Druce) and its promising effect on metabolic symptoms of
experimentally induced polycystic ovarian disease. Indian J
Pharmacol. 48:162–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mukudai Y, Zhang M, Shiogama S, Kondo S,
Ito C, Motohashi H, Kato K, Fujii M, Shintani S, Shigemori H, et
al: Methanol and butanol extracts of Paeonia lutea leaves
repress metastasis of squamous cell carcinoma. Evid Based
Complement Alternat Med. 2016:60872132016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jawaid T, Awasthi A and Kamal M:
Estrogenic activity of a hydro-alcoholic extract of Bambusa
arundinaceae leaves on female wistar rats. J Adv Pharm Technol
Res. 6:19–24. 2015. View Article : Google Scholar : PubMed/NCBI
|