Introduction
The reconstruction of large bone defects remains a
major orthopedic challenge (1). In
bone tissue engineering, osteogenic cell types are seeded onto
three-dimensional porous scaffolds to stimulate bone healing and
remodel bone defects (2).
The bone material requires chemical and physical
processing to minimize its antigenicity, maintain the physical
structure and preserve its osteogenic induction properties
(3). Besides the well-known
limitations regarding the origin of materials and seed cells, cell
adhesion to material is one of the problems that should be
addressed in bone tissue engineering.
Fibronectin (FN), which interacts with integrin, is
involved in the early stages of bone formation as a component of
the extracellular matrix (ECM) (4,5). Its
distribution in the areas of skeletogenesis mediates cell adhesion
and supports osteoblast differentiation to form mineralized
nodules. FN binds to osteoblast α5β1 integrin receptor through its
tri-peptide amino acid sequence Arg-Gly-Asp (RGD) (5–7). FN is
also required for the recruitment of growth factors and the
assembly of multiple ECM proteins through the RGD sequence of its
central-binding domain (8–10). The design of bioactive scaffolds
involves mimicking naturally occurring ECM signaling via modifying
the biomaterial with FN to enhance adhesion (11). Soluble Gly-Arg-Gly-Asp-Ser (GRGDS)
peptide competes with FN for binding to osteoblast α5β1 integrin
and inhibits integrin-mediated adhesion.
Basic fibroblast growth factor (bFGF) has been
recognized as a therapeutic agent to enhance bone regeneration
(12). bFGF may modulate bone
formation through regulating the proliferation and differentiation
of osteoblastic lineage cells, such as osteoblasts (12,13).
The objective of the present study was to
investigate whether FN and bFGF have an effect on the β1
integrin-mediated adhesion of osteoblasts in bio-deprived bone
materials through the ECM-integrin pathway.
Materials and methods
Preparation of rat calvarial
osteoblasts
A total of 10 healthy Sprague Dawley rats weighing
250–300 g (5 male and 5 female) were provided by Animal Center of
Nanjing Medical University (Nanjing, China). All animal protocols
were approved by the Animal Ethics Committee of First People's
Hospital of Jining City (Jining, China). They were anesthetized and
sacrificed by decapitation. Osteoblasts were isolated according to
a previously described procedure (9,14,15).
Calvariae were dissected out and immersed in PBS. The calvariae
were minced into 1-mm3 pieces and incubated with gentle
shaking for 20 min at 37°C in PBS containing 0.1% collagenase II
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Cells
dissociated by sequential enzymatic digestion were collected,
centrifuged, re-suspended and seeded in T-75 cm2 flasks,
maintained in Dulbecco's modified Eagle's medium/nutrient mixture
F-12 (DMEM/F-12) plus 10% fetal bovine serum (FBS) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.).
Staining of rat calvarial osteoblasts
for alkaline phosphatase (ALP)
Cells of the 2nd passage, which had been grown on
coverslips, were fixed with 95% ethanol for 10 min. ALP was stained
with the bromochloroindolyl phosphate/nitro blue tetrazolium kit
(Nanjing Jiancheng Biological Co., Nanjing, China) as previously
described (16).
Alizarin Red S staining of rat
calvarial osteoblasts
ECM mineralization was determined by Alizarin Red S
staining (17). Cells of the 2nd
passage growing to confluence on coverslips were further cultured
for three weeks and then fixed with 95% ethanol for 10 min. Cells
were stained with 0.1% Alizarin Red S-Tris HCl (pH 8.3) for 30 min
at 37°C and rinsed with distilled water to quantify calcium
deposition.
Preparation and identification of
bio-derived bone materials
The metaphysis of fresh porcine femur purchased from
Jining Slaughter Factory (Jining, China) was deprived of soft
tissue, periosteum and arthrodial cartilage, and then sliced into
elongated shapes of 0.5×0.5×1 cm3. After repeated
washing, the bone fragments were deproteinized with 30%
H2O2 for 48 h at 37°C and defatted with
chloroform/methanol (1:1) for 4 h. The bio-derived bone pieces were
cut into blocks sized 0.4×0.4×0.4 cm3. The material was
finally lyophilized in a vacuum freeze-drier following storage for
2 h in a −80°C freezer.
The resultant material was assessed by scanning
electronic microscopy (SEM) and X-ray diffraction analysis. The
specimens of the bio-derived bones were fixed with 3%
glutaraldehyde for 24 h, re-fixed with 1% osmium tetroxide for
another 2 h and rinsed in PBS. After drying naturally, the
specimens were mounted on aluminum stubs, sputter-coated with
gold-palladium and observed by S-3000N SEM (Hitachi, Tokyo, Japan).
X-ray diffraction analysis of the bio-derived bone materials was
performed on an energy dispersive D/Max-III X-ray diffractometer
(Rigaku Corp., Tokyo, Japan) in a Theta/Theta configuration and a
Cu anode with a scanner speed of 1°/min. The exposure conditions
were 40 kV and 40 mA (2,3,18–20).
Seeding of rat calvarial osteoblasts
onto bio-derived bone materials
FN and bFGF (Merck KGaA; Darmstadt, Germany) were
individually diluted in serum-free DMEM/F12 medium. After
sterilization with ethylene oxide, the bio-derived bone materials
were filled in 96-well plates (6 blocks/well), and optionally
modified with poly-L-lysine (PLL; Sigma-Aldrich; Merck KGaA) or
various concentrations of FN (0, 0.1, 1, 10 or 100 µg/ml) at 4°C
overnight.
The rat calvarial osteoblasts were incubated with
serum-free medium for 24 h and stimulated with various
concentrations of bFGF (0, 0.1, 1, 10 or 100 ng/ml) for another 24
h. The osteoblasts were then digested, centrifuged, re-suspended
and seeded into bio-derived bones at a density of 2×106
cells/ml with different concentrations of bFGF solution (0, 0.1, 1,
10 or 100 ng/ml). Thus, the study was designed to include 5 groups
of cells and 6 groups of scaffolds, resulting in 30 groups.
MTT assay
Cell proliferation was assessed using an MTT assay.
All procedures were performed according to the manufacturers'
protocols of the MTT kit (Sigma-Aldrich; Merck KGaA) (21). The optical density at 490 nm was
determined by microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Viable cell numbers on scaffolds were then
determined based on the absorbance.
Assessment of the effect of GRGDS
peptide on cell/scaffold construction
To explore whether the combination of FN and bFGF
influenced α5β1 integrin-mediated cell adhesion, 4 groups were
established: i) The non-stimulated and non-modified group as a
control; ii) 10 µg/ml FN-modified group; iii) 100 ng/ml
bFGF-stimulated group; iv) 100 ng/ml bFGF-stimulated + 10 µg/ml
FN-modified group. The osteoblasts were stimulated with bFGF and
bio-derived bone scaffolds were modified with FN according to the
methods mentioned above. The groups were then sub-divided into 2
groups, to one of which 2.1 mmol/l GRGDS was added. An MTT assay
was used to detect osteoblast proliferation or adhesion on
bio-derived bone scaffolds as described above.
Western blot analysis
The protein expression levels of
osteoblasts/bio-derived bone scaffold constructs were analyzed by
western blot analysis as previously described (22). Protein was extracted with
radioimmunoprecipitation assay lysis buffer with phenylmethane
sulfonylfluoride and quantified by using a bicinchoninic acid
protein assay kit (Cell Signaling Technology, Inc., Danvers, MA,
USA). A total of 30 µg protein was loaded per lane and separated by
10% SDS-PAGE before being transferred onto a polyvinylidene
difluoride membrane. Membranes were blocked with 5% nonfat dry milk
in Tris-buffered saline containing Tween-20 for 2 h at room
temperature and incubated with the rabbit integrin β1 antibody
(1:2,000 dilution; #4706, Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) overnight at 4°C. After washing, membranes were incubated
with secondary anti-rabbit horseradish peroxidase-conjugated
antibodies (1:2,000 dilution; #323-025-021; Zhongshan Goldenbridge
Bio, Inc., Haimen, China) for 1 h at room temperature. Bands were
visualized using a Phototope-HRP Western Blotting kit (Cell
Signaling Technology, Inc.) and X ray diffractometer (Rigaku,
Tokyo, Japan) and analyzed using a gel imaging system (UVP, LLC,
Upland, CA, USA) (23).
Statistical analysis
Values are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). One-way analysis of
variance was used to detect multivariate differences. Student's
t-test was used to analyze inter-group differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characteristics of the processed
osteoblasts and bio-derived bone scaffolds
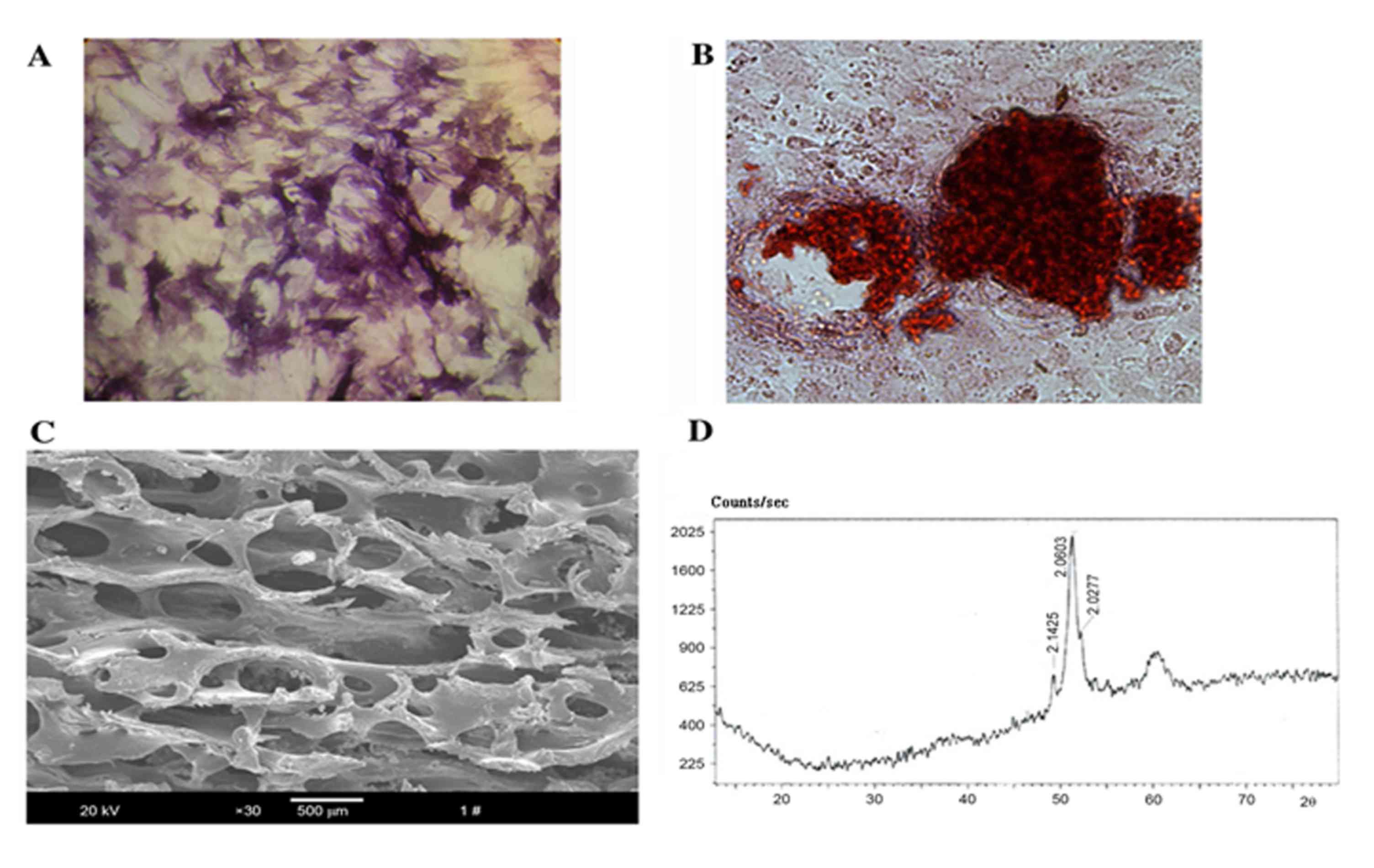
To identify the properties of the osteoblasts, ALP
activity was confirmed by staining using a commercially available
kit (Fig. 1A), while mineralized
nodules and calcium deposition were assessed by Alizarin Red S
staining (Fig. 1B). The
microstructure revealed by SEM showed that the bio-derived bone
scaffold materials maintained their natural interconnected pore
framework with an average pore size of 623.67±12.31 µm and a
porosity of 75±4.15% (Fig. 1C). The
X-ray diffraction pattern showed that curvilinear type of the
material was hydroxyapatite ceramic
[Ca10(OH)2(PO4)6]
(Fig. 1D).
bFGF and FN enhance adhesion of viable
cells in cell/scaffold constructs
To explore whether the combination of FN and bFGF
influenced cell adhesion to the material, osteoblasts stimulated
with bFGF were seeded on bio-derived bone scaffolds modified with
PLL or FN. The MTT results showed that modification with FN
significantly improved adhesion of osteoblasts seeded into
bio-derived bone scaffolds in comparison with that in the
unmodified group (P<0.01) and the PLL only group (P<0.01). In
addition, stimulation with bFGF slightly improved cell adhesion. Of
note, the combination of bFGF and FN significantly improved cell
adhesion compared with that in the unstimulated group and
unmodified group (P<0.01) The statistical and observed results
also revealed that cell adhesion was highest for osteoblasts with
100 ng/ml bFGF stimulation seeded into scaffolds modified with 10
µg/ml FN (Fig. 2).
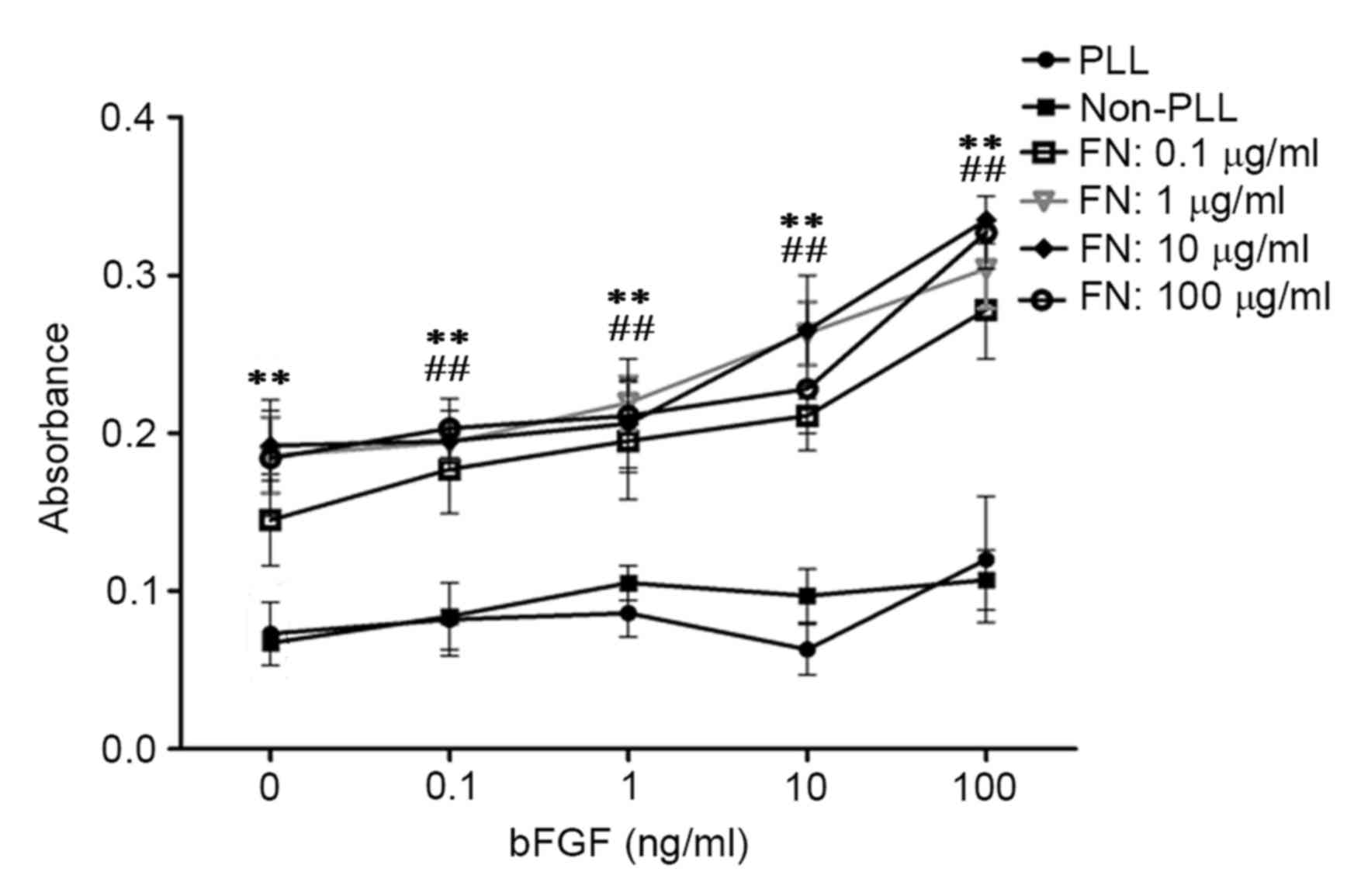 | Figure 2.Effects of bFGF and FN on osteoblasts
seeded into bio-derived bone scaffolds after culturing for 25 days
assessed by MTT assay. bFGF, basic fibroblast growth factor; FN,
fibronectin; PLL, poly-L-lysine. **P<0.01, FN-modified groups at
different concentrations of bFGN (0, 0.1, 1, 10, 100 ng/ml) vs. the
unmodified groups (Non-PLL and PLL-groups); ##P<0.01,
bFGF-stimulated groups (the groups whose concentration of bFGF were
above 0 ng/ml) at different concentrations of FN (0.1, 1, 10, 100
µg/ml) vs. the unstimulated groups (the groups whose concentration
of bFGF were 0 ng/ml). |
SEM results of cell/scaffold
constructs
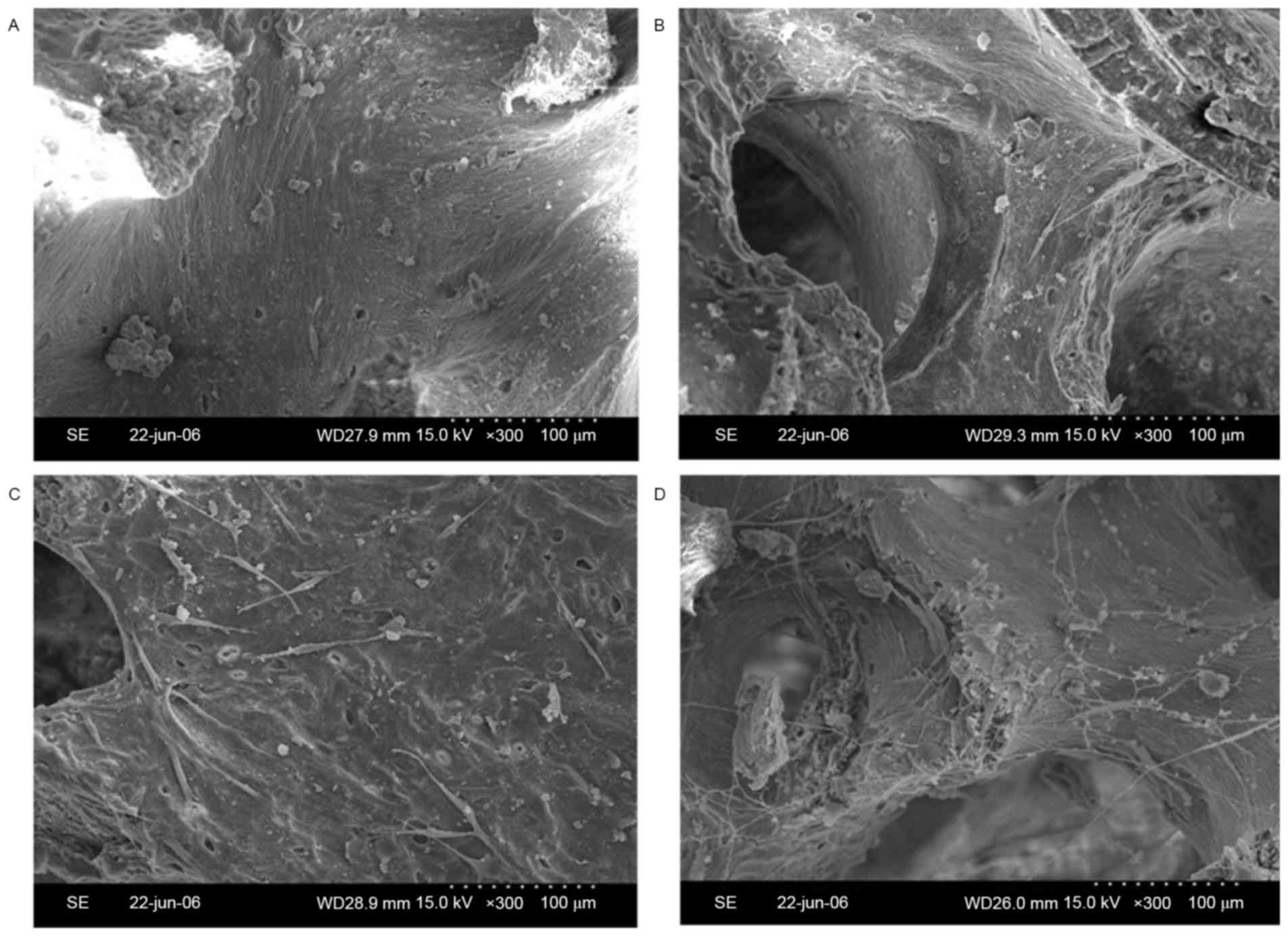
To observe the adhesion of osteoblasts to the
bio-derived bone scaffolds, specimens of cell/scaffold constructs
were visually observed by SEM. The number of spindle-shaped
osteoblasts in the 100 ng/ml bFGF-stimulated and 10 µg/ml
FN-modified group (Fig. 3A) was
higher than that in the 100 ng/ml bFGF-stimulated group (Fig. 3B), the 10 µg/ml FN-modified group
(Fig. 3C) and the non-stimulated and
non-modified group (Fig. 3D).
GRGDS peptide affects cell adhesion in
cell/scaffold constructs
To explore whether bFGF and FN have an effect on
cell adhesion through the integrin-mediated pathway, an MTT assay
was used to detect osteoblast proliferation, while the 1 mmol/l
GRGDS peptide was used to block RGD in the FN-integrin β1
interaction. Cell adhesion was significantly better in the groups
that were not treated with GRGDS in comparison with that in the
GRGDS-treated groups (P<0.01). Among the GRGDS-treated groups,
cell adhesion in the 10 µg/ml FN + 100 ng/ml bFGF group was
significantly higher compared with that in the 10 µg/ml FN-modified
group, the 100 ng/ml bFGF-stimulated group and the control group,
respectively (P<0.001). In addition, cell adhesion in the 10
µg/ml FN-modified group was better than that in the control group
(P<0.01; Fig. 4A).
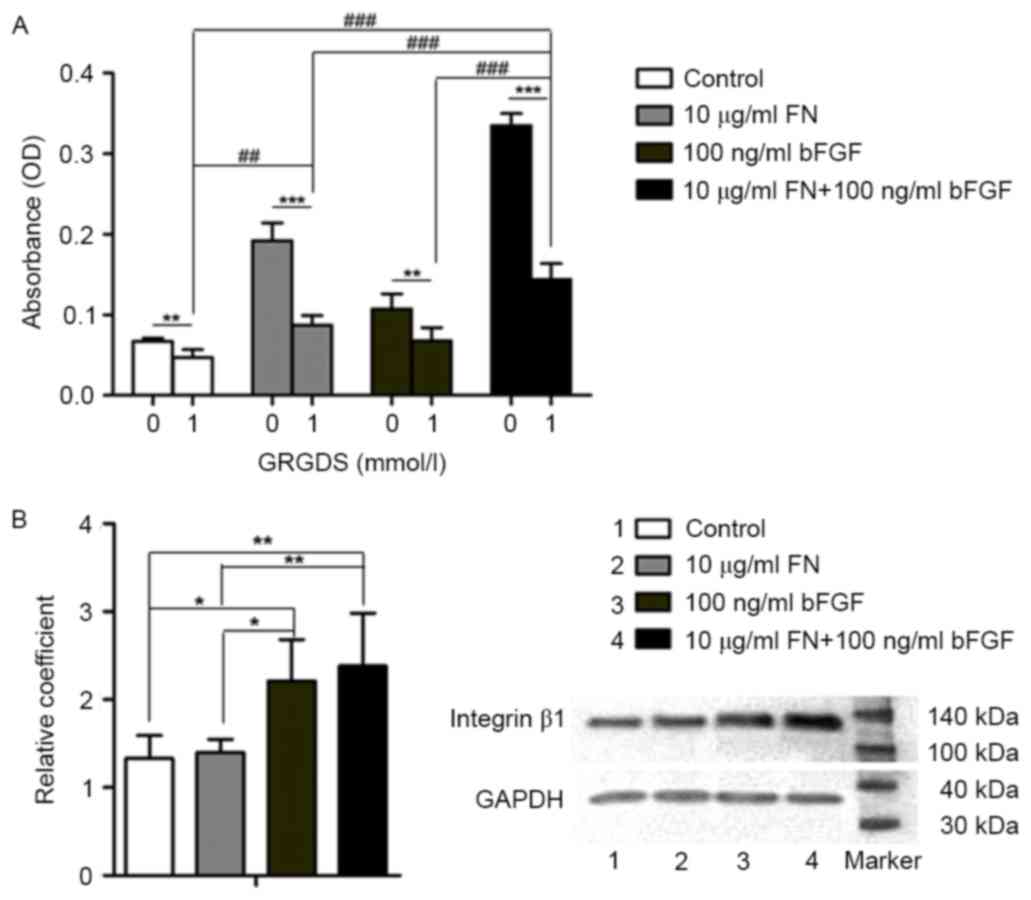 | Figure 4.Involvement of the integrin-β1 pathway
in the cell/scaffold constructs. (A) MTT results of osteoblasts
adhering to bio-derived bone scaffolds. Groups: 0, not treated with
GRGDS; 1, treated with 1 mmol/l GRGDS. (B) Western blot results of
integrin β1 in osteoblasts seeded into bio-derived bone scaffolds.
Lanes: 1, Control; 2, 10 µg/ml FN-modified group; 3, 100 ng/ml
bFGF-stimulated group; 4, 100 ng/ml bFGF-stimulated and 10 µg/ml
FN-modified group. ***P<0.001, **P<0.01, *P<0.05, 0 vs. 1;
###P<0.001, ##P<0.01 among the
respective GRGDS-treated groups. bFGF, basic fibroblast growth
factor; FN, fibronectin; OD, optical density. |
Integrin β1 has a role in osteoblast
attachment to bio-derived bone scaffolds
Western blot analysis was used to detect integrin β1
protein expression in the absence of GRGDS. The level of integrin
β1 protein in the 10 µg/ml FN + 100 ng/ml bFGF group was higher
than that in the 10 µg/ml FN-modified group (P<0.001) and that
in the control group (P<0.001). Furthermore, the level of
integrin β1 in the 100 ng/ml bFGF group was higher than that in the
10 µg/ml FN-modified group (P<0.05) and that in the control
group (P<0.05; Fig. 4B).
Discussion
Bone regeneration to restore the original bone's
form and function is limited by the size of the bone defect. The
general consensus is that a highly porous microstructure and large
surface area are conducive to tissue ingrowth (24,25). The
selection of the most promising scaffold material and seed cell
type to be used in bone regeneration remains critically important
for bone tissue engineering (26).
In the present study, osteoblasts we separated from
Sprague Dawley rat calvariae and bio-derived bone scaffolds were
manufactured from the metaphysis of fresh porcine femur.
Osteoblasts were identified via staining for ALP and Alizarin Red S
staining. ALP activity is used as an early phenotypic marker for
mature osteoblasts, while the formation of mineralized nodules is a
phenotypic marker for a later stage of osteogenic differentiation
(17). The deproteinized and
defatted bone materials were processed into small 0.4×0.4×0.4
cm3 blocks with an average pore size of 623.67±12.31 µm
and met the clinical requirements as indicated in the SEM images.
The X-ray diffraction pattern showed that the main component of
bio-derived bone materials was non-crystal phase hydroxyapatite
[Ca10(OH)2(PO4)6],
which was consistent with normal bone structure.
FN is an adhesive protein that contributes to the
structural stability of ECM. It has an important role in cell
attachment to surfaces of various materials through binding to
integrin (27). Thus, in the present
study, the bio-deprived bone scaffolds were modified with FN at
various concentrations. In addition, the effects of the FGF family
of proteins, particularly bFGF (also known as FGF-2) have been well
investigated in osteogenesis and bFGF is essential for bone
formation (24). Thus, in the
present study, rat calvarial osteoblasts were stimulated with
various concentrations of bFGF prior to seeding into the bone
scaffolds. To explore the most appropriate FN and bFGF
concentrations for cell-scaffold constructs, osteoblasts stimulated
with various concentrations of bFGF (0.1, 1, 10 or 100 ng/ml) were
seeded into bio-derived bone scaffolds modified with various
concentrations of FN (0.1, 1, 10 or 100 µg/ml) to quantify the
number of viable adherent cells by an MTT assay. The MTT assay
evaluates the number of living cells and the metabolic activity of
cells present. The results indicated that scaffold modification
with FN only or cell stimulation with bFGF only significantly
improved the adhesion of osteoblasts seeded into bio-derived bone
scaffolds. Cell adhesion was highest when a combination of
osteoblast stimulation with 100 ng/ml bFGF and scaffold
modification with 10 µg/ml FN was used.
Integrins, which trigger cell adhesion, are the
major class of receptors implicated in cell-ECM interactions, which
are mediated via the FN-integrin signaling pathway (6). The RGD peptide sequence derived from
ECM proteins such as FN has been used to modulate cell attachment,
proliferation and migration (11).
The RGD peptide of FN, to which cell adhesion ligands present in
the ECM attach, is blocked by the GRGDS peptide. The results of the
present study demonstrated that after GRGDS treatment, cell
adhesion in the 10 µg/ml FN-modified group and in the 10 µg/ml FN +
100 ng/ml bFGF group was significantly declined to a greater extent
than that in the control and 100 ng/ml bFGF-stimulated groups. This
result indicated that osteoblast adhesion to bio-derived bone
scaffolds may be enhanced through the ECM-FN-integrin pathway.
Western blot analysis further confirmed that the level of integrin
β1 was affected by bFGF, particularly by the combination of bFGF
and FN. As bFGF had a more significant effect than that of FN, this
may indicate that the mechanism is more complex and further
investigation is required to fully elucidate the mechanism
involved.
α5β1 integrin, a specific Fn receptor, mediates
critical interactions between osteoblasts and Fn required for bone
morphogenesis (28). The present
study showed that direct osteoblast interactions with the
extracellular matrix are mediated by a selective group of integrin
receptors, including α5β1, α3β1, αvβ3, and α4β1 (29). A study performed by Tang et al
(30) demonstrated that bFGF
increased Fn fibrillogenesis and the cell surface expression of α5
and β1 integrins via a PKC-dependent pathway. All the above studies
are consistent with our findings and further emphasize the
significance of our study.
In conclusion, the combined use of bFGF and FN has a
significant enhancing effect on osteoblast adhesion on bio-derived
bones. When 100 ng/ml bFGF-stimulated osteoblasts were seeded on
the 10 µg/ml FN-modified bio-derived bones, the greatest
enhancement of cell adhesion could be generated. The present
findings suggest the mechanism of the enhancing effect is likely
mediated by RGD-integrin α5β1 pathway.
References
|
1
|
Liu F, Zhang X, Yu X, Xu Y, Feng T and Ren
D: In vitro study in stimulating the secretion of angiogenic growth
factors of strontium-doped calcium polyphosphate for bone tissue
engineering. J Mater Sci Mater Med. 22:683–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang ZY, Teoh SH, Chong WS, Foo TT, Chng
YC, Choolani M and Chan J: A biaxial rotating bioreactor for the
culture of fetal mesenchymal stem cells for bone tissue
engineering. Biomaterials. 30:2694–2704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xie H, Yang F, Deng L, Luo J, Qin T, Li X,
Zhou GQ and Yang Z: The performance of a bone-derived scaffold
material in the repair of critical bone defects in a rhesus monkey
model. Biomaterials. 28:3314–3324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee S, Lee DS, Choi I, Le Pham BH and Jang
JH: Design of an osteoinductive extracellular fibronectin matrix
protein for bone tissue engineering. Int J Mol Sci. 16:7672–7681.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pegueroles M, Aparicio C, Bosio M, Engel
E, Gil FJ, Planell JA and Altankov G: Spatial organization of
osteoblast fibronectin matrix on titanium surfaces: Effects of
roughness, chemical heterogeneity and surface energy. Acta
Biomater. 6:291–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calderwood DA, Shattil SJ and Ginsberg MH:
Integrins and actin filaments: Reciprocal regulation of cell
adhesion and signaling. J Biol Chem. 275:22607–22610. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brunner M, Millon-Frémillon A, Chevalier
G, Nakchbandi IA, Mosher D, Block MR, Albigès-Rizo C and Bouvard D:
Osteoblast mineralization requires beta1 integrin/ICAP-1-dependent
fibronectin deposition. J Cell Biol. 194:307–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bentmann A, Kawelke N, Moss D, Zentgraf H,
Bala Y, Berger I, Gasser JA and Nakchbandi IA: Circulating
fibronectin affects bone matrix, whereas osteoblast fibronectin
modulates osteoblast function. J Bone Miner Res. 25:706–715.
2010.PubMed/NCBI
|
|
9
|
He L, Lin Y, Hu X, Zhang Y and Wu H: A
comparative study of platelet-rich fibrin (PRF) and platelet-rich
plasma (PRP) on the effect of proliferation and differentiation of
rat osteoblasts in vitro. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 108:707–713. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Houseman BT and Mrksich M: The
microenvironment of immobilized Arg-Gly-Asp peptides is an
important determinant of cell adhesion. Biomaterials. 22:943–955.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin H, Zygourakis K, Farach-Carson MC,
Yaszemski MJ and Mikos AG: Attachment, proliferation, and migration
of marrow stromal osteoblasts cultured on biomimetic hydrogels
modified with an osteopontin-derived peptide. Biomaterials.
25:895–906. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan Q, Kubo T, Doi K, Morita K, Takeshita
R, Katoh S, Shiba T, Gong P and Akagawa Y: Effect of combined
application of bFGF and inorganic polyphosphate on bioactivities of
osteoblasts and initial bone regeneration. Acta Biomater.
5:1716–1724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li P, Bai Y, Yin G, Pu X, Huang Z, Liao X,
Chen X and Yao Y: Synergistic and sequential effects of BMP-2, bFGF
and VEGF on osteogenic differentiation of rat osteoblasts. J Bone
Miner Metab. 32:627–635. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang DZ, Hou W, Zhou Q, Zhang M, Holz J,
Sheu TJ, Li TF, Cheng SD, Shi Q, Harris SE, et al: Osthole
stimulates osteoblast differentiation and bone formation by
activation of beta-catenin-BMP signaling. J Bone Miner Res.
25:1234–1245. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trivedi R, Kumar S, Kumar A, Siddiqui JA,
Swarnkar G, Gupta V, Kendurker A, Dwivedi AK, Romero JR and
Chattopadhyay N: Kaempferol has osteogenic effect in ovariectomized
adult sprague-dawley rats. Mol Cell Endocrinol. 289:85–93. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huo K, Zhang X, Wang H, Zhao L, Liu X and
Chu PK: Osteogenic activity and antibacterial effects on titanium
surfaces modified with Zn-incorporated nanotube arrays.
Biomaterials. 34:3467–3478. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao D, Xie XH, Wang XL, Wan C, Lee YW,
Chen SH, Pei DQ, Wang YX, Li G and Qin L: Icaritin, an exogenous
phytomolecule, enhances osteogenesis but not angiogenesis-an in
vitro efficacy study. PLoS One. 7:e412642012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang ZY, Teoh SH, Chong MS, Schantz JT,
Fisk NM, Choolani MA and Chan J: Superior osteogenic capacity for
bone tissue engineering of fetal compared with perinatal and adult
mesenchymal stem cells. Stem cells. 27:126–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin YC, Brayfield CA, Gerlach JC, Rubin JP
and Marra KG: Peptide modification of polyethersulfone surfaces to
improve adipose-derived stem cell adhesion. Acta Biomater.
5:1416–1424. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mariscal-Muñoz E, Costa CA, Tavares HS,
Bianchi J, Hebling J, Machado JP, Lerner UH and Souza PP:
Osteoblast differentiation is enhanced by a nano-to-micro hybrid
titanium surface created by Yb: YAG laser irradiation. Clin Oral
Investig. 20:503–511. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang YW, Wu Q and Chen GQ: Attachment,
proliferation and differentiation of osteoblasts on random
biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)
scaffolds. Biomaterials. 25:669–675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Li H, Li T, Fan J, Zhao RC and Weng
X: MicroRNA expression profile of dexamethasone-induced human bone
marrow-derived mesenchymal stem cells during osteogenic
differentiation. J Cell Biochem. 115:1683–1691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hendesi H, Barbe MF, Safadi FF, Monroy MA
and Popoff SN: Integrin mediated adhesion of osteoblasts to
connective tissue growth factor (CTGF/CCN2) induces cytoskeleton
reorganization and cell differentiation. PLoS One. 10:e01153252015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ishida K and Haudenschild DR: Interactions
between FGF21 and BMP-2 in osteogenesis. Biochem Biophys Res
Commun. 432:677–682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yoshimoto H, Shin YM, Terai H and Vacanti
JP: A biodegradable nanofiber scaffold by electrospinning and its
potential for bone tissue engineering. Biomaterials. 24:2077–2082.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang H, Li Y, Zuo Y, Li J, Ma S and Cheng
L: Biocompatibility and osteogenesis of biomimetic
nano-hydroxyapatite/polyamide composite scaffolds for bone tissue
engineering. Biomaterials. 28:3338–3348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ribeiro N, Sousa SR and Monteiro FJ:
Influence of crystallite size of nanophased hydroxyapatite on
fibronectin and osteonectin adsorption and on MC3T3-E1 osteoblast
adhesion and morphology. J Colloid Interface Sci. 351:398–406.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moursi AM, Globus RK and Damsky CH:
Interactions between integrin receptors and fibronectin are
required for calvarial osteoblast differentiation in vitro. J Cell
Sci. 110:2187–2196. 1997.PubMed/NCBI
|
|
29
|
Lim JY, Taylor AF, Li Z, Vogler EA and
Donahue HJ: Integrin expression and osteopontin regulation in human
fetal osteoblastic cells mediated by substratum surface
characteristics. Tissue Eng. 11:19–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang CH, Yang RS, Huang TH, Liu SH and Fu
WM: Enhancement of fibronectin fibrillogenesis and bone formation
by basic fibroblast growth factor via protein kinase c-dependent
pathway in rat osteoblasts. Mol Pharmacol. 66:440–449.
2004.PubMed/NCBI
|