Introduction
Kidney transplantation has become the most efficient
therapy for patients who suffer from end stage renal disease
(1). However, despite the
improvements of immunosuppressive methods on kidney
transplantation, the long-term graft survival rate, such as the
5-year survival rate, has not improved markedly (2). Chronic allograft nephropathy (CAN) is
one of the most common causes for loss of kidney graft function,
which accounts for 50–80% of late graft loss after the first year
of renal transplantation (3,4). The pathogenesis of CAN remains
intricate and poorly understood, as the condition is caused by
various immune and non-immune factors (5). For example, the immune factors include
acute clinical rejection, human leukocyte antigen match, whereas
non-immune factors consist of hypertension, glomerular
hypertension, delayed graft function and acute
calcineurin-inhibitor toxicity (6–8). These
factors lead to cumulative damage to the kidney, which results in
progressive allograft injury and consequently, dysfunction
(9,10). A previous study also suggested that
the effects of body mass index (BMI) 1 year after the kidney
transplantation were associated with CAN (11). CAN is the process of fibrotic
development with histological lesions, which can be characterized
by atherosclerosis, glomerulopathy, interstitial fibrosis and
tubular atrophy (6,9,12).
Considering the negative effects that CAN causes on renal
allograft, further insight on the pathology mechanisms is required
and may provide potential therapeutics for CAN.
The high mobility group box 1 protein (HMGB1), which
belongs to the most evolutionarily conserved proteins in the
nucleus and cytoplasm, is one of the members of high mobility group
nuclear protein family (13). HMGB1
has been demonstrated to act as a pro-inflammatory mediator and
participate in human immune systems diseases (14,15).
Studies indicated that innate immune cells, including macrophages
and monocytes, actively secreted HMGB1 (13,16). It
has been demonstrated that HMGB1 not only induces various
cytokines, such as interleukin (IL)-1, IL-6 and IL-8, but also
stimulates necrosis-induced inflammation. It indicates that HMGB1
has an important role in renal diseases, including acute kidney
injury, chronic kidney disease (CKD) and granulomatous nephritis
(17–20). However, the association between HMBG1
and CAN remains to be clarified. Furthermore, HMGB1 acts as the
endogenous ligand for toll-like receptor 2 (TLR2) and toll-like
receptor 4 (TLR4), and is associated with downstream translocation
of nuclear factor-κB (NF-κB) (21,22).
NF-κB is a protein complex, which has an important role in
transcription, immune responses and inflammation developments
(23,24). The NF-κB family is a family of
inducible transcription factors that have been demonstrated to
contribute to the process of renal inflammation and immunological
disease development (25,26). Transforming growth factor β (TGF-β)
is a multi-function cytokine complex with three isoforms, TGF-β1,
TGF-β2, and TGF-β3 (27). Among
them, TGF-β1 has an important role in interstitial fibrosis
development (28–30). Multiple studies have suggested that
the NF-κB pathway interacts with the TGF-β1/Smad pathway to form
the NF-κB/TGF-β1/Smad signaling pathway, which is involved in the
process of inflammation and renal tubulointerstitial fibrosis
development (31–33). Furthermore, the association between
TGF-β1 expression and CAN has been identified in animal models and
patients, demonstrating that there is an overexpression of TGF-β1
in patients with CAN (3,27). However, to the best of our knowledge,
the role of NF-κB in CAN has not been investigated previously.
Therefore, the present study aimed to investigate
whether HMGB1 and NF-κB in renal tissues are associated with CAN.
Due to the association between TGF-β1 and CAN, TGF-β1 was included
in the present study to investigate the relationship of TGF-β1 with
HMGB1 and NF-κB in CAN. Ultimately, the current case-control study
was conducted to accumulate data and determine the association
between HMGB1, NF-κB, TGF-β1 and CAN.
Materials and methods
Subjects
A total of 27 patients (age range, 18–54 years old;
mean age, 41±8 years; 15 males and 12 females) who were diagnosed
with CAN by histological biopsy diagnosis between September 2012
and November 2014 at Linyi People's Hospital (Linyi, China) were
enrolled into the study. All patients suffered graft kidney
dysfunction between 1 and 10 years after allograft renal
transplantation and the mean allograft survival time was 4.3 years.
Every CAN patient received renal biopsy guided by B ultrasound to
exclude other renal diseases and renal tissue specimens were
harvested from each CAN patient. In addition, 30 cases (age range,
29–63 years old; mean age, 44±9 years; 16 males and 14 females) who
received nephrectomy following trauma in the same time period were
selected as the control group for the current study. Normal renal
tissue specimens were collected through nephrectomy in the control
group. Non-anticoagulant blood was collected under fasting
conditions 1 day prior to renal biopsy to detect a number of
physical indications, including hemoglobin, high sensitivity
C-reactive protein (hs-CRP), IL-6, triglyceride (TG), low density
lipoprotein (LDL-C), highdensity lipoprotein (HDL-C) and fasting
plasma glucose (FPG). The present study was approved by the Ethics
and Clinical Committee of Linyi People's Hospital and written
informed consent was obtained from each patient.
Immunosuppressive regimens
Methylprednisolone (Pfizer Manufacturing Belgium,
Puurs, Belgium; imported drug registration number: H20080285) was
administered at a dose of 500 mg/day once a day by intravenous
injection in the first 3 days following renal transplant.
Subsequently, mycophenolate mofetil (Hangzhou Zhongmei Huadong
Pharmaceutical Co. Ltd, Zhejiang Sheng, China; permission number
approved by the state: H20052083) at 1.0–1.5 g/day was administered
orally 48 h after the surgery. The concentration of serum
creatinine (Cr) was assessed once a month using an automated
biochemical analyzer (AU5800; Beckman Coulter, Inc., Brea, CA,
USA). Cyclosporine A (CsA; Hangzhou Zhongmei Huadong Pharmaceutical
Co. Ltd; permission number: H10960122) was administered at 8
mg/kg/day when the concentration of Cr was <250 µmol/l; and then
adjusted to maintain the drug level at 300–350 ng/ml in the first 2
weeks following surgery, 250–300 ng/ml in weeks 2–4, 200–250 ng/ml
in weeks 4–12 and 150–200 ng/ml in weeks 12–48 following surgery.
Finally, prednisone (Zhejiang Xianju Pharmaceutical Co. Ltd.,
Zhejiang, China; permission number: H33021207) was used instead of
methylprednisolones at 0.6 mg/kg/day and the dose was adjusted to
maintain the blood drug concentration at 10–15 mg/day at 2 months
after renal transplantation.
Classification of chronic allograft
nephropathy
The diagnostic criteria and classification for all
the specimens was assured according to the Banff 07 working
classification (34). The
classifications based on histopathological findings were as
follows: Grade I (mild), mild interstitial fibrosis and tubular
atrophy; Grade II (moderate), moderate interstitial fibrosis and
tubular atrophy; and Grade III (severe), severe interstitial
fibrosis and tubular atrophy and moderate tubular loss.
Histomorphology
For microscopic observation, tissues were fixed in
10% formalin at 37°C for 24 h and conventionally dehydrated (70%
ethanol for 1 day, 80% ethanol for 45 min, 90% ethanol for 1 h, 95%
ethanol for 1 h, and ethanol 100% for 3 h). Tissues were embedded
in paraffin and 2-µm tissue sections were stained with hematoxylin
and eosin (HE). For electron microscopy observation, renal tissues
were fixed in 2.5% glutaraldehyde at 4°C for 4 h, and then fixed in
2% osmic acid (Seebio Biotech Co., Ltd., Shanghai, China) at 4°C
for 2 h. Tissues were then dehydrated by a graded acetone series.
Epon812 (Sangon Biotech Co., Ltd., Shanghai, China) was used for
saturating and embedding tissues, following the manufacturer's
protocol. An Ultra Rapid Tissue Processor (Histra-QS; Jokoh Co.,
Ltd., Tokyo, Japan) was used to produce 2-µm sections and uranyl
acetate and lead citrate were applied to stain the tissues. A
H-7500 transmission electron microscopy (magnification, ×7,000) was
used to observe and capture images of the tissue sections.
Immunohistochemistry
Expression of HMGB1, TGF-β1 and NF-κB in renal
tissue was detected by immunohistochemical staining
(streptavidin-perosidase). Polyclonal antibodies against HMGB1
(#BA2361; 1:200), TGF-β1 (#BM4876; 1:200) and NF-κB p65 (#BM3946;
1:200) were purchased from Wuhan Boster Biological Engineering Co.,
Ltd., (Wuhan, China) The procedure was performed following the
manufacturer's protocol of the VECTASTAIN® ABC kit
(#PK-6100; Vector Laboratories, Inc., Burlingame, CA, USA). Tissues
were fixed in 10% formalin at 37°C for 24 h and conventionally
dehydrated (70% ethanol for 1 day, 80% ethanol for 45 min, 90%
ethanol for 1 h, 95% ethanol for 1 h, and ethanol 100% for 3 h).
Tissues were embedded in paraffin and 3-µm tissue sections were
prepared. Following deparaffinization and rehydration to block
endogenous peroxidase, sections were soaked in 0.003 (volume ratio)
H2O2 in 80% methanol and washed with
phosphate-buffered saline (PBS) three times. Normal non-immune
serum was used to prevent unspecific binding. Subsequently, the
primary antibody was added to sections overnight at 4°C, followed
by overlaying with appropriate secondary antibody (#BM3895;
1:1,000; Wuhan Boster Biological Engineering Co., Ltd.) for 15 min
at 37°C. PBS was used as the negative control instead of primary
and secondary antibodies. Following 3,3′-diaminobenzidine (DAB)
staining and hematoxylin counterstaining, a section of renal tissue
was fixed with ethanol (75% for 1 min, 85% for 1 min, 95% for 1 min
and 100% for 4 min). A light microscope (magnification, ×100) was
used for observation and 10 high-power fields were randomly
selected in each section to record the number of dying cells and
calculate the positive signal rate. If there were no positive cells
in each of the high power field on average, a ‘−’ grade was given.
Positive cell numbers of <25%, 25–50% and >50% were graded as
‘+’, ‘++’ and ‘+++’, respectively.
Western blot analysis
Western blot analysis was used to detect the protein
expression levels of HMGB1, TGF-β1 and NF-κB. The procedure was
performed with the EasySee Western Blot kit (#DW101; Shanghai Bogoo
Biotechnology Co., Ltd., Shanghai, China) according to the
manufacturer's protocol. Polyclonal antibody rabbit anti-human
HMGB1 (#BA2361; 1:200), TGF-β1 (#BM4876; 1:200) NF-κB p65 (#BM3946;
1:200), and goat anti-rabbit IgG conjugated to horseradish
peroxidase (#BM3895; 1:1,000) were purchased from Wuhan Boster
Biological Engineering Co., Ltd. Samples were treated with RIPA
reagent (#P0013B; Beyotime Institute of Biotechnology, Shanghai,
China) for 30 min at 4°C for protein extraction. A total of 10 µg
cell plasma protein was separated by 10% SDS-PAGE. This was
transferred onto a polyvinylidene fluoride membrane under a steady
electric current (160 mA) for 20 min. The membrane was blocked with
TBS-Tween-20 (TBST; Beyotime Institute of Biotechnology) and 5%
skim milk at 37°C for 1 h. Then the samples were treated with
primary antibodies at 4°C overnight. After washing with TBST 3
times, samples were treated with secondary antibodies at 37°C for 1
h. ChemiDocTM XRS gel imaging system (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was used for capturing images of the gel
following DAB staining, and Image J software v1.48 (National
Institutes of Health, Bethesda, MD, USA) was used to read integral
absorbance values. Each sample was assessed with three replicates,
and β-actin was used as an internal reference.
Reverse transcription-quantification
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR was used to detect the relative expression
level of HMGB1, TGF-β1 and NF-κB mRNA relative to β-actin. Total
RNA was isolated from renal tissues using an RNAprep Pure Tissue
kit (#DP431; Tiangen Biotech Co., Ltd., Beijing, China), following
the manufacturers protocol. In a 25-µl total reaction volume, 2 µg
RNA was reverse transcribed into cDNA using an OligdT primer
(Takara Bio, Inc., Otsu, Japan), following the protocol of 37°C for
15 min and 85°C for 5 sec. All PCR primers sequences are presented
in the Table I. The full reaction
components were: 0.4 µl PCR forward primer (10 µM), 0.4 µl PCR
reverse primer (10 µM), 2 µl cDNA template, 7.2 µl RNase free
dH2O and 10 µl SYBR Premix Ex Taq TM II (2×) (Takara
Bio, Inc.). The amplification reaction was completed as follows:
Pre-degeneration for 5 min at 95°C; degeneration for 1 min in 94°C;
annealing for 60 sec at 59°C and extension for 60 sec at 72°C (35
cycles); and final extension for 8 min at 72°C. PCR product (5 µl)
was used for electrophoresis on 1% agarose gel, and ethidium
bromide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for DNA
stain. ChemiDocTM XRS gel imaging system (Bio-Rad Laboratories,
Inc.) was used to image the agarose gel and Image-J software v1.48
was used to read its integral absorbance values. The relative
expression level of mRNA was acquired by the ratio of relative
intensity between the PCR products electrophoresis banding and the
internal reference gene, β-actin.
 | Table I.Primer sequences for quantitative
polymerase chain reaction analysis. |
Table I.
Primer sequences for quantitative
polymerase chain reaction analysis.
| Gene | Primers | Product size
(bp) |
|---|
| HMGB1 | Forward:
5′-TCATCTGCTGCAGTGTTGTT-3′ | 285 |
|
| Reverse:
5′-CTCAGAGAGGTGGAAGA-3′ |
|
| TGF-β1 | Forward:
5′-TCCTGTGACAGCAGGGATAA-3′ | 298 |
|
| Reverse:
5′-TCCTGTGACAGCAGGGATAA-3′ |
|
| NF-κB | Forward:
5′-CTGAACCAGGGCATACCTGT-3′ | 197 |
|
| Reverse:
5′-GAGAAGTCCATGTCCGCAAT-3′ |
|
| β actin | Forward:
5′-AACCCTAAGGCCAACCGTGAAAAG-3′ | 240 |
|
| Reverse:
5′-TCATGAGGTAGTCTGTCAGGT-3′ |
|
Statistical analysis
SPSS 19.0 (IBM SPSS, Armonk, NY, USA) statistical
software was used to analyze the data. Measurement data was
expressed as mean ± standard deviation. The χ2 test was
applied for enumeration data and Student's t-test and analysis of
variance were applied for measurement data. Pearson correlation
analysis was used to assess the protein expression level and mRNA
relative expression level of HMGB1, TGF-β1 and NF-κB. Spearman
correlation analysis was applied to analyze the association between
CAN grade and HMGB1, TGF-β1 and NF-κB. A two-tailed P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline characteristics
Clinical characteristics of CAN patients and
controls are presented in Table II.
No statistically significant differences were observed for age,
sex, BMI index, hs-CRP, TG, LDL-C, HDL-C or FPG between CAN
patients and controls (P>0.05). The glomerular filtration rate
(GFR) and hemoglobin of CAN patients were significantly lower when
compared with controls (P<0.05), whereas IL-6 was significantly
higher in CAN patients than in controls (P<0.05; Table II).
 | Table II.Clinical characteristics of CAN
patients and controls. |
Table II.
Clinical characteristics of CAN
patients and controls.
| Characteristic | CAN (n=27) | Controls
(n=30) |
χ2/t | P-value |
|---|
| Age, years | 41±8 | 44±9 | 1.324 | 0.19 |
| Male/female | 15/12 | 16/14 | 0.028 | 0.87 |
| GFR | 27±10 | 87±20 | 14.540 | <0.05 |
| BMI | 24±4 | 26±4 | 1.885 | 0.07 |
| Hemoglobin,
g/l | 132±15 | 141±9 | 2.710 | <0.01 |
| Hs-CRP, mmol/l | 4.7±1.2 | 4.3±1.2 | 1.257 | 0.21 |
| IL-6, ng/l | 27.4±8.5 | 15.1±4.7 | 6.659 | <0.05 |
| TG, mmol/l | 2.1±1.4 | 1.9±0.9 | 0.634 | 0.53 |
| LDL-C, mmol/l | 2.9±1.3 | 2.6±1.1 | 0.943 | 0.35 |
| HDL-C, mmol/l | 1.3±0.6 | 1.4±0.5 | 0.686 | 0.50 |
| FPG, mmol/l | 5.5±0.5 | 5.3±0.6 | 1.358 | 0.18 |
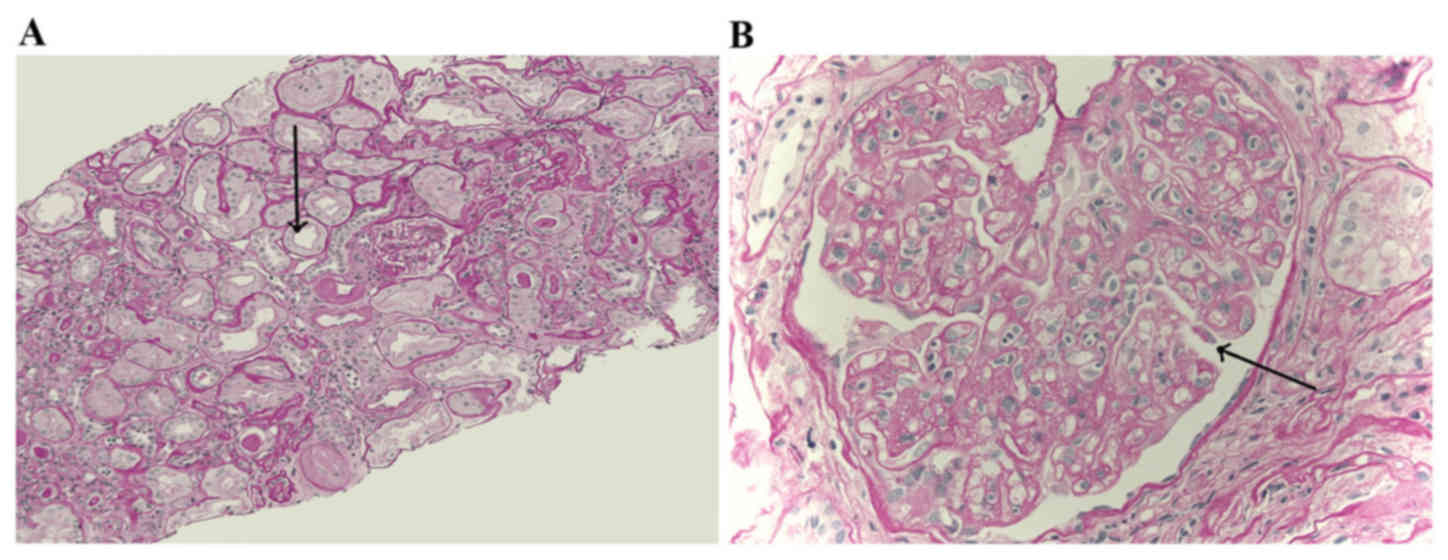
Histomorphology
In microscopic observations of paraffin sections
with HE staining, no characteristic changes were observed,
including abnormally elevated Cr induced by acute rejection or CsA
toxicosis. However, interstitial fibrosis and tubular atrophy were
observed based on the Banff 07 criteria, which were the primary
characteristics of chronic allograft nephropathy. Among 27 renal
tissues from CAN patients, there were 5 cases of CAN grade I, 12
cases of CAN grade II and 10 cases of CAN grade III. Observation
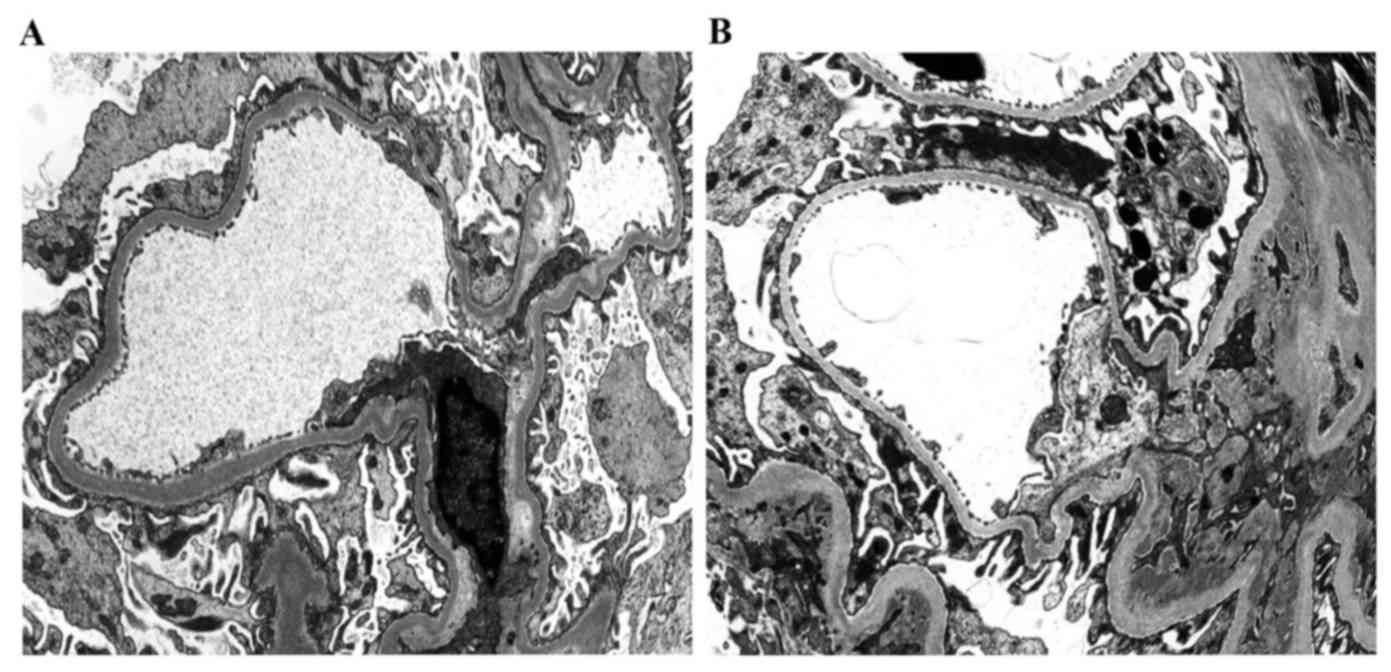
results of light microscopy and electron microscopy are presented
in Figs. 1 and 2, respectively.
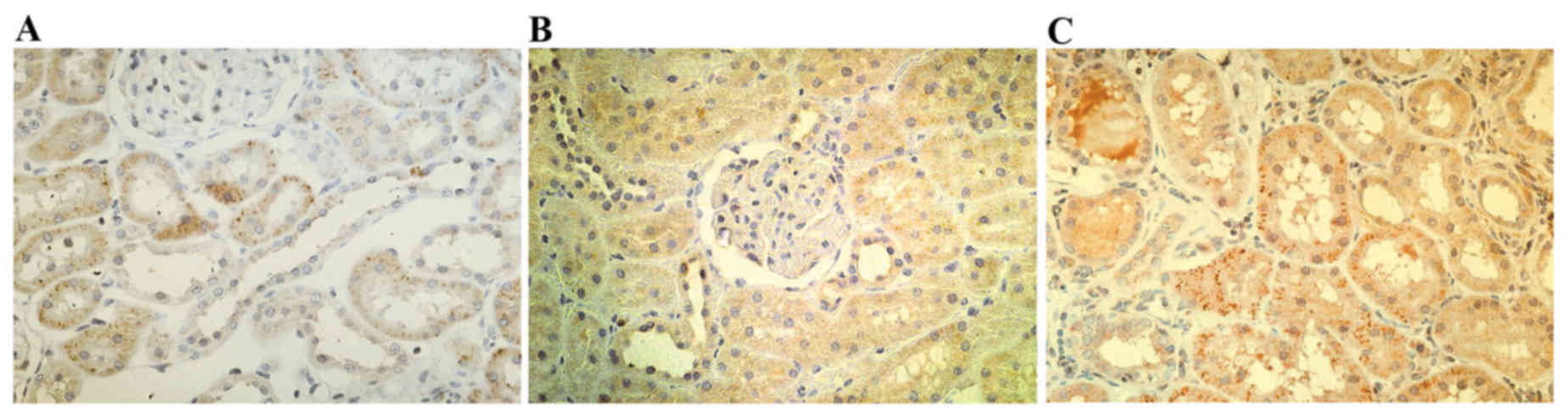
Immunohistology
In the renal tissues of patients with CAN, HMGB1,
TGF-β1 and NF-κB were markedly positively expressed in the cell
cytoplasm and membrane of renal tubular epithelial cells (Fig. 3). HMGB1, TGF-β1 and NF-κB in the
renal tissues of the CAN group revealed markedly higher positive
rates than normal renal tissues (Table
III). The positive rates of HMGB1, TGF-β1 and NF-κB in CAN
grade III were markedly higher than in CAN grade I (Table III).
 | Table III.Expression of HMGB1, TGF-β1 and NF-κB
in the CAN and control groups. |
Table III.
Expression of HMGB1, TGF-β1 and NF-κB
in the CAN and control groups.
|
|
| HMGB1 | TGF-β1 | NF-κB |
|---|
|
|
|
|
|
|
|---|
| Group | N | − | + | ++ | +++ | − | + | ++ | +++ | − | + | ++ | +++ |
|---|
| Controls | 30 | 19 | 11 | 0 | 0 | 18 | 12 | 0 | 0 | 18 | 12 | 0 | 0 |
| CAN I | 5 | 0 | 2 | 2 | 1 | 0 | 2 | 2 | 1 | 0 | 1 | 2 | 2 |
| CAN II | 12 | 0 | 2 | 4 | 6 | 0 | 2 | 6 | 4 | 0 | 2 | 7 | 3 |
| CAN III | 10 | 0 | 1 | 3 | 6 | 0 | 1 | 2 | 7 | 0 | 0 | 4 | 6 |
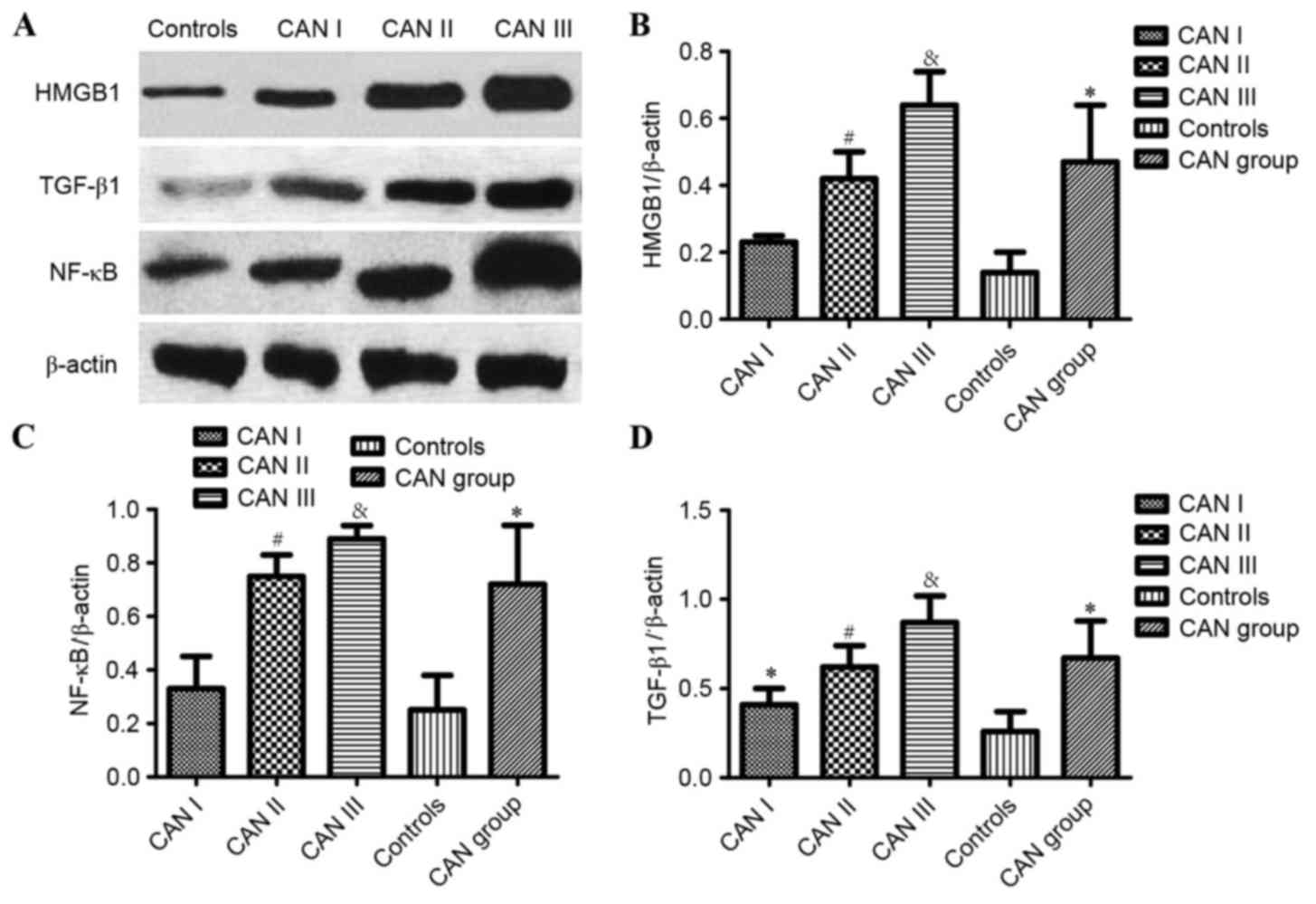
Western blot analysis
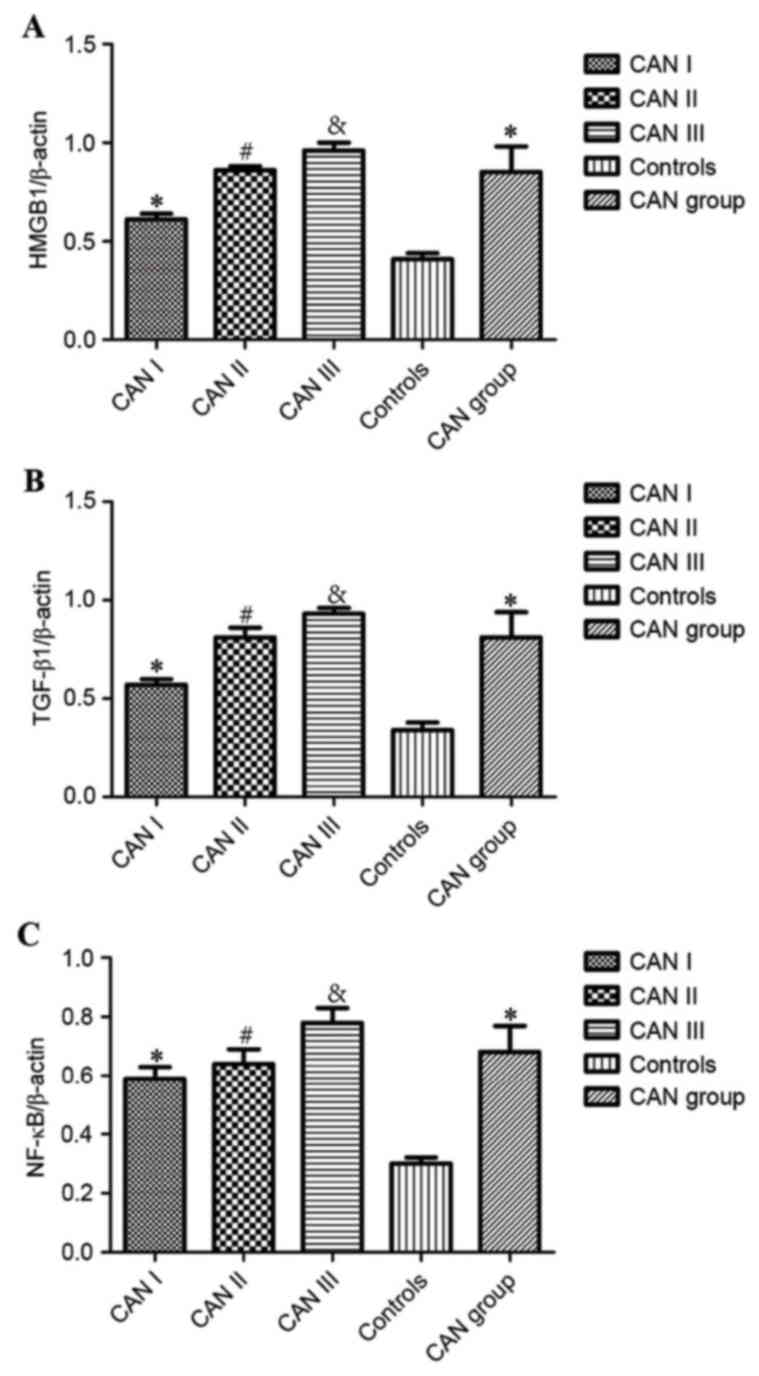
As demonstrated by western blot analysis (Fig. 4A), the expression of HMGB1, TGF-β1,
NF-κB was significantly higher in the renal tissues of patients in
the CAN group, compared with normal tissues from the control group
(P<0.05; Fig. 4B-D). Furthermore,
statistically significant differences in the expression of HMGB1,
TGF-β1 and NF-κB were identified among CAN grades I, II and III
(P<0.05; Fig. 4). The expression
of HMGB1 and NF-κB in the CAN grade I demonstrated no statistical
difference when compared with the controls (P>0.05; Fig. 4B and C, respectively), whereas TGF-β1
expression was significantly higher compared with the control group
(P<0.05; Fig. 4D). Furthermore,
expression of HMGB1, NF-κB and TGF-β1 in CAN grade III was
significantly higher than grade II (P<0.05; Fig. 4B-D, respectively).
RT-qPCR analysis
Compared with the control group, the relative
expression of HMGB1, TGF-β1 and NF-κB mRNA in the CAN group was
significantly elevated (P<0.05; Fig.
5A-C, respectively). Notably, there was significantly increased
expression of HMGB1, TGF-β1 and NF-κB mRNA in CAN grade II,
compared with grade I (P<0.05). Similarly, the expression of
HMGB1, TGF-β1, NF-κB mRNA in CAN grade III were significantly
higher compared with grade II (P<0.05; Fig. 5; Table
IV).
 | Table IV.Relative expression of HMGB1, TGF-β1
and NF-κB mRNA in the CAN and control groups. |
Table IV.
Relative expression of HMGB1, TGF-β1
and NF-κB mRNA in the CAN and control groups.
|
|
| CAN |
|
|---|
|
|
|
|
|
|---|
| Cytokine | CAN (n=27) | Grade I (n=5) | Grade II
(n=12) | Grade III
(n=10) | Controls
(n=30) |
|---|
| HMGB1 |
0.85±0.13a |
0.61±0.03b |
0.86±0.02c | 0.96±0.04 | 0.41±0.03 |
| TGF-β1 |
0.81±0.13a |
0.57±0.03b |
0.81±0.05c | 0.93±0.03 | 0.34±0.04 |
| NF-kB |
0.68±0.09a |
0.56±0.04b |
0.64±0.05c | 0.78±0.05 | 0.30±0.02 |
Correlation analysis among HMGB1,
TGF-β1 and NF-κB and CAN grade
Statistical analysis indicated positive associations
between the expression levels of HMGB1, TGF-β1 and NF-κB in renal
tissues (HMGB1 vs. TGF-β1: r=0.860, P<0.01; HMGB1 vs. NF-κB:
r=0.899, P<0.01; TGF-β1 vs. NF-κB: r=0.835, P<0.01). In the
renal tissues of patients with CAN, expression of HMGB1, TGF-β1 and
NF-κB was positively associated with CAN pathological grade (HMGB1:
r=0.894, P<0.01; TGF-β1: r=0.867, P<0.01; NF-κB: r=0.853,
P<0.01) and the expression level of HMGB1, TGF-β1 and NF-κB
tended to increase with the aggravation of the CAN pathological
grade. Furthermore, a positive association between the protein and
mRNA expression of HMGB1, TGF-β1 and NF-κB (r=0.904, P<0.01;
r=0.858, P<0.01; r=0.885, P<0.01, respectively) was
identified and the mRNA expression level of HMGB1, TGF-β1 and NF-κB
in renal tissues also increased with the progression of the CAN
pathological grade (Tables V and
VI).
 | Table V.Pearson correlation analysis of the
association between the protein and mRNA expression levels of
HMGB1, TGF-β1 and NF-κB (n=57). |
Table V.
Pearson correlation analysis of the
association between the protein and mRNA expression levels of
HMGB1, TGF-β1 and NF-κB (n=57).
| Analysis |
| HMGB1 | TGFB1 | NF-κB | HMGB1 mRNA | TGF-1 mRNA | NF-κB mRNA |
|---|
| HMGB1 | Pearson
correlation | 1 | 0.860 | 0.899 | 0.904 | – | – |
|
| P-value
(2-tailed) |
| <0.001 | <0.001 | <0.001 |
|
|
| TGF-tai | Pearson
correlation | 0.860 | 1 | 0.835 | – | 0.858 | – |
|
| P-value
(2-tailed) | <0.001 |
| <0.001 |
| <0.001 |
|
| NF-κB | Pearson
correlation | 0.899 | 0.835 | 1 | – | – | 0.885 |
|
| P-value
(2-tailed) | <0.001 | <0.001 |
|
|
| <0.001 |
| HMGB1 mRNA | Pearson
correlation | 0.904 | – | – | 1 | 0.979 | 0.962 |
|
| P-value
(2-tailed) | <0.001 |
|
|
| <0.001 | <0.001 |
| TGF-ailed)A | Pearson
correlation | – | 0.858 | – | 0.979 | 1 | 0.961 |
|
| P-value
(2-tailed) |
| <0.001 |
| <0.001 |
| <0.001 |
| NF-κB mRNA | Pearson
correlation | – | – | 0.885 | 0.962 | 0.961 | 1 |
|
| P-value
(2-tailed) |
|
| <0.001 | 0.000 | <0.001 |
|
 | Table VI.Spearman correlation analysis of the
association between the protein and mRNA expression levels of
HMGB1, TGF-β1 and NF-κB and CAN grade (n=57). |
Table VI.
Spearman correlation analysis of the
association between the protein and mRNA expression levels of
HMGB1, TGF-β1 and NF-κB and CAN grade (n=57).
| Variable | HMGB1 | TGFB1 | NF-κB | HMGB1 mRNA | TGF-1 mRNA | NF-κB mRNA |
|---|
| Association | 0.894 | 0.867 | 0.853 | 0.915 | 0.917 | 0.913 |
| P-value
(2-tailed) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Discussion
With the great developments made in transplantation
immunology, the short-term graft survival rate has markedly
improved, while the long-term survival rate has remained low
(34). Studies have revealed that
CAN is a leading factor, causing 50–80% of allograft loss in renal
function in the late period following kidney transplantation
(3,35). As CAN is caused by various immune and
non-immune factors, and is easily affected by a series of
donor-related factors including donor age, brain death and
consequences of ischemia-reperfusion injury, the pathogenesis and
mechanisms remain unclear (36–40).
Previous studies suggested that HMGB1, which is a
pro-inflammatory cytokine released from dying cells, would
accumulate as renal function deteriorates and have an important
role in CKD (17). The levels of
HMGB1 may be markedly overexpressed during the progression of CKD.
It was also demonstrated that HMGB1 functions are associated with
NF-κB in the inflammatory and immunostimulation response (41–43).
NF-κB has also been implicated in kidney diseases and is associated
with renal inflammatory injury and fibrosis (44). It is speculated that HMGB1-NF-κB may
function as a signaling pathway in renal diseases. Previous studies
revealed that TGF-β1 was regulated by NF-κB in certain signaling
pathways, which indicates a potential role for NF-κB (45,46).
Therefore, the present study aimed to investigate the association
between HMGB1, NF-κB, TGF-β1 and CAN.
The current study analyzed the HMGB1, NF-κB and
TGF-β1 staining intensity within graft kidneys. The results of
immunohistological staining indicated that HMGB1, NF-κB and TGF-β1
were predominantly expressed in the mesangium and glomerular
epithelium. The positive expression rate of HMGB1, NF-κB and TGF-β1
in CAN tissues was much higher when compared with controls,
indicating potential associations between HMGB1, NF-κB, TGF-β1 and
CAN. In the present study, the results of western blot analysis and
RT-qPCR also demonstrated that HMGB1, NF-κB and TGF-β1 were
significantly associated with CAN and specifically to the grade of
CAN. HMGB1, NF-κB and TGF-β1 were significantly overexpressed in
renal tissues of patients with CAN, compared with controls. The
expression of HMGB1, NF-κB and TGF-β1 increased with the
progression of CAN. Following the statistical analysis performed in
the current study, a significant association between HMGB1, NF-κB
and TGF-β1 in CAN progression was revealed.
TGF-β1 is one of the critical cytokines, which leads
to renal fibrosis, causing renal interstitial fibrosis in end-stage
renal disease (47). It has been
proven to be involved in CAN pathogenesis (28,48,49). To
the best of our knowledge, no studies have assessed the association
between NF-κB and CAN. However, NF-κB was indicated to have a
function in kidney diseases, including CKD (50). Furthermore, it has been demonstrated
that NF-κB was associated with hepatic stellate cell in the
apoptosis process, in which NF-κB activation stimulated TGF-β1,
leading to serious renal interstitial fibrosis (51–53).
These findings suggested that NF-κB and TGF-β1 may constitute an
important pathway in the pathogenesis of CAN, which may explain the
positive association between NF-κB and TGF-β1 in the current study.
HMGB1 has been studied in other renal diseases (54,55);
however, it is another novel inflammatory marker, which, to the
best of our knowledge, has not been studied in regard to CAN. The
concentration of HMGB1 serum levels in CKD was elevated in a
previous study (17). The
overexpression of HMGB1 in tissues in the present study also
demonstrated a marked association between HMGB1 and CAN. A
potential explanation for the positive association between HMGB1
and NF-κB in the present study is that HMGB1 induces the downstream
translocation of NF-κB by interacting with TLR2, TLR4 and RAGE
(41,56–58).
TLR2, TLR, HMGB1/NF-κB/TGF-β1 may form a signaling pathway to
induce the pathogenesis of CAN. The overexpression of HMGB1
stimulates NF-κB activation, leading to the overexpression of
TGF-β1, which causes renal interstitial fibrosis and a series of
immunostimulatory and inflammatory responses through the pathway,
resulting in CAN. With the accumulation of these factors in renal
tissues, the conditions of CAN progressively deteriorate.
Therefore, these factors (HMGB1, NF-κB and TGF-β1) in the tissues
of CAN Grade III would be higher than those observed in CAN Grade
I. Although this assumption is based on the present results and
other studies, more details regarding the function of HMGB1, NF-κB
and TGF-β1 in CAN pathogenesis are required to validate these
results in further studies.
In conclusion, the present study identified that the
levels of HMGB1, NF-κB and TGF-β1 in renal tissues are
significantly associated with CAN. Furthermore, expression of
HMGB1, NF-κB and TGF-β1 increases with CAN progression. Finally,
the positive associations among HMGB1, NF-κB and TGF-β1 indicate
that the HMGB1/NF-κB/TGF-β1 pathway may be one of the leading
causes of CAN. The present study therefore provides a novel way to
understand the mechanisms of CAN. Novel targets, including HMGB1
and NF-κB, were revealed in relation to CAN, and the
HMGB1/NF-κB/TGF-β1 pathway may be the foundation of a novel target
treatment for CAN. However, further studies are required to
validate the roles of HMGB1, NF-κB and TGF-β1 in CAN and to
investigate the mechanism of action in CAN pathogenesis. The
present study may lead to the identification of effective methods
to treat CAN and elevate the long-term graft survival rate
following kidney transplantation.
References
|
1
|
Cui Y, Huang Q, Auman JT, Knight B, Jin X,
Blanchard KT, Chou J, Jayadev S and Paules RS: Genomic-derived
markers for early detection of calcineurin inhibitor
immunosuppressant-mediated nephrotoxicity. Toxicol Sci. 124:23–34.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lamb KE, Lodhi S and Meier-Kriesche HU:
Long-term renal allograft survival in the United States: A critical
reappraisal. Am J Transplant. 11:450–462. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cassidy H, Slyne J, O'Kelly P, Traynor C,
Conlon PJ, Johnston O, Slattery C, Ryan MP and McMorrow T: Urinary
biomarkers of chronic allograft nephropathy. Proteomics Clin Appl.
9:574–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weir MR and Wali RK: Minimizing the risk
of chronic allograft nephropathy. Transplantation. 87 8
Suppl:S14–S18. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Birnbaum LM, Lipman M, Paraskevas S,
Chaudhury P, Tchervenkov J, Baran D, Herrera-Gayol A and
Cantarovich M: Management of chronic allograft nephropathy: A
systematic review. Clin J Am Soc Nephrol. 4:860–865. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lukenda V, Mikolasevic I, Racki S, Jelic
I, Stimac D and Orlic L: Transient elastography: A new noninvasive
diagnostic tool for assessment of chronic allograft nephropathy.
Int Urol Nephrol. 46:1435–1440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arndt R, Schmidt S, Loddenkemper C,
Grünbaum M, Zidek W, van der Giet M and Westhoff TH: Noninvasive
evaluation of renal allograft fibrosis by transient elastography-a
pilot study. Transpl Int. 23:871–877. 2010.PubMed/NCBI
|
|
8
|
Sayin B, Karakayali H, Colak T, Sevmis S,
Pehlivan S, Demirhan B and Haberal M: Conversion to sirolimus for
chronic allograft nephropathy and calcineurin inhibitor toxicity
and the adverse effects of sirolimus after conversion. Transplant
Proc. 41:2789–2793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haas M: Chronic allograft nephropathy or
interstitial fibrosis and tubular atrophy: What is in a name? Curr
Opin Nephrol Hypertens. 23:245–250. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Del Bello A, Rostaing L, Congy-Jolivet N,
Sallusto F, Gamé X and Kamar N: Kidney nephrectomy after allograft
failure. Nephrol Ther. 9:189–194. 2013.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang K and Liu QZ: Effect analysis of
1-year posttransplant body mass index on chronic allograft
nephropathy in renal recipients. Transplant Proc. 43:2592–2595.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Johnston O, Cassidy H, O'Connell S,
O'Riordan A, Gallagher W, Maguire PB, Wynne K, Cagney G, Ryan MP,
Conlon PJ and McMorrow T: Identification of β2-microglobulin as a
urinary biomarker for chronic allograft nephropathy using proteomic
methods. Proteomics Clin Appl. 5:422–431. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu P, Xie L, Ding HS, Gong Q, Yang J and
Yang L: High mobility group box 1 and kidney diseases (Review). Int
J Mol Med. 31:763–768. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srinivasan M, Banerjee S, Palmer A, Zheng
G, Chen A, Bosland MC, Kajdacsy-Balla A, Kalyanasundaram R and
Munirathinam G: HMGB1 in hormone-related cancer: A potential
therapeutic target. Horm Cancer. 5:127–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Gong Q, Zhong S, Wang L, Guo H,
Xiang Y, Ichim TE, Wang CY, Chen S, Gong F and Chen G:
Neutralization of the extracellular HMGB1 released by ischaemic
damaged renal cells protects against renal ischaemia-reperfusion
injury. Nephrol Dial Transplant. 26:469–478. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bruchfeld A, Qureshi AR, Lindholm B,
Barany P, Yang L, Stenvinkel P and Tracey KJ: High Mobility Group
Box Protein-1 correlates with renal function in chronic kidney
disease (CKD). Mol Med. 14:109–115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leelahavanichkul A, Huang Y, Hu X, Zhou H,
Tsuji T, Chen R, Kopp JB, Schnermann J, Yuen PS and Star RA:
Chronic kidney disease worsens sepsis and sepsis-induced acute
kidney injury by releasing High Mobility Group Box Protein-1.
Kidney Int. 80:1198–1211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zakiyanov O, Kriha V, Vachek J, Zima T,
Tesar V and Kalousova M: Placental growth factor,
pregnancy-associated plasma protein-A, soluble receptor for
advanced glycation end products, extracellular newly identified
receptor for receptor for advanced glycation end products binding
protein and high mobility group box 1 levels in patients with acute
kidney injury: A cross sectional study. BMC Nephrol. 14:2452013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oyama Y, Hashiguchi T, Taniguchi N,
Tancharoen S, Uchimura T, Biswas KK, Kawahara K, Nitanda T, Umekita
Y, Lotz M and Maruyama I: High-mobility group box-1 protein
promotes granulomatous nephritis in adenine-induced nephropathy.
Lab Invest. 90:853–866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bhatelia K, Singh K and Singh R: TLRs:
Linking inflammation and breast cancer. Cell Signal. 26:2350–2357.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou TB: Role of high mobility group box 1
and its signaling pathways in renal diseases. J Recept Signal
Transduct Res. 34:348–350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pateras I, Giaginis C, Tsigris C,
Patsouris E and Theocharis S: NF-κB signaling at the crossroads of
inflammation and atherogenesis: Searching for new therapeutic
links. Expert Opin Ther Targets. 18:1089–1101. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hayden MS and Ghosh S: Regulation of NF-κB
by TNF family cytokines. Semin Immunol. 26:253–266. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sanz AB, Sanchez-Niño MD, Ramos AM, Moreno
JA, Santamaria B, Ruiz-Ortega M, Egido J and Ortiz A: NF-kappaB in
renal inflammation. J Am Soc Nephrol. 21:1254–1262. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohammed-Ali Z, Cruz GL and Dickhout JG:
Crosstalk between the unfolded protein response and NF-κB-mediated
inflammation in the progression of chronic kidney disease. J
Immunol Res. 2015:4285082015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harris S, Coupes BM, Roberts SA, Roberts
IS, Short CD and Brenchley PE: TGF-beta1 in chronic allograft
nephropathy following renal transplantation. J Nephrol. 20:177–185.
2007.PubMed/NCBI
|
|
28
|
Daniel C, Vogelbacher R, Stief A, Grigo C
and Hugo C: Long-term gene therapy with thrombospondin 2 inhibits
TGF-β activation, inflammation and angiogenesis in chronic
allograft nephropathy. PLoS One. 8:e838462013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ding Y and Choi ME: Regulation of
autophagy by TGF-β: Emerging role in kidney fibrosis. Semin
Nephrol. 34:62–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi ME, Ding Y and Kim SI: TGF-β
signaling via TAK1 pathway: Role in kidney fibrosis. Semin Nephrol.
32:244–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Ge Y, Liu FY, Peng YM, Sun L, Li J,
Chen Q, Sun Y and Ye K: Norcantharidin, a protective therapeutic
agent in renal tubulointerstitial fibrosis. Mol Cell Biochem.
361:79–83. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ka SM, Yeh YC, Huang XR, Chao TK, Hung YJ,
Yu CP, Lin TJ, Wu CC, Lan HY and Chen A: Kidney-targeting Smad7
gene transfer inhibits renal TGF-β/MAD homologue (SMAD) and nuclear
factor κB (NF-κB) signalling pathways and improves diabetic
nephropathy in mice. Diabetologia. 55:509–519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lan HY and Chung AC: TGF-β/Smad signaling
in kidney disease. Semin Nephrol. 32:236–243. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Srinivas TR and Oppenheimer F: Identifying
endpoints to predict the influence of immunosuppression on
long-term kidney graft survival. Clin Transplant. 29:644–653. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia SQ, Fan Y, Tan MY and Zheng JH:
Five-year follow-up after conversion from calcineurin inhibitor to
sirolimus-based treatment in kidney transplant patients with
chronic allograft nephropathy. Int J Clin Exp Med. 8:3552–3558.
2015.PubMed/NCBI
|
|
36
|
Shrestha B and Haylor J: Experimental rat
models of chronic allograft nephropathy: A review. Int J Nephrol
Renovasc Dis. 7:315–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Timsit MO, Yuan X, Floerchinger B, Ge X
and Tullius SG: Consequences of transplant quality on chronic
allograft nephropathy. Kidney Int Suppl. S54–S58. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Leca N: Focal segmental glomerulosclerosis
recurrence in the renal allograft. Adv Chronic Kidney Dis.
21:448–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liao QB, Guo JQ, Zheng XY, Zhou ZF, Li H,
Lai XY and Ye JF: Test performance of sputum microRNAs for lung
cancer: A meta-analysis. Genet Test Mol Biomarkers. 18:562–567.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schinstock CA, Stegall M and Cosio F: New
insights regarding chronic antibody-mediated rejection and its
progression to transplant glomerulopathy. Curr Opin Nephrol
Hypertens. 23:611–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tan Y, Wang Q, She Y, Bi X and Zhao B:
Ketamine reduces LPS-induced HMGB1 via activation of the Nrf2/HO-1
pathway and NF-κB suppression. J Trauma Acute Care Surg.
78:784–792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shi Z, Lian A and Zhang F: Nuclear
factor-κB activation inhibitor attenuates ischemia reperfusion
injury and inhibits Hmgb1 expression. Inflamm Res. 63:919–925.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhou XJ, Dong ZG, Yang YM, Du LT, Zhang X
and Wang CX: Limited diagnostic value of microRNAs for detecting
colorectal cancer: A meta-analysis. Asian Pac J Cancer Prev.
14:4699–4704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Terhzaz S, Overend G, Sebastian S, Dow JA
and Davies SA: The D. Melanogaster capa-1 neuropeptide activates
renal NF-kB signaling. Peptides. 53:218–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kim HJ, Kim JG, Moon MY, Park SH and Park
JB: IκB kinase γ/nuclear factor-κB-essential modulator (IKKγ/NEMO)
facilitates RhoA GTPase activation, which, in turn, activates
Rho-associated KINASE (ROCK) to phosphorylate IKKβ in response to
transforming growth factor (TGF)-β1. J Biol Chem. 289:1429–1440.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jia QQ, Wang JC, Long J, Zhao Y, Chen SJ,
Zhai JD, Wei LB, Zhang Q, Chen Y and Long HB: Sesquiterpene
lactones and their derivatives inhibit high glucose-induced NF-κB
activation and MCP-1 and TGF-β1 expression in rat mesangial cells.
Molecules. 18:13061–13077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Oo YH, Shetty S and Adams DH: The role of
chemokines in the recruitment of lymphocytes to the liver. Dig Dis.
28:31–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Saigo K, Akutsu N, Maruyama M, Otsuki K,
Hasegawa M, Aoyama H, Matsumoto I, Asano T and Kenmochi T: Study of
transforming growth factor-β1 gene, mRNA, and protein in Japanese
renal transplant recipients. Transplant Proc. 46:372–375. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Assadiasl S, Ahmadpoor P, Nafar M,
Pezeshki Lessan M, Pourrezagholi F, Parvin M, Shahlaee A, Sepanjnia
A, Nicknam MH and Amirzargar A: Regulatory T cell subtypes and
TGF-β1 gene expression in chronic allograft dysfunction. Iran J
Immunol. 11:139–152. 2014.PubMed/NCBI
|
|
50
|
Liu H, Sun W, Wan YG, Tu Y, Yu BY and Hu
H: Regulatory mechanism of NF-kappaB signaling pathway on renal
tissue inflammation in chronic kidney disease and interventional
effect of traditional Chinese medicine. Zhongguo Zhong Yao Za Zhi.
38:4246–4251. 2013.(In Chinese). PubMed/NCBI
|
|
51
|
Braz MM, Ramalho FS, Cardoso RL, Zucoloto
S, Costa RS and Ramalho LN: Slight activation of nuclear factor
kappa-B is associated with increased hepatic stellate cell
apoptosis in human schistosomal fibrosis. Acta Trop. 113:66–71.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guicciardi ME and Gores GJ: Apoptosis as a
mechanism for liver disease progression. Semin Liver Dis.
30:402–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Qin L and Han YP: Epigenetic repression of
matrix metalloproteinases in myofibroblastic hepatic stellate cells
through histone deacetylases 4: Implication in tissue fibrosis. Am
J Pathol. 177:1915–1928. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liu GQ, Zuo XH, Jiang LN, Zhang YP, Zhang
LM, Zhao ZG and Niu CY: Inhibitory effect of post-hemorrhagic shock
mesenteric lymph drainage on the HMGB1 and RAGE in mouse kidney.
Ren Fail. 38:131–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qie GQ, Wang CT, Chu YF and Wang R:
Expression of HMGB1/RAGE protein in renal carcinoma and its
clinical significance. Int J Clin Exp Pathol. 8:6262–6268.
2015.PubMed/NCBI
|
|
56
|
Karuppagounder V, Arumugam S,
Thandavarayan RA, Pitchaimani V, Sreedhar R, Afrin R, Harima M,
Suzuki H, Nomoto M, Miyashita S, et al: Modulation of HMGB1
translocation and RAGE/NFκB cascade by quercetin treatment
mitigates atopic dermatitis in NC/Nga transgenic mice. Exp
Dermatol. 24:418–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kang N, Hai Y, Yang J, Liang F and Gao CJ:
Hyperbaric oxygen intervention reduces secondary spinal cord injury
in rats via regulation of HMGB1/TLR4/NF-κB signaling pathway. Int J
Clin Exp Pathol. 8:1141–1153. 2015.PubMed/NCBI
|
|
58
|
Sun J, Shi S, Wang Q, Yu K and Wang R:
Continuous hemodiafiltration therapy reduces damage of multi-organs
by ameliorating of HMGB1/TLR4/NFκB in a dog sepsis model. Int J
Clin Exp Pathol. 8:1555–1564. 2015.PubMed/NCBI
|