Introduction
Chemical burns are the second most common cause of
burns in China (1). According to a
previous retrospective study, ocular severe chemical damage leads
to devastating injury and accounts for a small but significant
percentage of corneal blindness in China (2), for which may be ascribed to the swiftly
developing industrialization in Shanghai (3). The failure of appropriate corneal
repair following severe eye injuries (such as those after chemical
burns) often leads to loss of vision. Alkali burns lead to some of
the most serious corneal injuries because alkaline chemicals
penetrate tissue more quickly than acids to reach the corneal
stroma and extirpate ocular tissues with severe inflammatory
response (4,5). Once corneal tissues are damaged by
severe chemical burn injuries, corneal neovascularization occurs
frequently. The mechanism of corneal neovascularization is thought
to involve an imbalance of proangiogenic and anti-angiogenic
factors (6,7). Even though corneal neovascularization
participates in the pathology of corneal alkali burns, the
molecular mechanism of these irreversible injuries is unknown.
MicroRNAs (miRNAs) are highly conserved small
noncoding RNAs (consisting of 19 to 25 nucleotides) that can
regulate gene transcription in certain kinds of animal and human
tissues (8,9). Studies have illustrated that numerous
of miRNAs are expressed in mammalian eyes and can display
distinctly different expression patterns and molecular functions
(10,11). Among them, miR-296 was shown to be
associated with angiogenic endothelial cells (12) and prostate cancer cells (13), and with upregulated VEGF and
downregulated Notch1 following cerebral ischemic injury (14). However, little is known about miR-296
involvement in the pathophysiology of chemical burns of the
cornea.
In the current study, we investigated whether
miR-296 participates in the pathobiology of alkali burns in cornea.
The results of our study can further our understanding of the
underlying molecular mechanisms of this type of ocular pathology,
and may help provide novel clinical treatments for patients with
corneal alkali burns.
Materials and methods
Animals
Care, use, and treatment of all of the animals
utilized in this study were in strict compliance with the Statement
for the Use of Animals in Ophthalmic and Vision Research. Male
Balb/c mice, 6 to 8 weeks old, were purchased from the Animal
Sciences Department of Nanchang University. The mice were
maintained on a 12-h light/dark cycle for at least 2 weeks prior to
the onset of experiments. All experimental procedures were reviewed
and approved by the Ethics Committee for Animal Research at the
Second Hospital Affiliated with Nanchang University, Nanchang,
China.
Experimental animal model of corneal
burns after exposure to alkali
The mice were studied while under general anesthesia
induced by intraperitoneal injection of 0.3 ml/kg chloral hydrate,
followed by topical anesthesia of the eye by drops of 0.5%
proparacaine hydrochloride (Alcon, Inc., Fort Worth, TX, USA). A
sheet of filter paper (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was cut to size in order to provide disk-shaped pieces
with diameters of 2 mm. These filter paper disks were presoaked in
1 N NaOH, and then applied to the center of the anesthetized cornea
of the right eye of each mouse for 10 sec, followed by immediate
eye irrigation with balanced salt solution (Alcon, Inc.), as
previously described (15), and the
animals were then randomly allocated to 1 of 3 different treatment
groups: Levofloxacin eye drops (KB2), levofloxacin and FK506 eye
drops (SY1), or levofloxacin and tobramycin dexamethasone eye drops
(SY2).
Total RNA sample preparation and
quantitative polymerase chain reaction (qPCR)
To quantify miR-296 mRNA expression levels, frozen
mice corneal tissues were dissolved in Trizol (Takara Biotechnology
Co., Ltd., Dalian, China) and treated 3 times with liquid nitrogen
for 5 min and then a 37°C water bath for 5 min. Total RNA was
extracted from the tissues according to the manufacturer's
guidelines. Purified total RNA (1 µg) was then reverse transcribed
into cDNA using a RevertAid™ First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc., Pittsburgh, PA,
USA). Specific primers (Table I)
were synthesized by Invitrogen; Thermo Fisher Scientific, Inc.
Real-time PCR was performed using the TransStart Tip Green qPCR
SuperMix system (TransGen Biotech, Beijing, China) using the
following conditions: Incubation at 95°C for 10 min, followed by 40
cycles of 15 sec at 95°C and 1 min at 60°C. All samples were tested
in triplicate. PCR reactions and the mean of the reactions were
used for calculating expression levels. All data were collected
from the linear range of amplification. Expression levels were
normalized for the average of U6 mRNA levels from the same
samples.
 | Table I.Primers list for mouse miR-296 gene
qPCR analysis. |
Table I.
Primers list for mouse miR-296 gene
qPCR analysis.
| Genes | Forward primers | Reverse primers |
|---|
| U6 |
5′-CTCGCTTCGGCAGCACATATACT-3′ |
5′-ACGCTTCACGAATTTGCGTGTC-3′ |
| miR-296 |
5′-ACACTCCAGCTGGGAGGGCCCCCCCTCAA-3′ |
5′-TGGTGTCGTGGAGTCG-3′ |
KEGG pathway enrichment analysis of
differentially expressed proteins
Kyoto Encyclopedia of Genes and Genomes (KEGG) is a
database for systematic analysis of gene function, linking genome,
and higher order information. This resource facilitates the study
of gene and expression data as a whole network. Our study was
performed using the KEGG database (http://www.genome.jp/kegg/) (16,17) and
Fisher's exact test. We selected the standard as the difference in
the number of genes on a term/pathway ≥5, P<0.05. The
term/pathway was drawn based on the enrichment factor value from
the descending order of size, taking into account the first 12
results.
Western blot analysis
To quantify FGF23 protein expression levels, frozen
mouse corneal tissues were dissolved in tissue RIPA lysis buffer
containing 50 mM Tris-HCl (pH 7.4); Igepal 1% (w/v); 0.25% (w/v)
Na-deoxy cholate; 1 mM EDTA, 150 mM NaCl; 1 µg/ml each of protease
inhibitors aprotinin, leupeptin and pepstatin; 1 mM
Na3VO4; and 1 mM NaF. Following ultrasonic
dispersion, the samples were centrifuged at 12,000 × g for 10 min
at 4°C. The supernatant was then collected, and total protein
concentration was determined using the Pierce BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). Protein samples of tissues were
loaded with an equal volume of 2X SDS-PAGE loading buffer (100 mM
Tris-HCl, pH 6.8; 4% SDS; 20% glycerol; 0.2% bromphenol blue and
0.14% β-mercaptoethanol) and boiled (for 5 min at 100°C), and the
protein samples were stored at −20°C until use. For immunoblotting,
20 µg of the total protein samples were resolved on 12% SDS-PAGE,
at a constant voltage of 100 V for 2 h. The proteins resolved in
the gel were then transferred to nitrocellulose membranes (EMD
Millipore, Billerica, MA, USA) using Tris/glycine buffer, pH 8.3
(25 mM Tris base, 192 mM glycine, 0.1% w/v SDS, 20% v/v methanol)
at 250 to 300 mA for 3 h. The membranes were then blocked for 1 h
in non-fat powdered milk (5%) in TBST washing buffer (10 mM Tris,
150 mM NaCl, 0.1% Tween-20), and incubated with the primary
antibody of interest overnight at 4°C. Rabbit polyclonal anti-FGF23
(1:200; Wuhan Boster Biological Technology, Ltd., Wuhan, China)
were used, and the membranes were then washed in TBS-Tween-20 three
times over 15 min and incubated with HRP-conjugated goat
anti-rabbit IgG (1:2,000; ZSGB-BIO; OriGene Technologies, Inc.,
Beijing, China) for 1 h at ambient temperature and washed in TBST
washing buffer as above. Immunoreactivity was visualized using film
exposure. To allow normalization for minor variances in protein
loading between sample lanes, the blots were re-probed for
anti-β-actin (1:1,000; ZSGB-BIO; OriGene Technologies, Inc.).
ImageJ software (National Institutes of Health, Bethesda, MD, USA)
was used to analyze immunoblotting images. Optical densities of all
bands were measured with the image background subtracted to give
the final densitometry values.
Statistical analysis
The data are presented as mean values ± standard
errors. The Kolmogorov-Smirnov test was used to check the normal
distribution of the datasets. The one-way analysis of variance
(ANOVA) for measurements was performed using GraphPad Prism version
5.0 (GraphPad Software, Inc., La Jolla, CA, USA), with Turkey's
post hoc test for multiple comparisons where appropriate. P<0.05
was considered to indicate a statistically significant difference.
All experiments were repeated at least three times.
Results
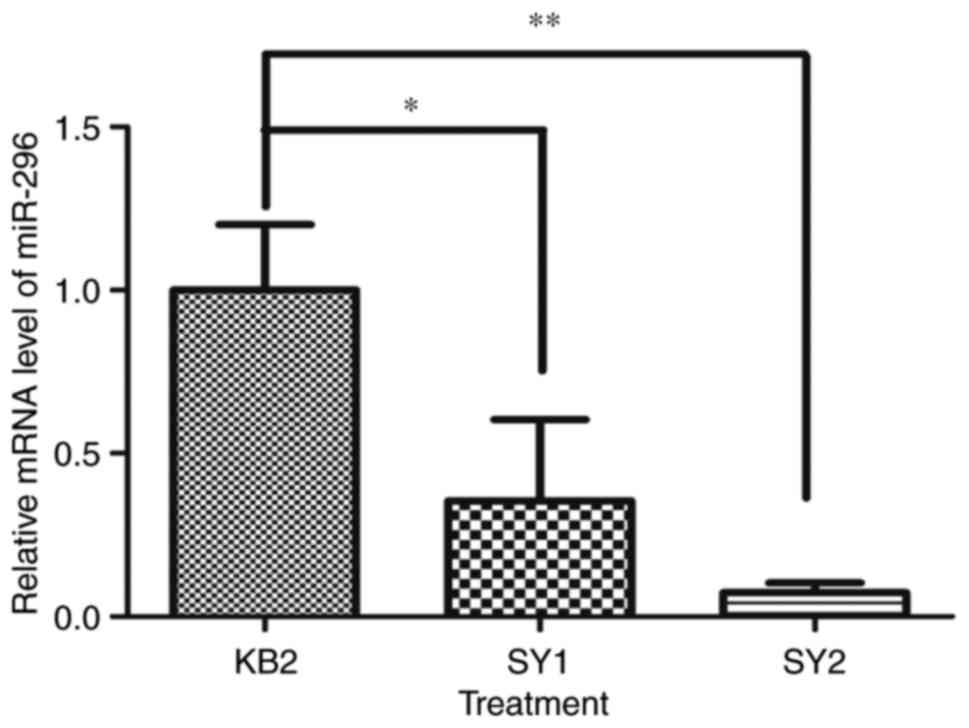
In this study of corneal chemical burn wounds, we
found that the expression levels of miR-296 mRNA varied
significantly in the different treatment groups of our mouse eye
model. The results revealed that the data were normally distributed
(P>0.05). There was a statistically significant difference
between miR-296 mRNA with KB2, SY1 and SY2, as determined by ANOVA
(P<0.01). Turkey's post-hoc test revealed the level of miR-296
mRNA expressed was significantly decreased in SY1 and SY2 compared
with KB2 (Fig. 1, P<0.05,
P<0.01). No significant difference was seen between SY1 and SY2.
This result suggested that the miR-296 gene may be involved in the
regulation of the pathophysiology of the alkali-burned cornea, and
levofloxacin and tobramycin dexamethasone eye drops (SY2) or
levofloxacin eye drops and FK506 eye drops (SY1) were both better
than the single-ingredient levofloxacin eye drops for treatment of
the alkali-burned cornea. Because we found that miR-296 was
actively involved in the reaction to alkali burns, we wondered
about the mechanism by which it affects the chemical injury in
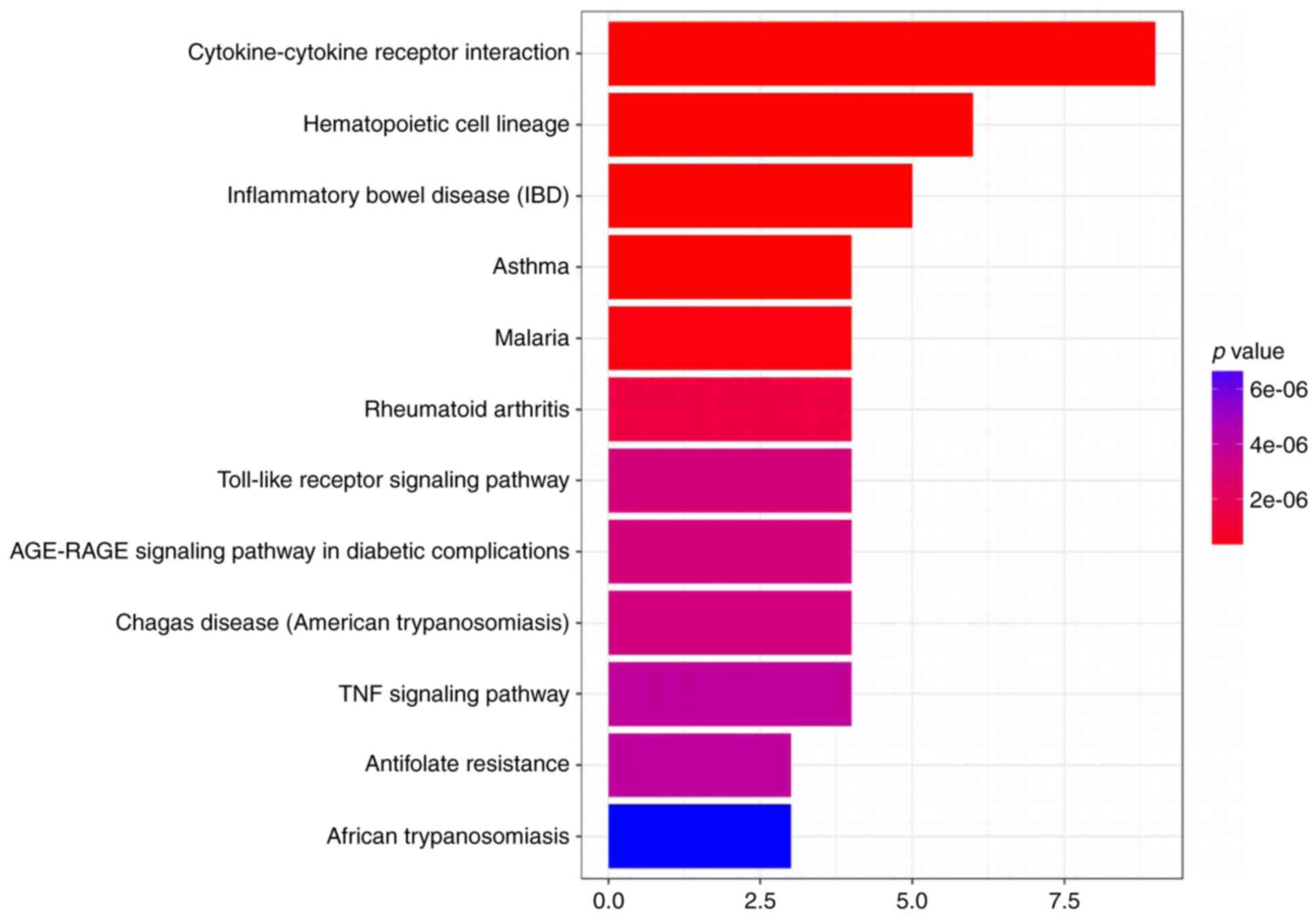
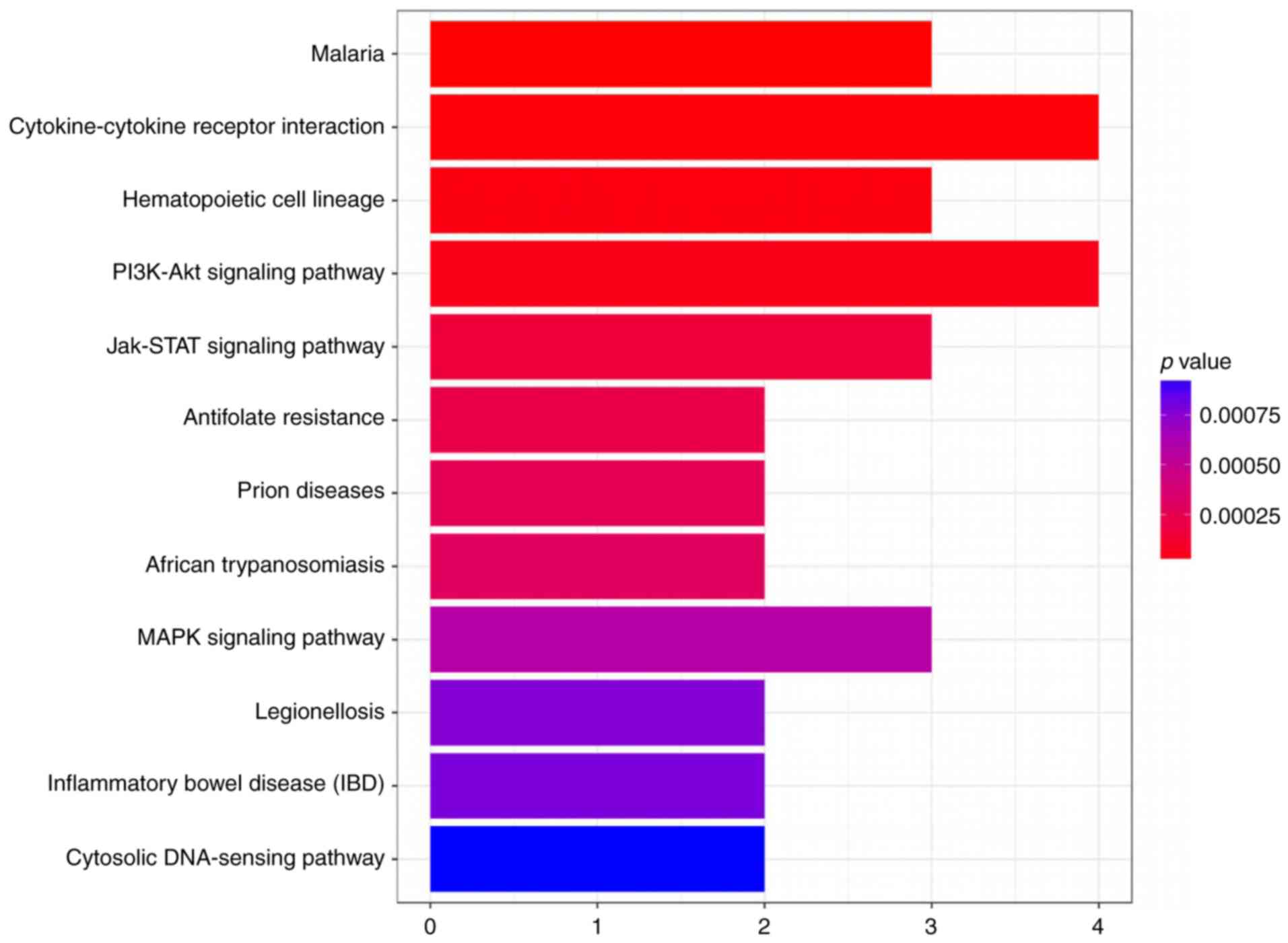
mouse cornea. Therefore, we evaluated the likely pathways that it
might affect utilizing a KEGG pathway analysis (Figs. 2 and 3).
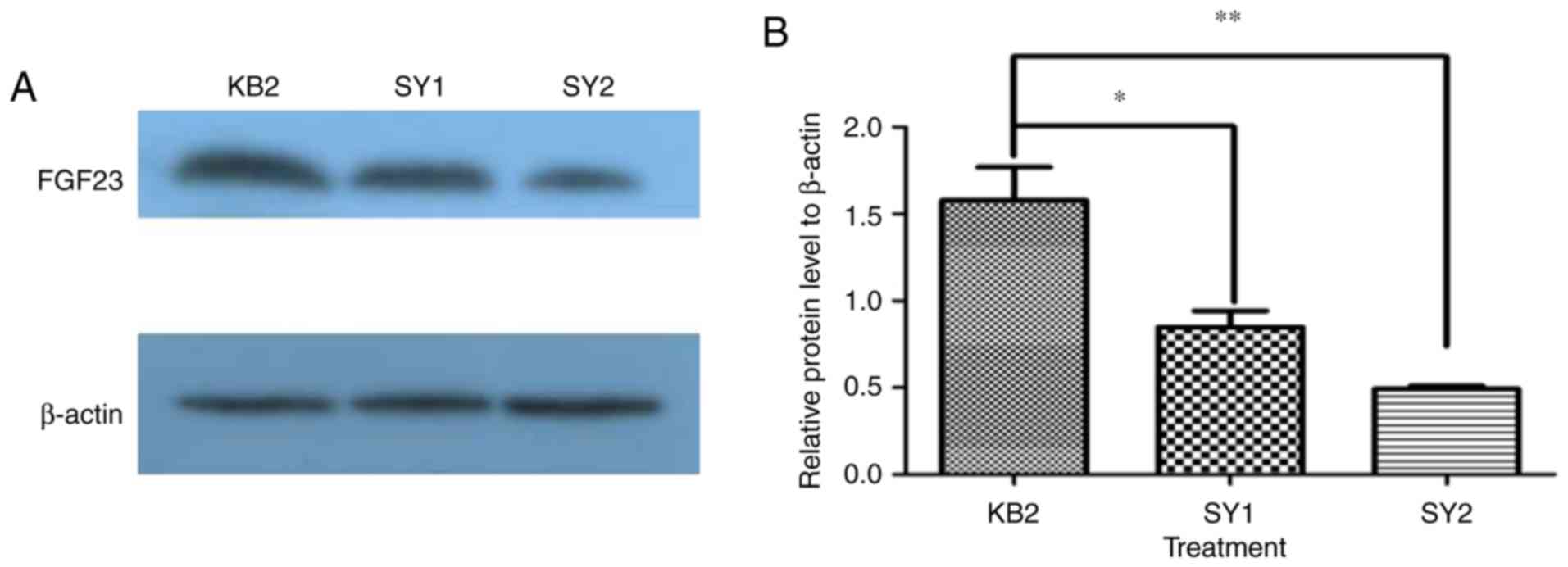
We found that FGF23 protein can be expressed
differently after different treatments of the corneal alkali burn
model. The results revealed that the data were normally distributed
(P>0.05). There was a statistically significant difference
between FGF23 protein with KB2, SY1 and SY2, as determined by ANOVA
(P<0.01). Turkey's post-hoc test indicated the level of FGF23
protein, in comparison with KB2, was significantly decreased in SY1
and SY2 (Fig. 4, P<0.05,
P<0.01). Furthermore, we found that SY2 exhibited lower FGF23
protein expression than SY1, but not significant (Fig. 4, P>0.05). This result demonstrated
that FGF23 factors may be involved in the regulation of the burned
cornea, and levofloxacin and tobramycin dexamethasone eye drops
(SY2) were better than levofloxacin eye drops and FK506 eye drops
(SY1) or levofloxacin eye drops (KB2) to modulate the damage in
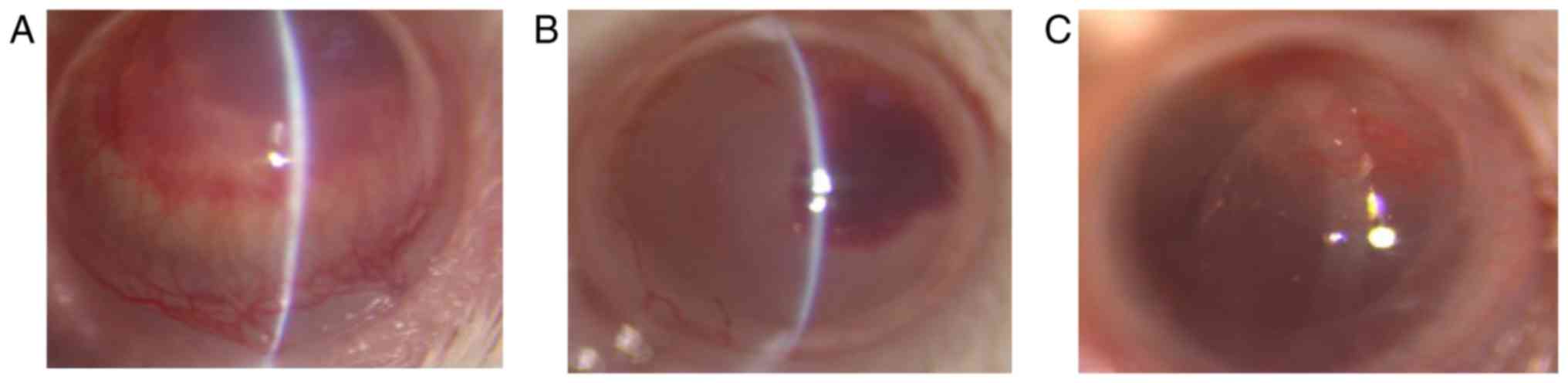
alkali burned cornea. Corneal neovascularization was
treatment-dependent with the different eye drops on day 7 after
corneal alkali burns. Although all mice developed corneal
neovascularization after corneal alkali burn, the SY2 exhibited
lower corneal neovascularization area compared to SY1 or KB2
(Fig. 5).
Discussion
Based on the present study, we report for the first
time that the miR-296 gene is expressed during the transient
inflammatory responses that occur in mouse cornea after alkali
burns. More importantly, we found that levofloxacin and tobramycin
dexamethasone eye drops (SY2) or levofloxacin eye drops and FK506
eye drops (SY1) were better than levofloxacin eye drops alone to
modulate miR-296 gene activity after alkali burns in the mouse
eye.
The advantages of employing knowledge base-driven
pathway analysis are becoming more and more appreciated by many
investigators in medical research, because it can reduce the
complexity of functional analysis by grouping thousands of genes
into just several hundred pathways. Such analysis can also increase
the explanatory power of experimental results by identifying active
pathways in different conditions (18). KEGG is an integrated database that is
helpful for biological interpretation of genome sequences and other
high-throughput data (19,20). In this study, we identified for the
first time a candidate for the most important pathway involved in
the miR-296 gene-related inflammatory responses in the mouse cornea
after alkali burns in the light of KEGG pathway. In addition, we
concluded that the most likely overlap pathway was a
cytokine-cytokine receptor interaction pathway. Then, we examined
the FGF23 protein that participates in the mouse cornea alkali burn
and found that SY2 was better than the other formulations (SY1 or
KB2) to ameliorate the effects of alkali on the mouse cornea.
Corneal neovascularization (CNV) is playing a critical aspect in
the pathophysiology of corneal alkali burns. Multistage and
distinct molecules contribute to CNV. In corneal diseases, a number
of cytokines and growth factors are upregulated and induce
infiltration of neutrophils, macrophages, and lymphocytes (21). CNV is also directly related to
Macrophages infiltration (22). The
current treatments for CNV include corticosteroids and anti-VEGF
(22). Dexamethasone, a synthetic
corticosteroid, is widely used as a potent anti-inflammatory drug
in various diseases including corneal angiogenesis (23). Dexamethasone can not only inhibit
IL-1β-induced angiogenesis via blocking NF-κB signaling, but also
partially inhibit VEGF-A (24).
Dexamethasone has a large therapeutic window. FK-506 (Tacrolimus),
an immunosuppressive neutral macrolide, is isolated from
streptomyces tsukubaensis (25). A
study pointed out FK-506 can suppress VEGF in a rabbit CNV model
(22). At present, we firstly
compared SY2 with SY1, and we concluded that SY2 is SY2 was better
to ameliorate the effects of alkali on the mouse cornea. We also
found that the IL6 protein was not expressed in this experimental
model. Corneal neovascularization was decreased, but we did not
quantify it on day 7 after the alkali burn. Thus, in subsequent
research, we aim to investigate the mechanism of miR-296-mediated
corneal neovascularization in the mouse model of alkali burns.
Taken together, we investigated the miR-296 gene and
a possible pathway involved in the transient inflammatory responses
of mouse cornea following experimental trauma from alkali exposure.
The insights from this study may lead to new therapeutic strategies
for treatment corneal alkali burns in the clinical setting.
Acknowledgements
This study was supported by Natural Science
Foundation of Jiangxi (grant no. 20161BAB205270).
References
|
1
|
Xie Y, Tan Y and Tang S: Epidemiology of
377 patients with chemical burns in Guangdong province. Burns.
30:569–572. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang C and Xu J: Indications for
penetrating keratoplasty in East China, 1994–2003. Graefes Arch
Clin Exp Ophthalmol. 243:1005–1009. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong J, Qiu T, Wei A, Sun X and Xu J:
Clinical characteristics and visual outcome of severe ocular
chemical injuries in Shanghai. Ophthalmology. 117:2268–2272. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saika S, Kobata S, Hashizume N, Okada Y
and Yamanaka O: Epithelial basement membrane in alkali-burned
corneas in rats. Immunohistochemical study. Cornea. 12:383–390.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishizaki M, Zhu G, Haseba T, Shafer SS and
Kao WW: Expression of collagen I, smooth muscle alpha-actin, and
vimentin during the healing of alkali-burned and lacerated corneas.
Invest Ophthalmol Vis Sci. 34:3320–3328. 1993.PubMed/NCBI
|
|
6
|
Pandya NM, Dhalla NS and Santani DD:
Angiogenesis-a new target for future therapy. Vascul Pharmacol.
44:265–274. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Groot H, Schmit-Eilenberger V, Kirchhof
J and Augustin AJ: Angiostatic and angiogenic factors. Dev
Ophthalmol. 46:1–3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ranganathan K and Sivasankar V:
MicroRNAs-Biology and clinical applications. J Oral Maxillofac
Pathol. 18:229–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryan DG, Oliveira-Fernandes M and Lavker
RM: MicroRNAs of the mammalian eye display distinct and overlapping
tissue specificity. Mol Vis. 12:1175–1184. 2006.PubMed/NCBI
|
|
11
|
Karali M, Peluso I, Gennarino VA, Bilio M,
Verde R, Lago G, Dollé P and Banfi S: miRNeye: A microRNA
expression atlas of the mouse eye. BMC Genomics. 11:7152010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Würdinger T, Tannous BA, Saydam O, Skog J,
Grau S, Soutschek J, Weissleder R, Breakefield XO and Krichevsky
AM: miR-296 regulates growth factor receptor overexpression in
angiogenic endothelial cells. Cancer Cell. 14:382–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei JJ, Wu X, Peng Y, Shi G, Basturk O,
Yang X, Daniels G, Osman I, Ouyang J, Hernando E, et al: Regulation
of HMGA1 expression by microRNA-296 affects prostate cancer growth
and invasion. Clin Cancer Res. 17:1297–1305. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Huang T, Huang Q, Chen H, Li Y, He
W, Wang GB, Zhang L, Xia J, Zhang N and Liu Y: Pro-angiogenic
microRNA-296 upregulates vascular endothelial growth factor and
downregulates Notch1 following cerebral ischemic injury. Mol Med
Rep. 12:8141–8147. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bian F, Pelegrino FS, Henriksson JT,
Pflugfelder SC, Volpe EA, Li DQ and de Paiva CS: Differential
effects of dexamethasone and doxycycline on inflammation and MMP
production in alkali-burned corneas associated with dry eye. Ocul
Surf. 14:242–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Draghici S, Khatri P, Tarca AL, Amin K,
Done A, Voichita C, Georgescu C and Romero R: A systems biology
approach for pathway level analysis. Genome Res. 17:1537–1545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kanehisa M, Goto S, Kawashima S, Okuno Y
and Hattori M: The KEGG resource for deciphering the genome.
Nucleic Acids Res. 32(Database issue): D277–D280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du J, Li M, Yuan Z, Guo M, Song J, Xie X
and Chen Y: A decision analysis model for KEGG pathway analysis.
BMC Bioinformatics. 17:4072016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanehisa M, Sato Y, Kawashima M, Furumichi
M and Tanabe M: KEGG as a reference resource for gene and protein
annotation. Nucleic Acids Res. 44:D457–D462. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Khatri P, Sirota M and Butte AJ: Ten years
of pathway analysis: Current approaches and outstanding challenges.
PLoS Comput Biol. 8:e10023752012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wilson SE, Mohan RR, Mohan RR, Ambrósio R
Jr, Hong J and Lee J: The corneal wound healing response:
Cytokine-mediated interaction of the epithelium, stroma, and
inflammatory cells. Prog Retin Eye Res. 20:625–637. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JH, Joo CK and Chung SK: Comparative
study of tacrolimus and bevacizumab on corneal neovascularization
in rabbits. Cornea. 34:449–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakao S, Hata Y, Miura M, Noda K, Kimura
YN, Kawahara S, Kita T, Hisatomi T, Nakazawa T, Jin Y, et al:
Dexamethasone inhibits interleukin-1beta-induced corneal
neovascularization: Role of nuclear factor-kappaB-activated stromal
cells in inflammatory angiogenesis. Am J Pathol. 171:1058–1065.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakao S, Zandi S, Lara-Castillo N, Taher
M, Ishibashi T and Hafezi-Moghadam A: Larger therapeutic window for
steroid versus VEGF-A inhibitor in inflammatory angiogenesis:
Surprisingly similar impact on leukocyte infiltration. Invest
Ophthalmol Vis Sci. 53:3296–3302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan J, Zhai JJ, Chen JQ, Ye CT and Zhou
SY: Preparation of 0.05% FK506 suspension eyedrops and its
pharmacokinetics after topical ocular administration. J Ocul
Pharmacol Ther. 25:345–350. 2009. View Article : Google Scholar : PubMed/NCBI
|