Introduction
Subarachnoid hemorrhage (SAH) is a common disease in
neurosurgery, accounting for 10% of acute strokes and 20% of
hemorrhagic strokes around the world (1,2).
Ischemic brain damage secondary to cerebral vasospasm (CVS) is a
common and dangerous complication of SAH and is one of the most
common reasons for morbidity and mortality after SAH (1–3). The
prevention and treatment of cerebral vasospasm primarily relies on
nimodipine administration and optimization of blood volume and
cardiac performance, pharmacologically induced hypertension
combined with volume is the established first-line therapy for
delayed cerebral vasospasm (4).
However, occasionally medically refractory delayed cerebral
vasospasm may occur, defined as failure to respond adequately to
these measures (5). Although there
are multiple domestic and international studies focusing on the
mechanism underlying the development of CVS after SAH, its
pathophysiological basis has not been fully elucidated. Studies
have demonstrated that a large amount of calcitonin gene-related
peptide (CGRP) is released from nerve endings into human blood to
oppose vasospasm at the early stage of CVS (6–8). With
the progression of time, CGRP at nerve endings is depleted, and CVS
develops progressively. It is speculated that the depletion of
endogenous CGRP may be a key reason for CVS development after SAH.
As a neuropeptide containing 37 amino acids, CGRP has strong
vasodilation and neuronal protection functions (6,9). Animal
experiments and early clinical trials have shown that
intra-cisternal injection or intravenous injection of exogenous
CGRP is able to significantly relieve vasospasm and improve nerve
function (10–14). However, due to its protein features,
CGRP has a very short plasma half-life of only 7–10 min, which
restricts its clinical application (15).
In the present study, genetic engineering technology
was used to clone the CPGR gene into the adeno-associated virus
(AAV) to construct a eukaryotic expressing vector containing the
CGRP gene (rAAV-CGRP). Through the allantoic cavity injection
approach, the feasibility of using rAAV-CGRP to improve umbilical
artery vasospasm in the chick embryo allantoic cavity was
demonstrated.
Materials and methods
Experimental grouping
A total of 80 specific pathogen-free fresh
fertilized chicken eggs (Merial Vital Laboratory Animal Technology,
Beijing, China) were selected. The mean weight was 60±2.9 g. Eggs
were randomly divided into the rAAV group, the empty vector AAV
group, and the control group, with 24 eggs in each group. Other
eggs were used as a reserve supply. Each group was further randomly
divided into three subgroups: 3-, 5- and 7-day groups, with 8 eggs
in each subgroup. Fertilized chicken eggs were hatched in an
automatic incubator. Hatching parameters were as follows:
Temperature, 37.8°C; humidity, 60%; and rotation of eggs, once
every 90 min. After five days of hatching, the chick embryos were
observed under an egg-candling lamp in a dark room. A total of
three chick embryos failed to hatch were excluded and replaced with
fertilized eggs from the reserve supply. All animal experimental
operations were approved by the Ethics Committee of Jilin
University (Changchun, China).
Establishment of an umbilical artery
vasospasm model in the chick embryo allantoic cavity
A previous study by our group confirmed that
allantoic cavity hemorrhage caused by puncture of chorioallantoic
membrane (CAM) blood vessels can induce umbilical artery vasospasm
that presents certain features of the pathological changes of CVS
after SAH in mammals (16). For
model establishment, fertilized chicken eggs at 11 days of age were
selected. Chick embryos were observed under an egg-candling lamp in
a dark room through the blunt end of the air chamber. CAM blood
vessels in chick embryos were clearly observed. At the top of the
chicken embryo, a large vein departed the CAM, after multiple
vascular branches congregated, and extended toward the deep
allantoic cavity to connect to the chicken embryo, which is the
umbilical vein. A relatively large CAM vessel was selected for
needle puncturing. The surface of the fertilized eggs was wiped
with alcohol-moistened cotton balls. The eggshell was initially
drilled using a 16-G syringe needle; the CAM and blood vessels were
not damaged. The CAM blood vessel was subsequently punctured using
a 26-G syringe needle. Obvious bleeding was observed under an
egg-candling lamp. The fertilized egg was gently rotated to evenly
disperse the blood in the allantoic cavity, which made the
allantoic fluid turbid. In the control group, the eggshell was only
drilled using a 16-G syringe needle, and the CAM vein was not
punctured. The eggs were returned to the incubator for hatching
until being sacrificed (see Sample collection and fixation of
umbilical arteries in the allantic cavity) at 3, 5 and 7 days,
respectively. Each egg was exposed to an egg-candling lamp every
day to remove chick embryos that did not survive.
Construction and vaccination of
rAAV
The construction of the chick rAAV-CGRP virus is
described in previous literature. Briefly, the forward and reverse
primers of chick CGRP were designed and synthesized by Sangon
Biotech, Co., Ltd. (Shanghai, China; forward,
5′-CCGGAATTCATGGTCATGCTGAAGATTTCATC-3′ and reverse,
5′-CAAGCTTCTAGTTGTTTCCTAGGGTTTCCCCA-3′). Using polymerase chain
reaction with Takara Ex Taq DNA polymerase (Takara Bio, Inc., Otsu,
Japan) with a 7500 Fast thermocycler (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), the fragment encoding
CGRP was produced, including the EcoRI and HindIII
restriction enzyme sites. The PCR procedures as follow:
Denaturation at 94°C for 60 sec, annealing at 37°C for 60 sec,
extension at 72°C for 80 sec for 30 cycles, followed by 5 min at
72°C for the final extension. Next, the synthesized fragments were
added to the pGEM-T-Easy vectors (Promega Corporation, Madison, WI,
USA), and the connection products were transformed into
Escherichia coli TOP10 bacterial strains. The positive clone
was identified using EcoRI and HindIII restriction
enzymes (Takara Bio, Inc., Otsu, Japan), and the cloned amplified
fragments were sequenced by the dideoxy-mediated chain-termination
method. Cloned CGRP cDNAs were compared with the GenBank sequence
using DNASIS software (Version 2.7; MiraiBio Group, San Francisco,
CA, USA). Subsequently, pGEM-T-CGRP and shuttle vector pSSCMV
(Huaguang, China) were digested by EcoRI and HindIII,
respectively. Once treated with T4 DNA ligase, CGRP and linearized
pSS-CMV were recovered on a low melting point gel, and the novel
products were transformed into E. coli DH5α-competent cells.
Eventually, the recombinant vectors were collected and digested by
EcoRI and HindIII, and the positive clones were
subsequently identified by advanced glycation end product. To
acquire the rAAV, HEK293 cells were co-transfected with pSSCMV-CGRP
and aid vector (Helper plasmid, to provide protein necessary for
AVV replication and packaging) pAAV-Ad by calciumphosphate
co-precipitation. After three days, the rAAV was collected from the
supernatant by repeatedly freezing/thawing the culture medium
containing the virus embolus. HEK293 cells were treated by multiple
dilutions of rAAV, and the virus titer was estimated by dot-blot
hybridization (17). The virus titer
of the recombinant group was 1.15×1012 pfu/ml. The titer
of the empty vector virus in the empty vector AAV group was
1.13×1012 pfu/ml. Syringes were used to inject 1 ml of
recombinant virus solution or empty vector virus solution into the
chick embryo allantoic cavity after 24 h of model establishment.
The pSSCMV viral vector, adenovirus vector pAAV/Ad, E. coli
TOP10, E. coli DH5α and HEK293 cell lines were provided by
Xi'an Huaguang Biological Engineering Co., Ltd., (Xi'an,
China).
Detection of CGRP concentrations in
the allantoic fluid using ELISA
On days 3, 5 and 7 after model establishment,
fertilized eggs were broken from the air chamber end with tweezers
to expose the inner shell membrane and the tightly attached CAM
below. Cotton swabs dipped in PBS were used to gently wipe the
inner shell membrane. The CAM blood vessels inside could be clearly
seen. Caution was taken to avoid the large vessels as the inner
eggshell membrane and the attached CAM was gently torn up with a
16-G syringe needle to enter the allantoic cavity. Allantoic fluid
was aspirated using a syringe, and the CGRP levels in the allantoic
fluid were detected using a chicken CGRP ELISA kit (MBS2513672;
MyBioSource, San Diego, CA, USA). Procedures were performed
according to the instruction manual provided with the reagent kit.
Standard concentrations were assigned to the horizontal axis, and
optical density (OD) values were assigned to the vertical axis. The
standard curve was plotted on coordinate paper, and the linear
regression equation of the standard curve was calculated. The OD
values of the samples were introduced into the equation to
calculate the sample concentrations. The results multiplied by the
dilution fold were the actual sample concentrations.
Sample collection and fixation of
umbilical arteries in the allantoic cavity
On days 3, 5 and 7 after model establishment, the
fertilized eggs were carefully opened from the air chamber end
using forceps to expose the shell membrane and the tightly attached
CAM below. Cotton swabs dipped in PBS were used to gently wipe the
inner shell membrane. Inside, the CAM blood vessels could be
clearly observed. Care was taken to avoid the large vessels as the
inner eggshell membrane and the attached CAM was gently torn up
with a 16-G syringe needle to enter the allantoic cavity. Allantoic
fluid was aspirated as much as possible. A syringe was used to
inject 4% paraformaldehyde (in 0.1 mol/l PBS; pH 7.4), precooled at
4°C, into the allantoic cavity for fixation at 4°C for 2 h. The
eggshell was opened and the umbilical arteries in the allantoic
cavity that were connected to the ventral side of the chick embryo
could be observed. Subsequently, ~1 cm of the proximal end of the
right branch of the umbilical artery after bifurcation was
collected and placed in 4% paraformaldehyde for fixation and
storage.
Measurement of the cross-sectional
area (CSA) of the inner diameter and vessel wall thickness of the
umbilical arteries
The paraformaldehyde-fixed umbilical artery was
evenly divided into three segments (proximal, distal and middle
segments), dehydrated, cleared and embedded. Cross sections of the
blood vessels were prepared at a 5-µm thickness; sections were
stained with hematoxylin and eosin, observed, and images were
captured under a microscope at ×200 magnification (Olympus Corp.,
Tokyo, Japan). The CSA of the inner and outer diameters of the
blood vessels was measured separately by two individuals using
ImageJ software (Version 1.48; National Institutes of Health,
Bethesda, MA, USA). The mean value of the CSA of three segments was
obtained, and the mean value of the measurement results from the
two individuals was subsequently calculated. Vessel wall thickness
was calculated using a geometric formula according to the CSA of
the inner and outer diameters.
Statistical analysis
SPSS 21.0 statistical software (IBM SPSS, Armonk,
NY, USA) was used for statistical analysis. Measurement data are
presented as the mean ± standard deviation. Comparison of
measurement data between two groups was performed using the
Student's t-test. Countable data were compared using the
Chi-square test. P<0.05 was considered to indicate a
statistically significant difference.
Results
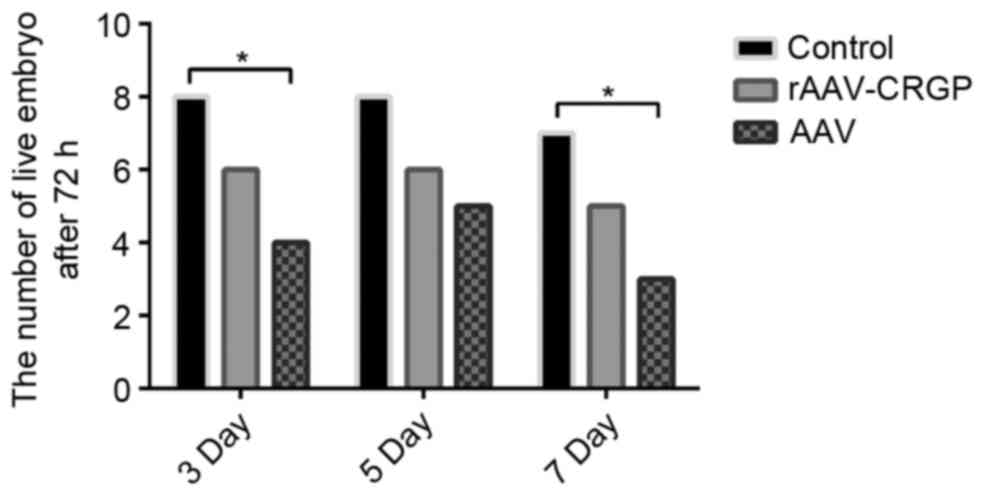
Mortality rate of the umbilical artery
vasospasm model in the chick embryo allantoic cavity after 72
h
At 72 h after model establishment, only one chick
embryo in the 7-day subgroup of the control group did not survive.
Following rAAV-CGRP injection, the mortality rates in the 3-, 5-
and 7-day subgroups were 25% (2/8), 25% (2/8) and 37.5% (3/8),
respectively, which were lower than the values of 50% (4/8), 37.5%
(3/8) and 62.5% (5/8) in the 3-, 5- and 7-day subgroups after AAV
injection. There was no statistical significance between these two
groups (P>0.05). Notably, the mortality rates in the 3- and
7-day subgroups of the chick embryo hemorrhage model after AAV
injection were significantly higher than those in the control group
(P<0.05; Fig. 1).
Improvement of vasospasm after
rAAV-CGRP transfection
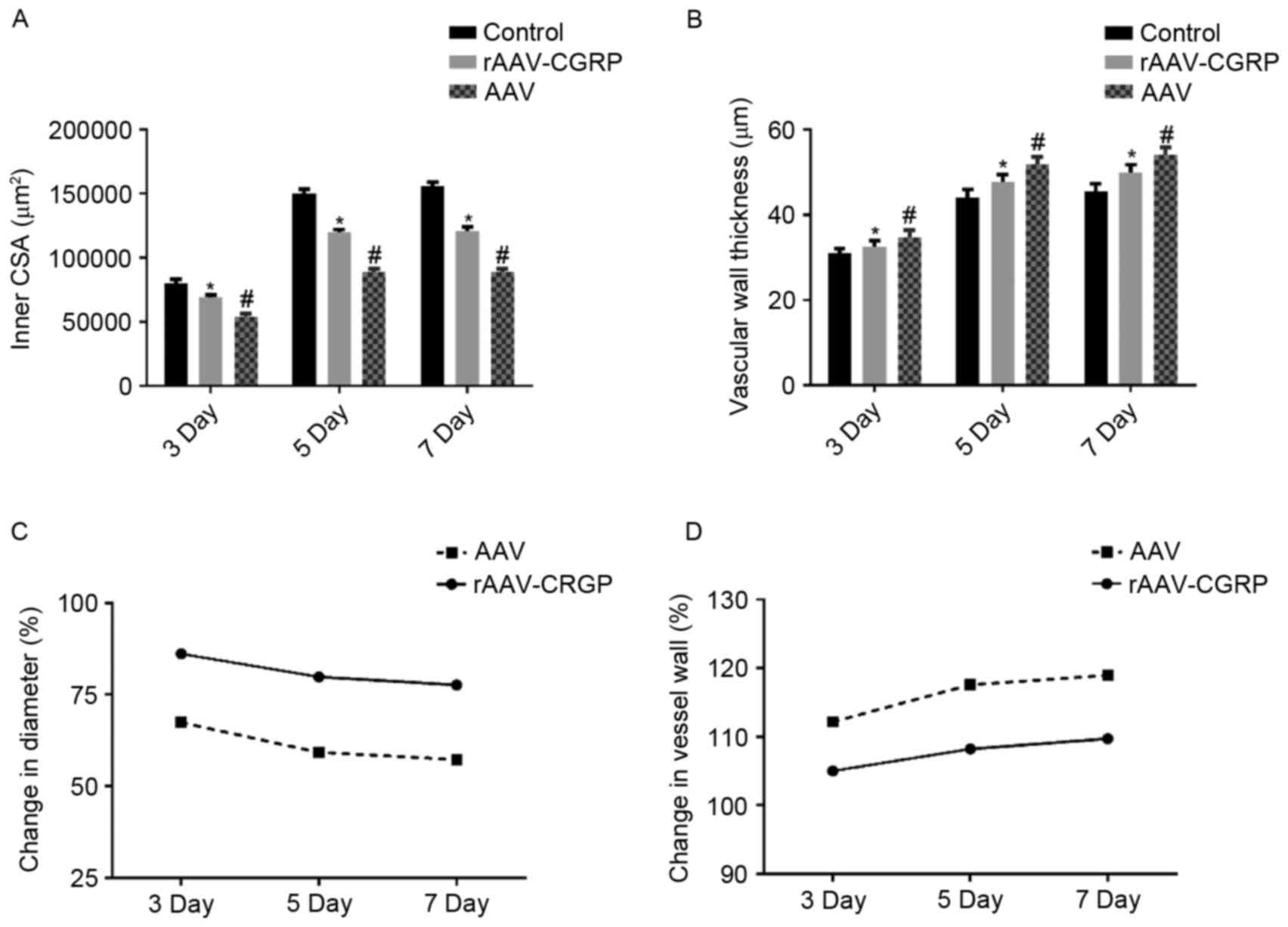
Inner CSA and vessel wall thickness of the umbilical
artery in each group are presented in Tables I and II respectively. Typical sections of each
group are presented in Fig. 2.
Compared with the control group, the inner CSA in the AAV group
markedly decreased, and the vessel wall became thicker and
exhibited wave-form changes. Compared with the AAV group, the inner
CSA in the rAAV-CGRP group increased, and the vessel wall
thickening displayed a certain degree of improvement; however,
compared with the control group, the inner CSA of the rAAV-CGRP
group decreased and the vessel wall became thicker. The inner CSA
and vessel wall thickness of the umbilical artery in the chick
embryo allantoic cavity of each group are illustrated in Fig. 3A and B. These results revealed that
the AAV model group exhibited severe vasospasm of the umbilical
artery in the chick embryo allantoic cavity. In addition, the inner
CSA values of the umbilical artery were only 67.33, 59.19 and
57.22%, respectively, of the CSA of those in the age-matched
control group (Fig. 3C), and the
umbilical artery vessel wall thickness increased by 12.17, 17.59
and 18.95%, respectively, of those in the age-matched control group
(Fig. 3D). Compared with the AAV
group, the degrees of vasospasm in the 3-, 5- and 7-day subgroups
of the rAAV-CGRP group after model establishment significantly
improved (P<0.05); the CSA values of the umbilical arteries were
86.11, 79.75 and 77.60%, respectively, of the CSA of those in the
age-matched control group (Fig. 3C).
The vessel wall thickness values of the umbilical arteries
increased by 5.00, 8.21 and 9.73% in the 3-, 5- and 7-day subgroups
of the rAAV-CGRP group, respectively, of those in the age-matched
control group (Fig. 3D). However,
compared with the control group, rAAV-CGRP did not completely
reverse the vasospasm induced by allantoic cavity hemorrhage caused
by puncture. These findings indicated that the CSA and vessel wall
thickness values of the age-matched umbilical arteries among these
three groups displayed significant differences.
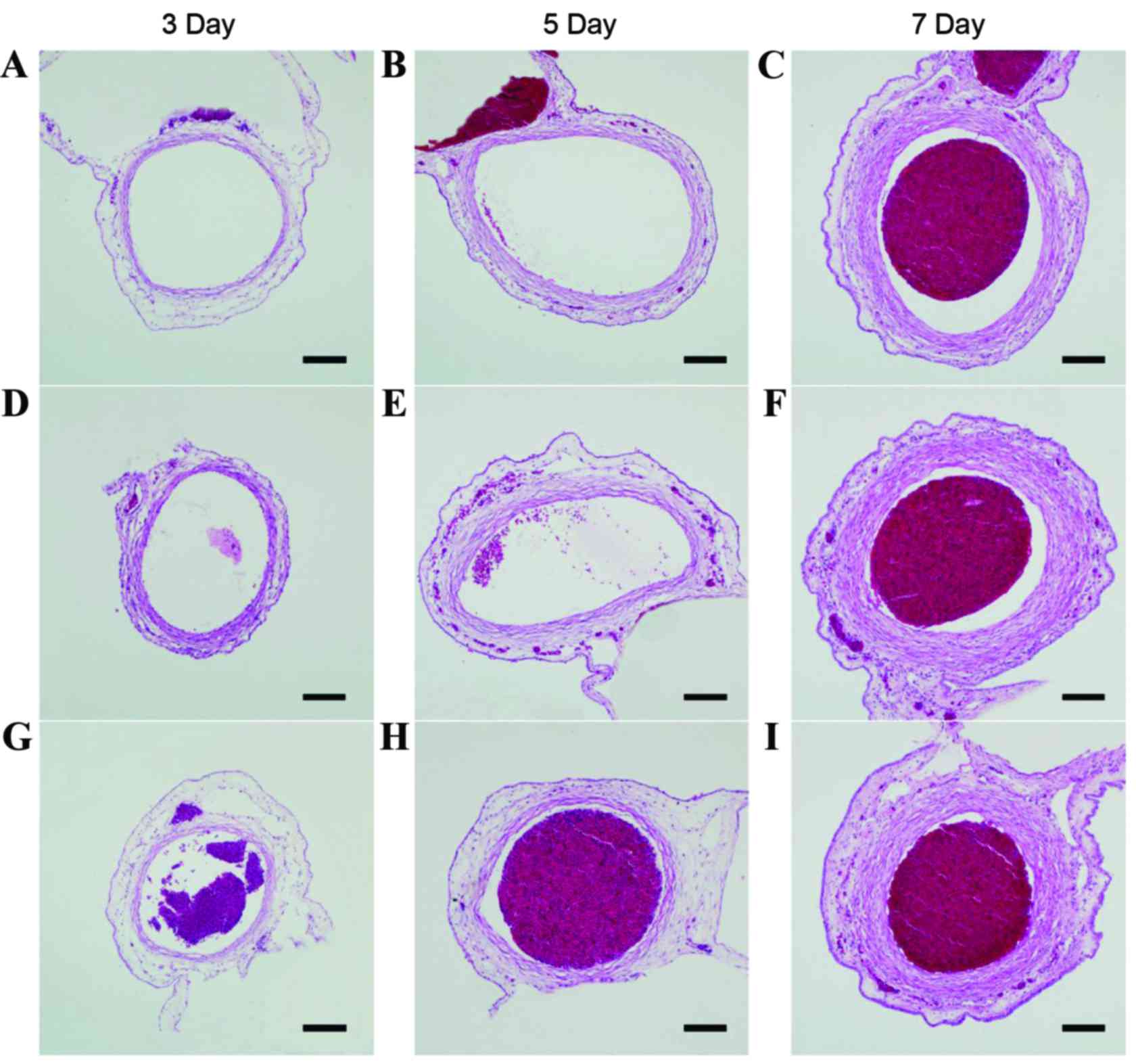 | Figure 2.Typical pathological sections of
umbilical arteries in all subgroups. Typical pathological sections
of umbilical arteries in the allantoic cavity in the (A) 3-, (B) 5-
and (C) 7-day subgroups of the control group. Typical pathological
sections of umbilical arteries in the allantoic cavity in the (D)
3-, (E) 5- and (F) 7-day subgroups of the rAAV-CGRP group. Typical
pathological sections of umbilical arteries in the allantoic cavity
in the (G) 3-, (H) 5- and (I) 7-day subgroups of the empty vector
AAV group. Compared with structures in the control group, the lumen
in the AAV group markedly decreased, and the vessel wall became
thicker and displayed a wave-form change. Compared with structures
in the AAV group, the lumen in the rAAV-CGRP group became thinner,
and the vessel wall thickening exhibited a certain degree of
improvement. However, compared with structures in the control
group, the lumen still became thinner, and the vessel wall became
thicker. Scale bar, 100 µm. rAAV, recombinant adeno-associated
virus; AAV, adeno-associated virus; CGRP, calcitonin gene-related
peptide. |
 | Table I.Inner CSA (µm2) of the
umbilical artery in each group. |
Table I.
Inner CSA (µm2) of the
umbilical artery in each group.
| Group | 3 day | 5 day | 7 day |
|---|
| Control |
80193.62±3059.49 |
150329.14±2989.76 |
155615.73±3202.46 |
| rAAV-CGRP |
69056.64±2093.75a |
119891.61±2095.39a |
120762.51±3001.47a |
| AAV |
53992.81±2398.62b |
88976.16±2207.95b |
89045.38±2309.93b |
 | Table II.Vessel wall thickness of the umbilical
artery in each group (µm). |
Table II.
Vessel wall thickness of the umbilical
artery in each group (µm).
| Group | 3 day | 5 day | 7 day |
|---|
| Control |
30.99±1.13 |
44.07±1.83 |
45.44±1.82 |
| rAAV-CGRP |
32.54±1.37a |
47.69±1.75a |
49.86±1.91a |
| AAV |
34.76±1.61b |
51.82±1.80b |
54.05±1.79b |
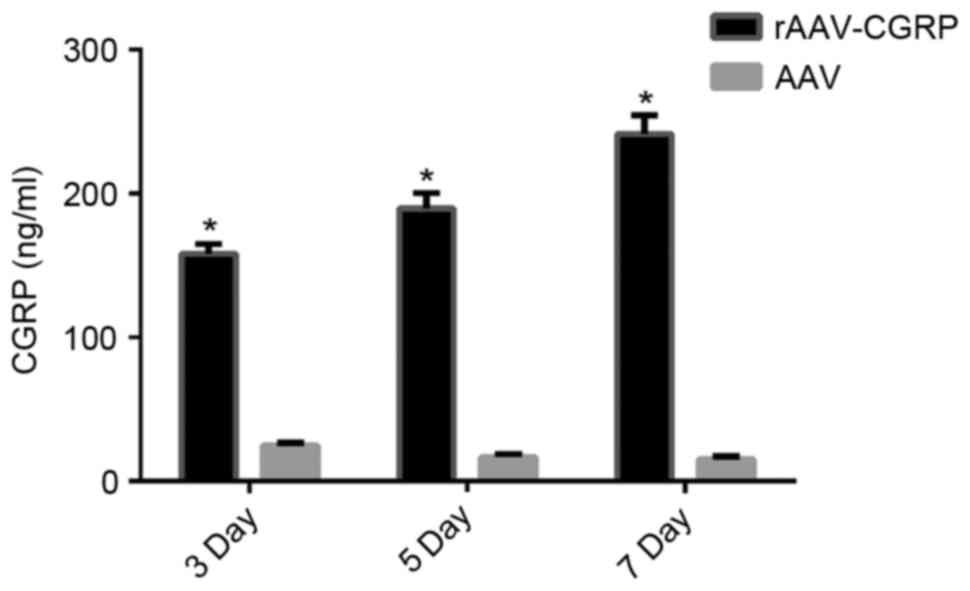
Increase in CGRP release in the
allantoic fluid after rAAV-CGRP transfection
The chick embryos were opened, and the allantoic
fluid was collected from the chick embryos of all groups. CGRP
concentrations in the chick embryo allantoic fluid on days 3, 5 and
7 after model establishment were detected using ELISA reagent kits.
The AAV group exhibited very low levels of CGRP. The CGRP
concentration in the rAAV-CGRP group significantly increased
(P<0.05) to be six-fold higher than that of the AAV group
(Fig. 4).
Discussion
CGRP, which is a polypeptide containing 37 amino
acids, is a translated product of calcitonin gene expression in
nerve tissues. Mature CGRP is currently the strongest known
endogenously active vasodilator substance (6,7,9). Studies have revealed that, CGRP is
released into the blood from nerve endings at the early stage of
CVS after SAH to oppose vasospasm and thereby maintain the blood
supply in the brain; therefore, CGRP concentrations in the blood
and cerebrospinal fluid (CSF) are increased (18–20).
With disease progression, CGRP levels in nerve endings are depleted
and CGRP concentrations in the blood gradually decrease, leading to
the progressive aggravation of CVS. It has been confirmed that CGRP
fibers significantly decrease in a CVS animal model (21,22).
These studies support the hypothesis that the synthesis and release
of CGRP is closely associated with vasospasm after SAH. Depletion
of endogenous CGRP may be an important factor for CVS development
after SAH. Exogenous administration of CGRP in animal models also
confirmed that CGRP is able to improve CVS after SAH. Nozaki et
al (10) conducted an
intra-cisternal injection of CGRP on day 3 after the establishment
of a cerebral arterial spasm model in dogs and showed that basilar
artery spasm improved within 5 min of injection. Between the doses
of 10−11 and 2×10−10 mol/kg, the effect was
even more prominent as the dosage increased; in addition, spasms
were completely reversed at 2×10−10 mol/kg. Toshima
et al (11) demonstrated that
both intra-cisternal and intravenous administration of CGRP
improves CVS in rabbits; however, intravenous administration
induced significant hypotension. Ahmad et al (23) and Inoue et al (24) implanted a CGRP slow-release tablet in
to the cisterna magna to extend the duration of action of CGRP in
animal CVS models. In addition, the early clinical trials of
intravenously administered exogenous CGRP into CVS patients after
SAH confirmed the improvement of CVS and recovery of neurological
function within a short period (12–14).
However, the intravenous application of CGRP easily induced
complications, such as hypotension and increased heart rate
(12–14). Furthermore, due to its protein
features, CGRP has a short plasma half-life of only 7–10 min. These
phenomena have limited the clinical application of CGRP (15).
With the development of genetic engineering
technology, studies on CGRP gene therapy for CVS after SAH have
received more attention and have become a hot topic in clinical
studies. Toyoda et al (25)
injected the CGRP gene mediated by an adenovirus into the cisterna
magna of rabbits in a SAH model. After five days, the CGRP
concentration in the CSF of the CGRP transgene treatment group
increased 400-fold, the basilar artery diameter increased by 25%,
and the degree of spasm significantly improved compared with the
values in the control group. Satoh et al (26) applied the same method in a canine CVS
model. Compared with the control group, the CGRP concentration in
the CSF of the CGRP transgene treatment group increased 115-fold
and the basilar artery diameter increased by 25%. These results
further confirmed that adenovirus-mediated CGRP gene therapy
improves CVS after SAH. Tian et al (7) injected Tat-gelatin siloxane
nanoparticles encapsulating the expression plasmid vector
pLXSN-CGRP into the cisterna magna of a CVS model in rats. Compared
with the control group, the CGRP expression levels in the CSF of
the treatment group increased, the perimeter of the basilar artery
lumen increased by 23%, the vessel wall thickness decreased by 76%
and the neurological function significantly improved. These
findings support the feasibility of CGRP gene therapy in CVS after
SAH.
In the present study, the CGRP gene was cloned into
AAV using genetic engineering technology to construct a eukaryotic
expressing vector, rAAV-CGRP, containing the CGRP gene. Through the
allantoic cavity injection route, the feasibility of improving
umbilical artery vasospasm in the chick embryo allantoic cavity
induced by allantoic cavity hemorrhage using rAAV-CGRP was
demonstrated. The embryonic chick allantois is a sac formed by a
bulge from the ventral side of the embryo during chick embryo
incubation; this sac is filled with allantoic fluid. Internally, it
is wrapped with umbilical arteries and veins. The allantoic
umbilical artery travels through the allantoic cavity and is
divided into two branches. After entering the CAM, the allantoic
umbilical artery gradually branches to form a dense CAM vascular
network, which gradually converges into the CAM veins and finally
returns to the body through the CAM veins (27,28). The
natural fluid environment of the umbilical arteries in the
allantoic cavity is similar to the subarachnoid CSF environment of
cerebral blood vessels in the skull base. Allantoic umbilical
artery spasm induced by allantoic cavity hemorrhage using CAM vein
puncture is able to simulate the pathological changes of CVS after
SAH. These phenomena have been confirmed in our previous studies
(16,27). As a commonly used gene transfer
vector, AAV has the advantages of excellent safety, a broad host
range, a specific integration function, and long-term stable
expression of exogenous genes; therefore, AAV is widely used in
gene therapy experimentation of mammalian CVS models (25,26) and
can be used for the stable expression of gene vectors in chick
embryos (29,30).
The present study injected constructed rAAV-CGRP
into the chick embryo allantoic cavity after 24 h of establishment
of the chick embryo umbilical artery vasospasm model. The CGRP
concentrations in the allantoic fluid after 3, 5 and 7 days of
model establishment were detected using ELISA reagent kits. The
results showed that the CGRP concentration in allantoic fluid in
the rAAV-CGRP group significantly increased and exhibited a gradual
increasing trend compared with that of the empty vector AAV group.
The CGRP concentration in the allantoic fluid in the empty vector
AAV group exhibited a decreasing trend. These results indicated
that rAAV-CGRP was stably expressed in chick embryos; the CGRP
concentration in the allantoic fluid gradually increased, whereas
endogenous CGRP in the empty vector AAV group gradually depleted.
These results were consistent with previous studies in mammals
(7,25,26).
Pathological sections of umbilical arteries also revealed that the
chick embryo allantoic umbilical artery developed severe vasospasm
in the model group injected with empty vector AAV. On days 3, 5 and
7 after model establishment, the inner CSA of the umbilical
arteries were only 67.33, 59.19 and 57.22%, respectively, of those
in the age-matched control group, and the vessel wall thickness of
the umbilical arteries increased by 12.17, 17.59 and 18.95%,
respectively, of those in the age-matched control group. In the
rAAV-CGRP injection group, on days 3, 5, and 7 after model
establishment, the degrees of vasospasm significantly improved, and
the inner CSA of the umbilical arteries were only 86.11, 79.75 and
77.60%, respectively, of those in the age-matched control group;
similarly, the vessel wall thickness values of the umbilical
arteries increased to 5.00, 8.21 and 9.73%, respectively, of those
in the age-matched control group. The above results suggested that
rAAV-CGRP significantly increased CGRP expression levels in the
chick embryo allantoic cavity and improved umbilical artery
vasospasm induced by allantoic cavity hemorrhage. However,
rAAV-CGRP was not able to completely reverse the vasospasm
resulting from allantoic cavity hemorrhage caused by puncture. In
addition, the statistical results of the successful rate of
umbilical artery vasospasm models induced by chick embryo allantoic
cavity hemorrhage demonstrated that, after 72 h of model
establishment, the control group contained one chick embryo that
did not survive in the 7-day subgroup. Following model
establishment, the mortality rates in the 3-, 5- and 7-day
subgroups of the rAAV-CGRP group were 25, 25 and 37.5%,
respectively, which were lower than the values of 50, 37.5 and
62.5%, respectively, in the age-matched subgroups of the empty
vector AAV group. The differences between these two groups were not
statistically significant. However, the mortality rates in the 3-
and 7-day subgroups in the chick embryo model of the empty vector
AAV group were significantly higher than those in the control
group. These results suggest that injection of rAAV-CGRP through
the allantoic cavity may improve the oxygen supply and blood supply
during chick embryo development and increase the success rate of
the umbilical artery vasospasm model induced by chick embryo
allantoic cavity hemorrhage, possibly by relieving umbilical artery
vasospasm.
The present study used an umbilical artery vasospasm
model induced by chick embryo allantoic cavity hemorrhage to
validate the effectiveness of rAAV-CGRP treatment. This model had
the advantages of simple manipulation, ease of feeding, low cost,
short cycles, and fewer ethical restrictions compared with previous
mammalian vasospasm models. Notably, although AAV is a commonly
used vector for gene therapy of CVS in mammalian models and has
high transfection efficiency, it induces cytotoxicity and
immunogenicity; in particular, it may induce a systemic toxic
reaction or immune and inflammatory reactions (25,26) that
interfere with experimental results. The deficiency of innate
immunity in chick embryos may compensate for this shortcoming
(31,32). The present study also had certain
limitations. The umbilical artery vasospasm model induced by chick
embryo allantoic cavity hemorrhage would not be able to completely
simulate vasospasm after SAH in human experimentation. Compared
with mammalian models, the model used in this study did not
evaluate the neurologic impairment by behavior after rAAV-CGRP
treatment, and the treatment results could only be evaluated by
pathological change.
In conclusion, the present study demonstrated that
rAAV-CGRP is able to increase CGRP expression in the allantoic
cavity in a vasospasm model induced by allantoic cavity hemorrhage
in the chick embryo, improve vasospasm induced by allantoic cavity
hemorrhage, and increase the success rate of the vasospasm model
induced by allantoic cavity hemorrhage in the chick embryo. These
results further validate the feasibility of gene therapy for CVS
after SAH. In addition, the utilization of this simple vasospasm
model induced by allantoic cavity hemorrhage in the chick embryo
may reduce the use of mammalian SAH models and reduce experimental
costs. This model also has the advantage of natural immunity
deficiency to prevent the inflammatory response of viral vectors.
Although there are certain limitations, this model may be used as a
simple and low-cost vasospasm model for preliminary studies to
identify other target genes in the pursuit of effective gene
therapy.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81200888).
References
|
1
|
Becker KJ: Epidemiology and clinical
presentation of aneurysmal subarachnoid hemorrhage. Neurosurg Clin
N Am. 9:435–444. 1998.PubMed/NCBI
|
|
2
|
Przybycien-Szymanska MM and Ashley WW Jr:
Biomarker discovery in cerebral vasospasm after aneurysmal
subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 24:1453–1464.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Budohoski KP, Guilfoyle M, Helmy A,
Huuskonen T, Czosnyka M, Kirollos R, Menon DK, Pickard JD and
Kirkpatrick PJ: The pathophysiology and treatment of delayed
cerebral ischaemia following subarachnoid haemorrhage. J Neurol
Neurosurg Psychiatry. 85:1343–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gathier CS, van den Bergh WM and Slooter
AJ; HIMALAIA-Study Group, : HIMALAIA (Hypertension Induction in the
Management of AneurysmaL subArachnoid haemorrhage with secondary
IschaemiA): A randomized single-blind controlled trial of induced
hypertension vs. no induced hypertension in the treatment of
delayed cerebral ischemia after subarachnoid hemorrhage. Int J
Stroke. 9:375–380. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Francoeur CL and Mayer SA: Management of
delayed cerebral ischemia after subarachnoid hemorrhage. Crit Care.
20:2772016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun BL, Shen FP, Wu QJ, Chi SM, Yang MF,
Yuan H, Xie FM, Zhang YB, Chen J and Zhang F: Intranasal delivery
of calcitonin gene-related peptide reduces cerebral vasospasm in
rats. Front Biosci (Elite Ed). 2:1502–1513. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian XH, Wang ZG, Meng H, Wang YH, Feng W,
Wei F, Huang ZC, Lin XN and Ren L: Tat peptide-decorated
gelatin-siloxane nanoparticles for delivery of CGRP transgene in
treatment of cerebral vasospasm. Int J Nanomedicine. 8:865–876.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kolias AG, Sen J and Belli A: Pathogenesis
of cerebral vasospasm following aneurysmal subarachnoid hemorrhage:
Putative mechanisms and novel approaches. J Neurosci Res. 87:1–11.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu N, Wu Y, Chen BZ, Han JF and Zhou MT:
Protective effect of stellate ganglion block on delayed cerebral
vasospasm in an experimental rat model of subarachnoid hemorrhage.
Brain Res. 1585:63–71. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nozaki K, Uemura Y, Okamoto S, Kikuchi H
and Mizuno N: Relaxant effect of calcitonin gene-related peptide on
cerebral arterial spasm induced by experimental subarachnoid
hemorrhage in dogs. J Neurosurg. 71:558–564. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Toshima M, Kassell NF, Tanaka Y and
Dougherty DA: Effect of intracisternal and intravenous calcitonin
gene-related peptide on experimental cerebral vasospasm in rabbits.
Acta Neurochir (Wien). 119:134–138. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Juul R, Aakhus S, Bjornstad K, Gisvold SE,
Brubakk AO and Edvinsson L: Calcitonin gene-related peptide (human
alpha-CGRP) counteracts vasoconstriction in human subarachnoid
haemorrhage. Neurosci Lett. 170:67–70. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Juul R, Edvinsson L, Gisvold SE, Ekman R,
Brubakk AO and Fredriksen TA: Calcitonin gene-related peptide-LI in
subarachnoid haemorrhage in man. Signs of activation of the
trigemino-cerebrovascular system? Br J Neurosurg. 4:171–179.
1990.PubMed/NCBI
|
|
14
|
Effect of calcitonin-gene-related peptide
in patients with delayed postoperative cerebral ischaemia after
aneurysmal subarachnoid haemorrhage. European CGRP in Subarachnoid
Haemorrhage Study Group. Lancet. 339:831–834. 1992.PubMed/NCBI
|
|
15
|
Kokkoris S, Andrews P and Webb DJ: Role of
calcitonin gene-related peptide in cerebral vasospasm, and as a
therapeutic approach to subarachnoid hemorrhage. Front Endocrinol
(Lausanne). 3:1352012.PubMed/NCBI
|
|
16
|
Yuan Y, Yang S, Li C, Xu K, Luo Q and Yu
J: The chicken embryo umbilical artery is a promising in vivo model
system for the study of vasospasm. Int J Clin Exp Med. 9:1139–1149.
2016.
|
|
17
|
Pallás V, Más P and Sánchez-Navarro JA:
Detection of plant RNA viruses by nonisotopic dot-blot
hybridization. Methods Mol Biol. 81:461–468. 1998.PubMed/NCBI
|
|
18
|
Edvinsson L, Ekman R, Jansen I, McCulloch
J, Mortensen A and Uddman R: Reduced levels of calcitonin
gene-related peptide-like immunoreactivity in human brain vessels
after subarachnoid haemorrhage. Neurosci Lett. 121:151–154. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schebesch KM, Herbst A, Bele S, Schödel P,
Brawanski A, Stoerr EM, Lohmeier A, Kagerbauer SM, Martin J and
Proescholdt M: Calcitonin-gene related peptide and cerebral
vasospasm. J Clin Neurosci. 20:584–586. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Juul R, Hara H, Gisvold SE, Brubakk AO,
Fredriksen TA, Waldemar G, Schmidt JF, Ekman R and Edvinsson L:
Alterations in perivascular dilatory neuropeptides (CGRP, SP, VIP)
in the external jugular vein and in the cerebrospinal fluid
following subarachnoid haemorrhage in man. Acta Neurochir (Wien).
132:32–41. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edvinsson L, Delgado-Zygmunt T, Ekman R,
Jansen I, Svendgaard NA and Uddman R: Involvement of perivascular
sensory fibers in the pathophysiology of cerebral vasospasm
following subarachnoid hemorrhage. J Cereb Blood Flow Metab.
10:602–607. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Imaizumi S, Shimizu H, Ahmad I, Kaminuma
T, Tajima M and Yoshimoto T: Effect of calcitonin gene-related
peptide on delayed cerebral vasospasm after experimental
subarachnoid hemorrhage in rabbits. Surg Neurol. 46:263–271. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ahmad I, Imaizumi S, Shimizu H, Kaminuma
T, Ochiai N, Tajima M and Yoshimoto T: Development of calcitonin
gene-related peptide slow-release tablet implanted in CSF space for
prevention of cerebral vasospasm after experimental subarachnoid
haemorrhage. Acta Neurochir (Wien). 138:1230–1240. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inoue T, Shimizu H, Kaminuma T, Tajima M,
Watabe K and Yoshimoto T: Prevention of cerebral vasospasm by
calcitonin gene-related peptide slow-release tablet after
subarachnoid hemorrhage in monkeys. Neurosurgery. 39:984–990. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toyoda K, Faraci FM, Watanabe Y, Ueda T,
Andresen JJ, Chu Y, Otake S and Heistad DD: Gene transfer of
calcitonin gene-related peptide prevents vasoconstriction after
subarachnoid hemorrhage. Circ Res. 87:818–824. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Satoh M, Perkins E, Kimura H, Tang J, Chun
Y, Heistad DD and Zhang JH: Posttreatment with adenovirus-mediated
gene transfer of calcitonin gene-related peptide to reverse
cerebral vasospasm in dogs. J Neurosurg. 97:136–142. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yuan YJ, Xu K, Wu W, Luo Q and Yu JL:
Application of the chick embryo chorioallantoic membrane in
neurosurgery disease. Int J Med Sci. 11:1275–1281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tufan AC and Satiroglu-Tufan NL: The chick
embryo chorioallantoic membrane as a model system for the study of
tumor angiogenesis, invasion and development of anti-angiogenic
agents. Curr Cancer Drug Targets. 5:249–266. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tutykhina IL, Bezborodova OA, Shmarov MM,
Logunov DY, Neugodova GL, Nemtsova ER, Naroditsky BS, Yakubovskaya
RI and Gintsburg AL: Production of recombinant human lactoferrin in
the allantoic fluid of embryonated chicken eggs and its
characteristics. Protein Expr Purif. 65:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Sun H, Shen P, Zhang X and Xia X:
Effective inhibition of infectious bursal disease virus replication
by recombinant avian adeno-associated virus-delivered microRNAs. J
Gen Virol. 90:1417–1422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ribatti D: Chicken chorioallantoic
membrane angiogenesis model. Methods Mol Biol. 843:47–57. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Palmer TD, Lewis J and Zijlstra A:
Quantitative analysis of cancer metastasis using an avian embryo
model. J Vis Exp. 28152011.PubMed/NCBI
|