Introduction
A varicocele (VC) is defined as the abnormal
expansion, elongation and tortuosity of the spermatic vein and is
considered to be the primary cause of infertility in males
(1). The exact mechanism of
testicular dysfunction and infertility induced by VC has not yet
been clearly defined. It may be associated with a number of
factors, including microcirculation disturbance of the testis,
vasoactive substance reflux, oxidative stress, an increase in
nitric oxide (NO) concentration, hypoxia, hyperthermia,
inflammation or apoptosis (2). It
has been reported that the excessive production of reactive oxygen
species (ROS) causes germinal cell necrosis, endothelial damage and
DNA damage, leading to sperm dysfunction in VC-induced rats
(3). Furthermore, elevated
spermatogenic cell apoptosis and testicular inflammation are
closely associated with male infertility (4).
Hydrogen sulfide (H2S) is a colourless,
water-soluble, volatile gas with a characteristic smell of rotten
eggs and was initially considered to be a toxic gas (5). It has been suggested that
H2S may be a third type of endogenous gaseous
transmitter, such as carbon monoxide (CO) and NO, and may induce
antioxidant, anti-inflammatory and anti-apoptotic effects in
various systems (6,7). NaHS has been widely used in clinical
experiments to evaluate the biological effects of H2S
(5,8). However, NaHS releases large amounts of
H2S over a short period of time and cannot effectively
imitate the biological course of naturally produced H2S
(9). Morpholin-4-ium 4 methoxyphenyl
(morpholino) phosphonodithioate (GYY4137) was one of a series of
compounds synthesized that were based on the chemical structure of
Lawesson's compound, which releases H2S in organic
solvents (10). GYY4137 is novel
H2S donor that, unlike NaHS, decomposes and over a
period of a few hours produces small amounts of H2S
under physiological conditions, thus potentially mimicking
H2S release in vitro and in vivo (10). Meng et al (11) reported that GYY4137 may significantly
increase the ventricular ejection fraction and reduce the ischemic
area in rats, thus protecting against myocardial
ischemia-reperfusion injury by attenuating oxidative stress and
apoptosis. Additionally, Tang et al (12) demonstrated that GYY4137 serves an
anti-inflammatory role in the process of acute lung injury in rats.
Therefore, the current study hypothesized that GYY4137 has the
ability to ameliorate oxidative stress, inflammation and
spermatogenic cell apoptosis.
Materials and methods
Animals
The current study involved 48 adult male Sprague
Dawley rats (8–10 weeks), weighing 250–300 g, obtained from the
Hubei Center for Disease Control (Hubei, China). Prior to the
experiment, rats had free access to food and water in a 12 h
light/dark cycle with a constant temperature (22±2°C) and humidity
(40–70%). All experimental procedures adhered to the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (13) and were approved by
the Animal Care and Use Committee of Wuhan University (Wuhan,
China).
Experimental grouping and surgical
procedures
All rats were anaesthetized by intraperitoneal
administration of 2% sodium phenobarbital (50 mg/kg; Propbs
Biotechnology. Co., Ltd., Beijing, China) and were then placed on a
homoeothermic table to maintain a rectal temperature of 37–38°C.
The 48 rats were then randomly divided into 6 different groups (all
n=8). The rats in group A (control) did not undergo surgery. The
rats in group B (sham group) underwent an abdominal median
incision. The left renal vein was separated and there was no
additional intervention. The rats in group C (VC group), underwent
a procedure in which the left renal vein was carefully separated on
the inside of the adrenal vein and the spermatic vein, and a metal
probe (0.5–0.85 mm diameter) was placed parallel to the left renal
vein. The vein and metal probe were ligated together with 4–0 silk
suture, reducing the vein diameter to ~50% of its original
diameter. The metal probe was then removed and the left renal vein
was reversibly constricted. This procedure followed the procedure
previously performed by Turner (14)
and induced the following characteristics in the rats: Abnormal
expansion, elongation and tortuosity of the left renal and
spermatic vein; diameter of the left renal vein >1 mm and no
pathological lesion in the left kidney. The rats in groups D, E and
F underwent the procedures performed in group C followed by the
intraperitoneal administration of 5, 10 or 20 mg/kg GYY4137
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), once a day
respectively.
A total of 4 weeks following the successful
establishment of the VC model, the left testis of the rat from the
all groups was removed and divided into two halves. One half was
fixed in 4% paraformaldehyde at 4°C for 12 h prior to histological
examination. The other half was immediately frozen at −80°C for
later analysis.
Hematoxylin and eosin (H&E)
staining
Following fixation in 4% paraformaldehyde, the
testis tissue was embedded in paraffin and cut into 4-µm thick
sections following standard protocols. Following routine dewaxing
and hydrating, sections were stained with hematoxylin for 5 min and
eosin for 2 min at the room temperature.
Biochemical evaluation
Frozen testicular tissues were homogenized and
centrifuged at 500 × g for 10 min at 4°C, and the supernatants were
collected. Testicular Malondialdehyde (MDA) content and superoxide
dismutase (SOD) activity were measured spectrophotometrically using
thiobarbituric acid (TBA) and xanthine oxidase methods following
the manufacturer's protocol (A003-1; Malondialdehyde assay kit;
Jiancheng Bioengineering Institute, Nanjing, China). MDA content
was detected by measuring absorbance at 532 nm and expressed as
nmol/g protein. SOD activity was determined by measuring absorbance
at 550 nm using a microplate reader and presented as U/mg
protein.
TUNEL assays
A TUNEL assay was performed to detect spermatogenic
cell apoptosis in the testes using the transferase-mediated dUTP
nick-end labelling (TUNEL) method with an in situ apoptosis
detection kit (Roche Diagnostics, Basel, Switzerland) following the
manufacturer's protocol. Briefly, the rat testis tissue was fixed
in 4% paraformaldehyde/PBS solution (pH 7.4) at 4°C overnight and
then placed into 70% ethanol at 20°C for 24 h. Following washing 3
times with PBS, the samples were immersed in a permeabilization
buffer for 15 min. Subsequently, they were incubated with 50 ml
reaction buffer (TdT Enzyme 5 ml + Labeling Safe Buffer 45 ml) at
37°C for 90 min. Nuclei that stained brown were considered to be
TUNEL-positive cells as observed under a Zeiss LSM 510 confocal
laser scanning microscope (Zeiss AG, Oberkochen, Germany). Five
high-power fields of vision were randomly selected in each slice
and the average number of apoptotic cells per 200 cells was
calculated. The apoptosis index (AI) was expressed as follows:
AI=(positive cells/total cells counted) × 100%.
Immunohistochemistry
Caspase-3 and Bax expression were measured using
immunohistochemical staining. Tissues were fixed in 4%
paraformaldehyde, embedded in paraffin and then cut in 4 µm
thickness, and endogenous peroxidase activity was blocked with 3%
hydrogen peroxide at 37°C for 10 min. The sections were incubated
with 10% normal goat serum in Tris-buffered saline (TBS) for 30 min
at 37°C. Staining was performed using rabbit polyclonal
anti-caspase-3 (sc7148; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) and rabbit polyclonal anti-Bax (sc493; Santa Cruz
Biotechnology, Inc.) antibodies. After being washed three times
with PBS, all sections were incubated in DAB reagents and
counterstained with haematoxylin. All steps were performed
following the manufacturer's instructions and the results were
evaluated by comparing the staining intensity with an Olympus BX50
light microscope (Olympus Corporation, Tokyo, Japan).
Western blot analysis
All testicular tissue proteins were extracted with
radioimmunoprecipitation assay lysis buffer (P0013B; Beyotime
Institute of Biotechnology, Haimen, China) then quantified using a
bicinchoninic acid assay. Briefly, 40 µg/lane protein samples were
separated by 10% SDS-PAGE and transferred to a polyvinylidene
difluoride membrane. The membrane was blocked at 37°C for 2 h with
5% non-fat milk in TBST buffer and then incubated with the
following primary antibodies at 4°C overnight: Bax (sc-493; 1:500;
Santa Cruz Biotechnology, Inc.), cleaved-caspase-3 (sc7148;
1:1,000; Santa Cruz Biotechnology, Inc.), tumor necrosis factor
(TNF)-α (ab6671; 1:500; Abcam, Cambridge, UK) and interleukin
(IL)-1β (ab100768; 1:200; Abcam). Following three washes with TBST
buffer, membranes were incubated with secondary goat anti-rabbit
antibodies conjugated with horseradish peroxidase (LK2001/LK2003;
1:100; Sungene Biotech, Co., Ltd., Tianjin, China) at room
temperature for 1 h. All specific bands were visualized using an
enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Optical densities were
detected using ImageJ software version 1.48 (National Institute of
Health, Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the testicular tissue
samples from each group using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) and RNA concentration was detected using
spectrophotometry. First-strand cDNA was synthesized using a cDNA
synthesis kit (Promega Corporation, Madison, WI, USA), following
the manufacturer's protocol. Subsequently, qPCR was performed using
an Applied Biosystems SYBR Green mix kit on an ABI 7900 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The primer sequences for TNF-α and IL-1β were as follows: TNF-α
forward, 5′-ATCCGCGACGTGGAACTG-3′, and reverse,
5′-ACCGCCTGGAGTTCTGGAA-3′; IL-1β forward,
5′-GAGCACCTTCTTTTCCTTCATCTT-3′, and reverse,
5′-TCACACACCAGCAGGTTATCATC-3′. GAPDH was used as a housekeeping
gene and the primer sequences for GAPDH were as follows: Forward,
5′-ACAGCAACAGGGTGGTGGAC-3′ and reverse, 5′-TTTGAGGGTGCAGCGAACTT-3′.
The data were presented as a ratio to GAPDH mRNA. qPCR was
performed with 40 cycles of 94°C for 30 sec, followed by 56°C for
30 sec and 72°C for 25 sec. The quantitative analysis was conducted
using the 2−ΔΔCq method (15).
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed using SPSS 19.0 (IBM
Corp, Armonk, NY, USA). The means were compared using one-way
analysis of variance followed by the Student-Newman-Keuls test for
the different groups. P<0.05 was considered to indicate a
statistically significant difference. All experiments were
performed at least 3 times.
Results
GYY4137 alleviates VC-induced
histopathological damage
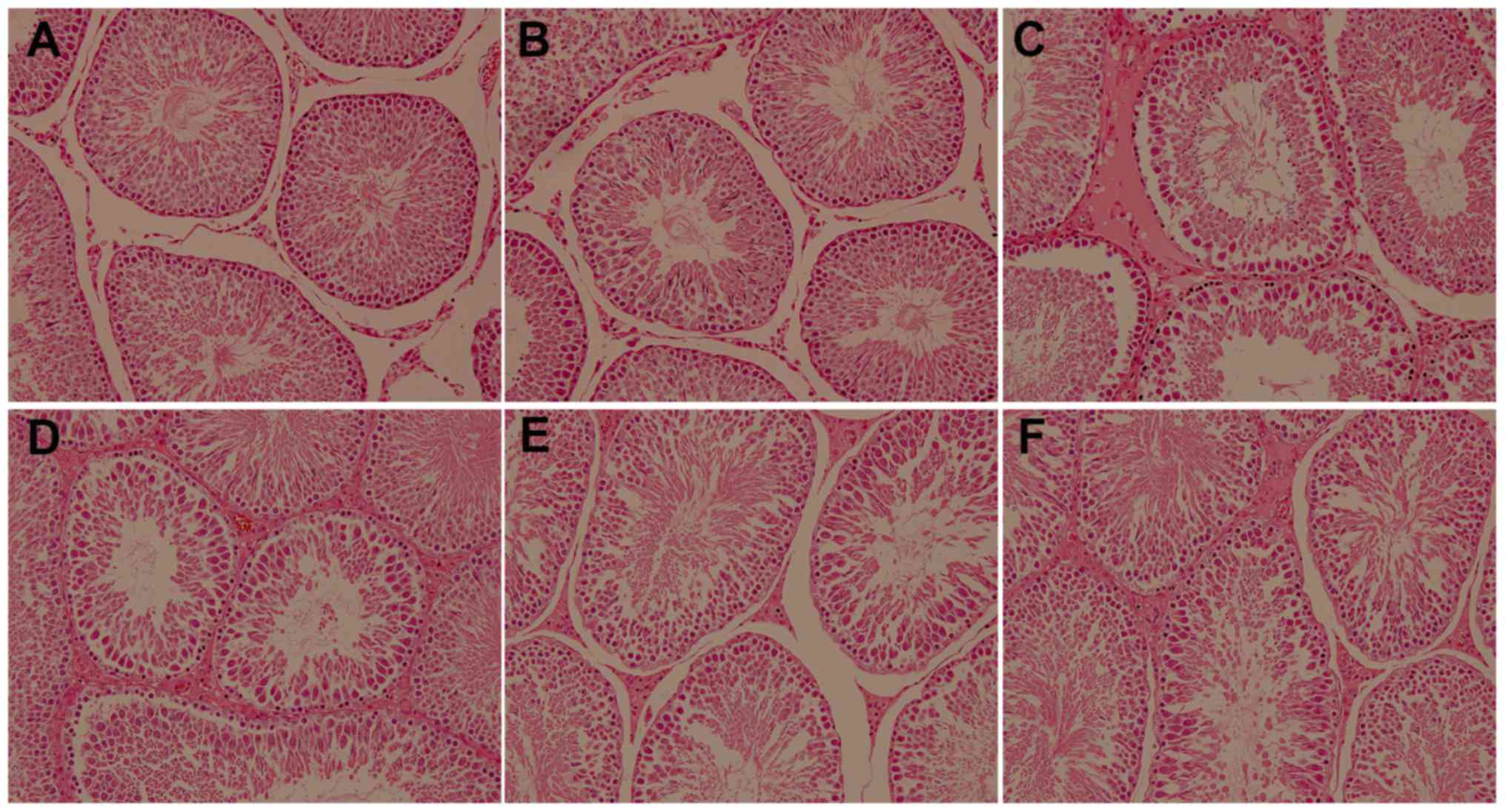
H&E staining indicated that there were no marked
morphological changes in the left testicular tissue of rats in
groups A and B. The spermatogenic cells in the seminiferous tubules
were arranged in order, including the primary and secondary
spermatocytes, spermatids and spermatozoa. Rats in group C
exhibited marked damage in their spermatogenic function, as
determined by the detachment of epithelial cells in the lumen, the
disordered arrangement of spermatogenic cells and extensive damage
of the seminiferous epithelium. However, treatment with GYY4137
reduced severe testicular damage and there were fewer spermatogenic
cells and seminiferous epithelium changes in group C compared with
groups D, E and F (Fig. 1).
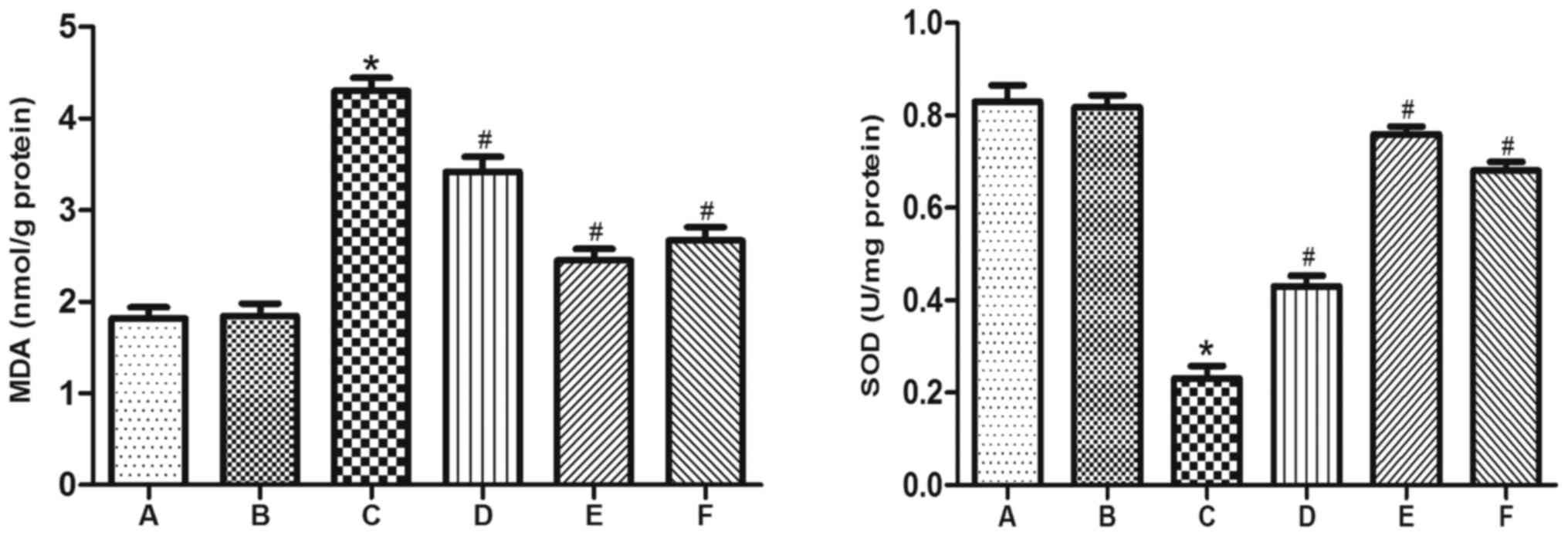
GYY4137 reduces the increases in MDA
content and reduction in SOD activity induced by VC
To evaluate levels of oxidative stress in VC-induced
rats, MDA content and SOD activity were measured in testicular
tissue. In group C, MDA content was significantly increased and SOD
activity was significantly decreased, compared with groups A and B.
However, treatment GYY4137 significantly reversed the increase in
MDA content and the reduction in SOD activity induced by VC in
groups D, E and F (Fig. 2).
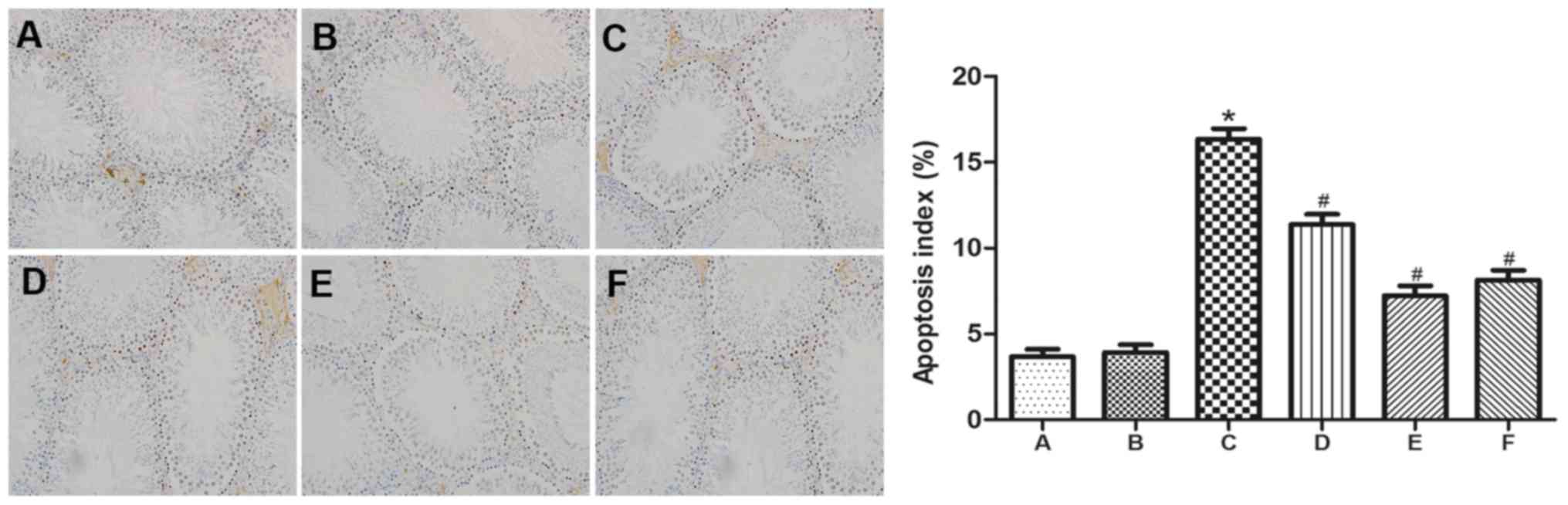
GYY4137 inhibits the apoptosis of
spermatogenic cells induced by VC
To assess apoptosis and determine the AI, the
expression of Bax and caspase-3 in testicular spermatogenic cells
was evaluated by TUNEL, immunohistochemistry and Western blot
analysis. There was a significant increase in the number of
TUNEL-positive spermatogenic cells in group C compared with groups
A and B (Fig. 3). Furthermore,
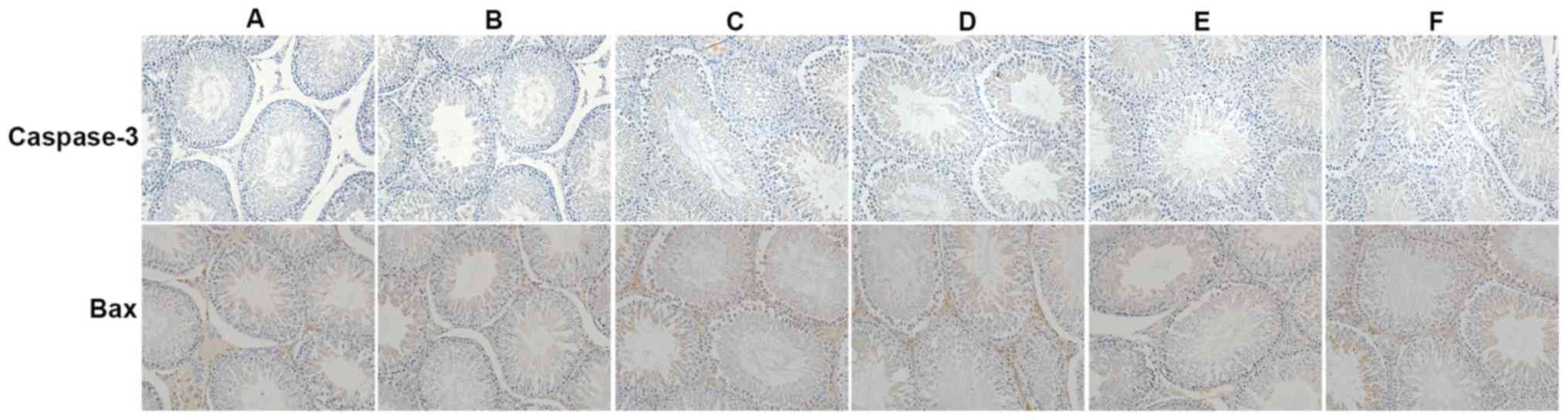
immunohistochemistry indicated that caspase-3 and Bax levels were
markedly increased in group C compared with groups A and B.
However, following treatment with GYY4137, significantly fewer
TUNEL-positive spermatogenic cells were detected. Additionally,
treatment with GYY4137 decreased caspase-3 and Bax expression.
Testis taken from mice in group E that received 10 mg/kg/day
GYY4127 experienced an ideal therapeutic effect on damaged testes,
evident by a significant reduction of TUNEL-positive spermatogenic
cells (Fig. 3) and marked reduction
in the expression of caspase-3 and Bax (Fig. 4).
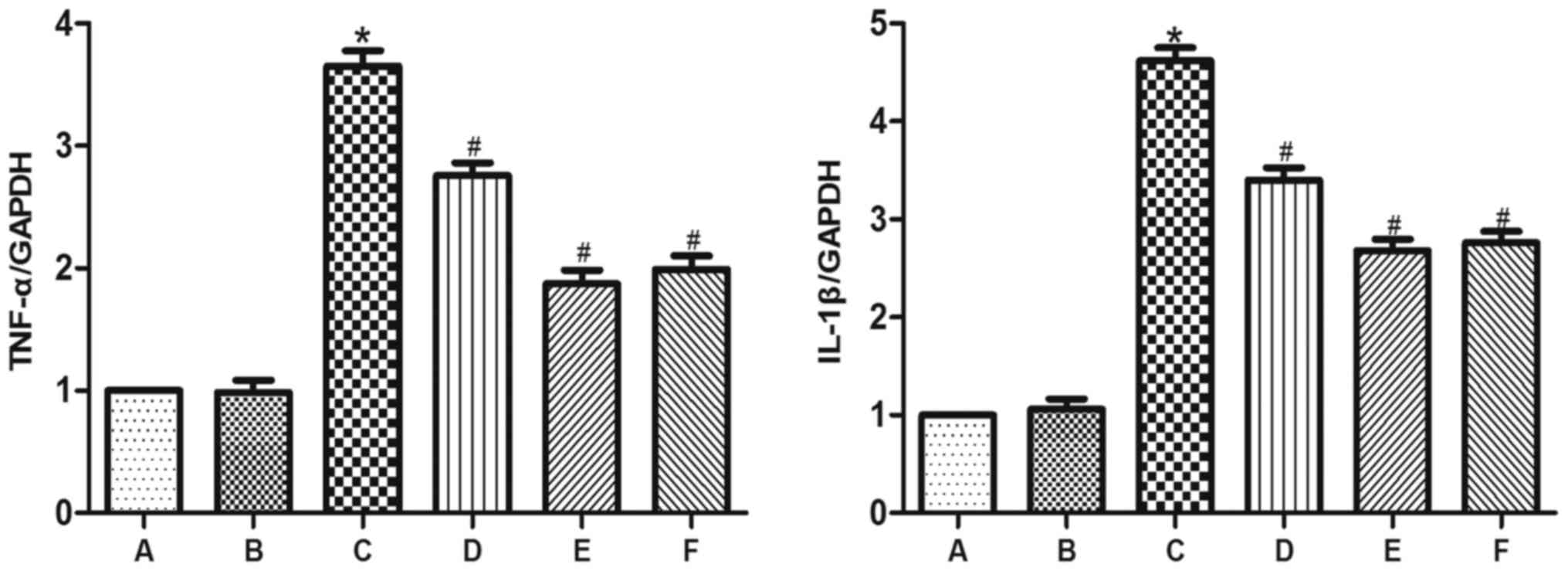
GYY4137 attenuates the VC-induced
inflammatory response
The expression of TNF-α, IL-1β, Bax and cleaved
caspase-3 was significantly higher in group C than in groups A and
B (Figs. 5 and 6). However, administration of GYY4137
significantly decreased the expression of TNF-α, IL-1β, Bax and
cleaved caspase-3 in groups D, E and F compared with group C. The
results demonstrated that GYY4137 was most effective at reducing
the expression of inflammation-related genes at a dose of 10
mg/kg/day (Figs. 5 and 6).
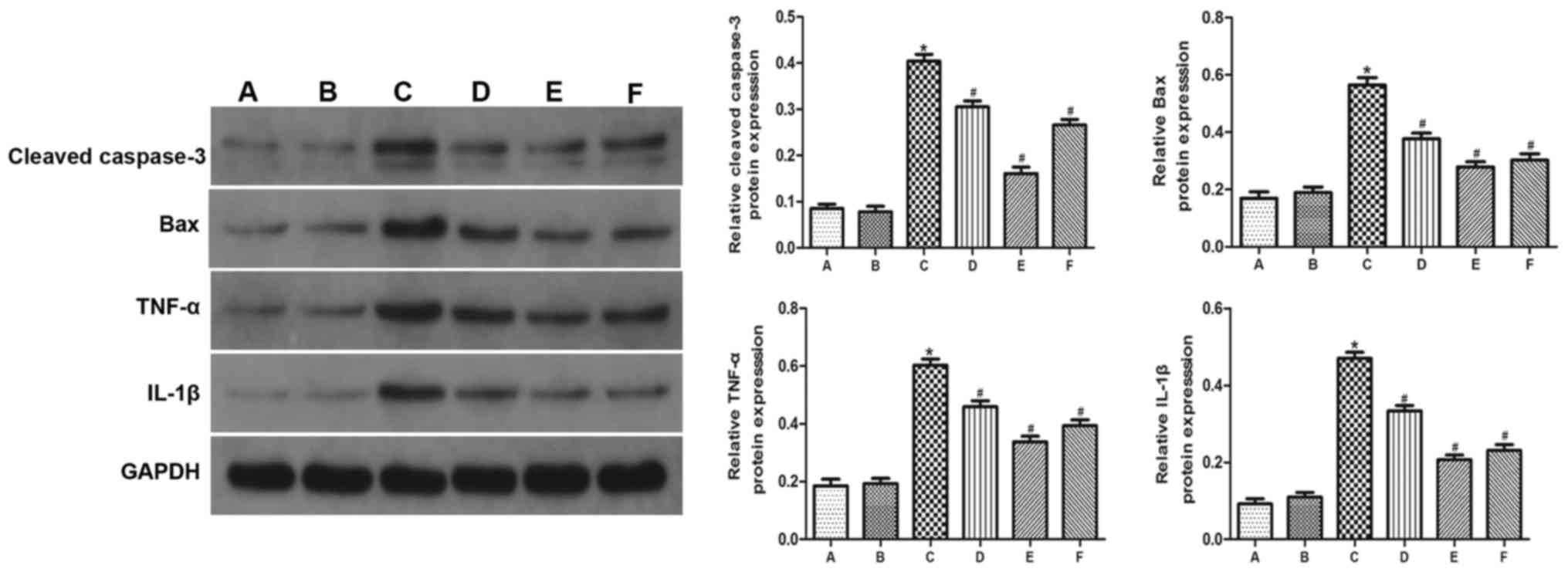 | Figure 5.The expression of Bax, cleaved
caspase-3, TNF-α and IL-1β was detected by western blotting. GAPDH
was as a loading control. (A) Control group, (B) sham group, (C) VC
group, (D) VC+5 mg/kg/day GYY4137 group, (E) VC+10 mg/kg/day
GYY4137 group, (F) VC+20 mg/kg/day GYY4137 group. *P<0.05 vs.
group A; #P<0.05 vs. group C. VC, varicocele;
GYY4127, Morpholin-4-ium 4 methoxyphenyl (morpholino)
phosphonodithioate; TNF-α, tumor necrosis factor α; IL-1β,
interleukin 1β. |
Discussion
VC is described as the abnormal expansion,
elongation and tortuosity of the spermatic vein and is considered
to be the primary cause of infertility in adolescent males
(1). The incidence of VC in the
general population is ~15%, however, in infertile men it is much
higher, at 35% (16). Nevertheless,
the mechanism of testicular dysfunction and infertility associated
with VC remains unclear, therefore, a number of studies
investigating the pathophysiology of this condition have been
performed. GYY4137 is a novel H2S-releasing molecule and
induces a wide range of pharmacological effects, including
antioxidant, anti-apoptosis, anti-inflammatory and
immune-modulating activities, in a wide range of different organs
(17–19). The current study initially evaluated
the effect of GYY4137 on the histological changes that occur in the
testes of rats with a VC. The results demonstrated that VC severely
damages testicular spermatogenic function, in accordance with the
results of previous studies (20,21).
However, different doses of GYY4137 ameliorated histological injury
to different extents and the ideal effect was induced following the
administration of 10 mg/kg/day GYY4137 in experimental rats with a
VC.
Oxidative stress is a result of the accumulation of
ROS in tissues, which leads to the deregulation of antioxidant
mechanisms (22). Under
physiological situations, the accumulation of ROS is maintained at
a low level throughout the antioxidant defence system and has
specific regulatory effects on cell growth, development,
differentiation and death. However, in pathological circumstances,
including ischemia and hypoxia, the overproduction of ROS can
result in the oxidation of cellular membrane proteins, lipids and
DNA to cause a range of cellular dysfunction and cell death
(22,23). Mammalian testes are highly sensitive
to oxidative stress and the pathology of VC is closely associated
with the overproduction of ROS (24). MDA, a major product of lipid
peroxidative decomposition generated by ROS, is typically used to
evaluate the extent of cellular damage under conditions of
oxidative stress (25). SOD is a
critical component in the processes of cell growth, differentiation
and protection, and has the ability to convert a superoxide anion
radical into hydrogen peroxide; its activity reflects the
scavenging capacity of ROS in organisms (26). It has been demonstrated that
H2S serves a key role in reducing ROS production and has
therapeutic potential in preventing oxidative stress damage in
different types of organs (27). In
the current study, treatment with the novel H2S donor,
GYY4137, alleviated oxidative stress in VC testes by reducing the
expression of MDA and increasing the expression of SOD.
Apoptosis is a normal physiological phenomenon that
serves an important role in maintaining homeostasis during
spermatogenesis (28). However, the
pathological process of VC usually leads to widespread
spermatogenic cell apoptosis, which may induce further testicular
dysfunction and cause infertility (29). Zheng et al (30) demonstrated that the incidence of
apoptosis in the ipsilateral testis was greatly raised in a rat
model of VC and that there was a positive correlation between the
extent of apoptosis and injury duration. It has been demonstrated
that the process of apoptosis is regulated by gene expression and
its activation may stimulate the degradation of cellular substrates
and participate in a variety of pathogeneses, including acting as a
functional obstacle of spermatogenesis and decreasing sperm
motility and DNA levels (31).
Furthermore, Bax may promote the release of cytochrome c
from the mitochondria to induce apoptosis (32). Mostafa et al (33) indicated that the expression of Bax
was significantly increased in infertile men with a VC.
Additionally, caspase-3 is an inactive zymogen among the cysteine
proteases and the convergence point of multiple
apoptosis-stimulating signals. Its activation is a sign of an
irreversible commitment to cellular apoptosis (30). It has been demonstrated in an
androgen-deficiency model that the decrease in testosterone induced
by spermatogenic cell apoptosis in testicular tissue is caspase-3
dependent, indicating that caspase-3 activation induces
spermatogenic cell apoptosis (34).
Furthermore, it has been demonstrated that exogenous H2S
inhibits the activation of caspase-3 to induce an anti-apoptotic
effect in renal ischemia-reperfusion injury (35). The damage induced by VC has
complicates the pathological course and inflammation has been
recognized as an important factor in the onset and development of
VC (36). TNF-α and IL-1β are key
inflammatory cytokines involved in the pathological process of VC.
Germ cell-derived TNF-α increases tissue damage and the
inflammatory response and may control physiological spermatogenic
cell apoptosis by regulating Fas ligand levels (37). IL-1β is an immune-derived cytokine
and promotes its own secretion under ischemia and hypoxia and the
increased expression of IL-1β induces detrimental effects in the
testes of infertile males with VC (38,39). In
the present study, it was hypothesised that GYY4137 inhibits the
expression of Bax, caspase-3, TNF-α and IL-1β, thereby alleviating
apoptosis and inflammatory responses in a rat model of VC.
In conclusion, the present study demonstrated that
GYY4137 attenuates testicular dysfunction by alleviating oxidative
stress, inflammation and apoptosis in a rat model of VC. GYY4137
was most effective at attenuating this dysfunction at a dose of 10
mg/kg/day. However, the specific underlying protective mechanisms
of GYY4137 in VC remain unknown and further studies are
required.
References
|
1
|
Chan CC, Sun GH, Shui HA and Wu GJ:
Differential spermatozoal protein expression profiles in men with
varicocele compared to control subjects: Upregulation of heat shock
proteins 70 and 90 in varicocele. Urology. 81:1379.e1–e8. 2013.
View Article : Google Scholar
|
|
2
|
Fretz PC and Sandlow JI: Varicocele:
Current concepts in pathophysiology, diagnosis, and treatment. Urol
Clin North Am. 29:921–937. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fazlioglu A, Yilmaz I, Mete O, Kurtulus F,
Parlakkilic O, Güctas O and Cek M: The effect of varicocele repair
on experimental varicocele-induced testicular germ cell apoptosis.
J Androl. 29:29–34. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khosravanian H, Razi M, Farokhi F and
Khosravanian N: Simultaneous administration of dexamethasone and
vitamin E reversed experimental varicocele-induced impact in
testicular tissue in rats; correlation with Hsp70-2 chaperone
expression. Int Braz J Urol. 41:773–790. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kimura H: The physiological role of
hydrogen sulfide and beyond. Nitric Oxide. 41:4–10. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lobb I, Zhu J, Liu W, Haig A, Lan Z and
Sener A: Hydrogen sulfide treatment ameliorates long-term renal
dysfunction resulting from prolonged warm renal
ischemia-reperfusion injury. Can Urol Assoc J. 8:E413–E418. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L, Bhatia M and Moore PK: Hydrogen
sulphide-a novel mediator of inflammation? Curr Opin Pharmacol.
6:125–129. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li G, Xie ZZ, Chua JM, Wong PC and Bian J:
Hydrogen sulfide protects testicular germ cells against
heat-induced injury. Nitric Oxide. 46:165–171. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu Z, Han Y, Li L, Lu H, Meng G, Li X,
Shirhan M, Peh MT, Xie L, Zhou S, et al: The hydrogen sulfide
donor, GYY4137, exhibits anti-atherosclerotic activity in high fat
fed apolipoprotein E(−/-) mice. Br J Pharmacol. 169:1795–1809.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Whiteman M, Guan YY, Neo KL, Cheng
Y, Lee SW, Zhao Y, Baskar R, Tan CH and Moore PK: Characterization
of a novel, water-soluble hydrogen sulfide-releasing molecule
(GYY4137): New insights into the biology of hydrogen sulfide.
Circulation. 117:2351–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meng G, Wang J, Xiao Y, Bai W, Xie L, Shan
L, Moore PK and Ji Y: GYY4137 protects against myocardial ischemia
and reperfusion injury by attenuating oxidative stress and
apoptosis in rats. J Biomed Res. 29:203–213. 2015.PubMed/NCBI
|
|
12
|
Tang B, Ma L, Yao X, Tan G, Han P, Yu T,
Liu B and Sun X: Hydrogen sulfide ameliorates acute lung injury
induced by infrarenal aortic cross-clamping by inhibiting
inflammation and angiopoietin 2 release. J Vasc Surg.
65:501–508.e1. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
U.S. National Institutes of Health, .
Laboratory animal welfare: Public Health Service policy on humane
care and use of laboratory animals by awardee institutions; notice.
Fed Regist. 50:19584–19585. 1985.PubMed/NCBI
|
|
14
|
Turner TT: The study of varicocele through
the use of animal models. Hum Reprod Update. 7:78–84. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2^(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
16
|
Kilinç F, Kayaselcuk F, Aygun C, Guvel S,
Egilmez T and Ozkardes H: Experimental varicocele induces hypoxia
inducible factor-1alpha, vascular endothelial growth factor
expression and angiogenesis in the rat testis. J Urol.
172:1188–1191. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rose P, Dymock BW and Moore PK: GYY4137, a
novel water-soluble, H2S-releasing molecule. Methods Enzymol.
554:143–167. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH,
Li L, Moore PK and Deng LW: The slow-releasing hydrogen sulfide
donor, GYY4137, exhibits novel anti-cancer effects in vitro and in
vivo. PLoS One. 6:e210772011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan S, Shen X and Kevil CG: Beyond a
Gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular
health and immune response. Antioxid Redox Signal. 27:634–653.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pastuszak AW and Wang R: Varicocele and
testicular function. Asian J Androl. 17:659–667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakamoto H and Ogawa Y: Does a clinical
varicocele influence the relationship between testicular volume by
ultrasound and testicular function in patients with infertility?
Fertil Steril. 92:1632–1637. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turkmen S, Mentese A, Karaguzel E, Karaca
Y, Kucuk A, Uzun A, Yulug E and Turedi S: A comparison of the
effects of N-acetylcysteine and ethyl pyruvate on experimental
testicular ischemia-reperfusion injury. Fertil Steril. 98:626–631.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Southorn PA and Powis G: Free radicals in
medicine. I. Chemical nature and biologic reactions. Mayo Clin
Proc. 63:pp. 381–389. 1988; View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang M, Wen J, Dong Q, Zhao LG and Shi
BK: Testicular hypofunction caused by activating p53 expression
induced by reactive oxygen species in varicocele rats. Andrologia.
47:1175–1182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dogan F, Armagan A, Oksay T, Akman T,
Aylak F and Bas E: Impact of micronised purified flavonoid fraction
on increased malondialdehyde and decreased metalloproteinase-2 and
metalloproteinase-9 levels in varicocele: Outcome of an
experimentally induced varicocele. Andrologia. 46:380–385. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sakamoto Y, Ishikawa T, Kondo Y, Yamaguchi
K and Fujisawa M: The assessment of oxidative stress in infertile
patients with varicocele. Bju Int. 101:1547–1552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen CQ, Xin H and Zhu YZ: Hydrogen
sulfide: Third gaseous transmitter, but with great pharmacological
potential. Acta Pharmacol Sin. 28:1709–1716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vaux DL and Korsmeyer SJ: Cell death in
development. Cell. 96:245–254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka H, Fujisawa M, Tanaka H, Okada H
and Kamidono S: Apoptosis-related proteins in the testes of
infertile men with varicocele. Bju Int. 89:905–909. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng Y, Zhang X, Zhou J, Cheng F and Zhou
B: Effects on the ipsilateral testis during progression of
experimental varicocele in rat. Med Sci Monit. 14:BR122–BR126.
2008.PubMed/NCBI
|
|
31
|
Arnoult D, Parone P, Martinou JC,
Antonsson B, Estaquier J and Ameisen JC: Mitochondrial release of
apoptosis-inducing factor occurs downstream of cytochrome c release
in response to several proapoptotic stimuli. J Cell Biol.
159:923–929. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang H, Yu F, Tong Z, Yuan B and Wang C:
Effect of ischemia post-conditioning on skeletal muscle oxidative
injury, mTOR, Bax, Bcl-2 proteins expression, and HIF-1α/β-actin
mRNA, IL-6/β-actin mRNA and caveolin-3/β-actin mRNA expression in
ischemia-reperfusion rabbits. Mol Biol Rep. 40:507–514. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mostafa T, Rashed L, Nabil N and Amin R:
Seminal BAX and BCL2 gene and protein expressions in infertile men
with varicocele. Urology. 84:590–595. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JM, Ghosh SR, Weil AC and Zirkin BR:
Caspase-3 and caspase-activated deoxyribonuclease are associated
with testicular germ cell apoptosis resulting from reduced
intratesticular testosterone. Endocrinology. 142:3809–3816. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bos EM, Leuvenink HG, Snijder PM,
Kloosterhuis NJ, Hillebrands JL, Leemans JC, Florquin S and van
Goor H: Hydrogen sulfide-induced hypometabolism prevents renal
ischemia/reperfusion injury. J Am Soc Nephrol. 20:1901–1905. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zeinali M, Amree A Hadian, Khorramdelazad
H, Karami H and Abedinzadeh M: Inflammatory and anti-inflammatory
cytokines in the seminal plasma of infertile men suffering from
varicocele. Andrologia. 49:2016.
|
|
37
|
Pentikäinen V, Erkkila K, Suomalainen L,
Otala M, Pentikäinen MO, Parvinen M and Dunkel L: TNFalpha
down-regulates the Fas ligand and inhibits germ cell apoptosis in
the human testis. J Clin Endocrinol Metab. 86:4480–4488. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sahin Z, Celik-Ozenci C, Akkoyunlu G,
Korgun ET, Acar N, Erdogru T, Demir R and Ustunel I: Increased
expression of interleukin-1alpha and interleukin-1beta is
associated with experimental varicocele. Fertil Steril. 85 Suppl
1:S1265–S1275. 2006. View Article : Google Scholar
|
|
39
|
Wang Y, Ge P and Zhu Y: TLR2 and TLR4 in
the brain injury caused by cerebral ischemia and reperfusion.
Mediators Inflamm. 2013:1246142013. View Article : Google Scholar : PubMed/NCBI
|