Introduction
Retinal vein occlusion (RVO) is one of most common
vision-threatening retinal vascular diseases and can be divided
into two primary categories: i) Central retinal vein occlusion
(CRVO) and ii) branch retinal vein occlusion (BRVO) (1). Previous studies have confirmed that the
increased expression of angiogenic growth factors such as vascular
endothelial cell growth factor (VEGF) caused by hypoxia secondary
to RVO leads to vascular hyperpermeability with subsequent
breakdown of the blood-retina barrier and macular edema (ME). The
development of ME contributes to visual deterioration (2–5).
The introduction of VEGF inhibitors is the beginning
of a new era in the treatment of ME secondary to RVO targeting the
disease at the molecular level (6).
Ranibizumab has been applied successfully to reduce ME due to RVO
(7–13). However, treatment success is often
temporary. Some patients experience no effect on the resolution of
ME, and some patients have a poor visual outcome despite complete
resolution of the ME under ranibizumab therapy, despite multiple
intravitreal injections. Therefore, the predictive factors for
visual outcome after ranibizumab therapy have become very important
(7,14–22).
Some factors are thought to be associated with the
post-treatment best-corrected visual acuity (BCVA) prognosis of ME
due to RVO under intravitreal anti-VEGF agent injections, such as
the baseline BCVA, age, and macular microstructure (2). Optical coherence tomography (OCT) is a
noninvasive method that visualizes the macular microstructure
clearly. Spectral domain optical coherence tomography (SD-OCT)
machines now attain 5 µm resolution, which allows layer-by-layer
evaluation of the retina, such as the ellipsoid zone, external
limiting membrane (ELM), retina pigment epithelium (RPE), and
choroid (23). Among these ocular
structures, the central foveal thickness (CFT), ellipsoid zone, and
ELM integrity were reported to be associated with post-treatment
BCVA (24–27).
However, previously, the extent of the ellipsoid
zone and ELM damage was assessed mainly by dividing the
hyperreflective line within the 1 mm diameter circle centered on
the fovea into completely visible, partially visible, and invisible
(25). Thus, the important effect of
integrity beneath the center of the fovea was not considered
adequately, which is very important to visual acuity. In the
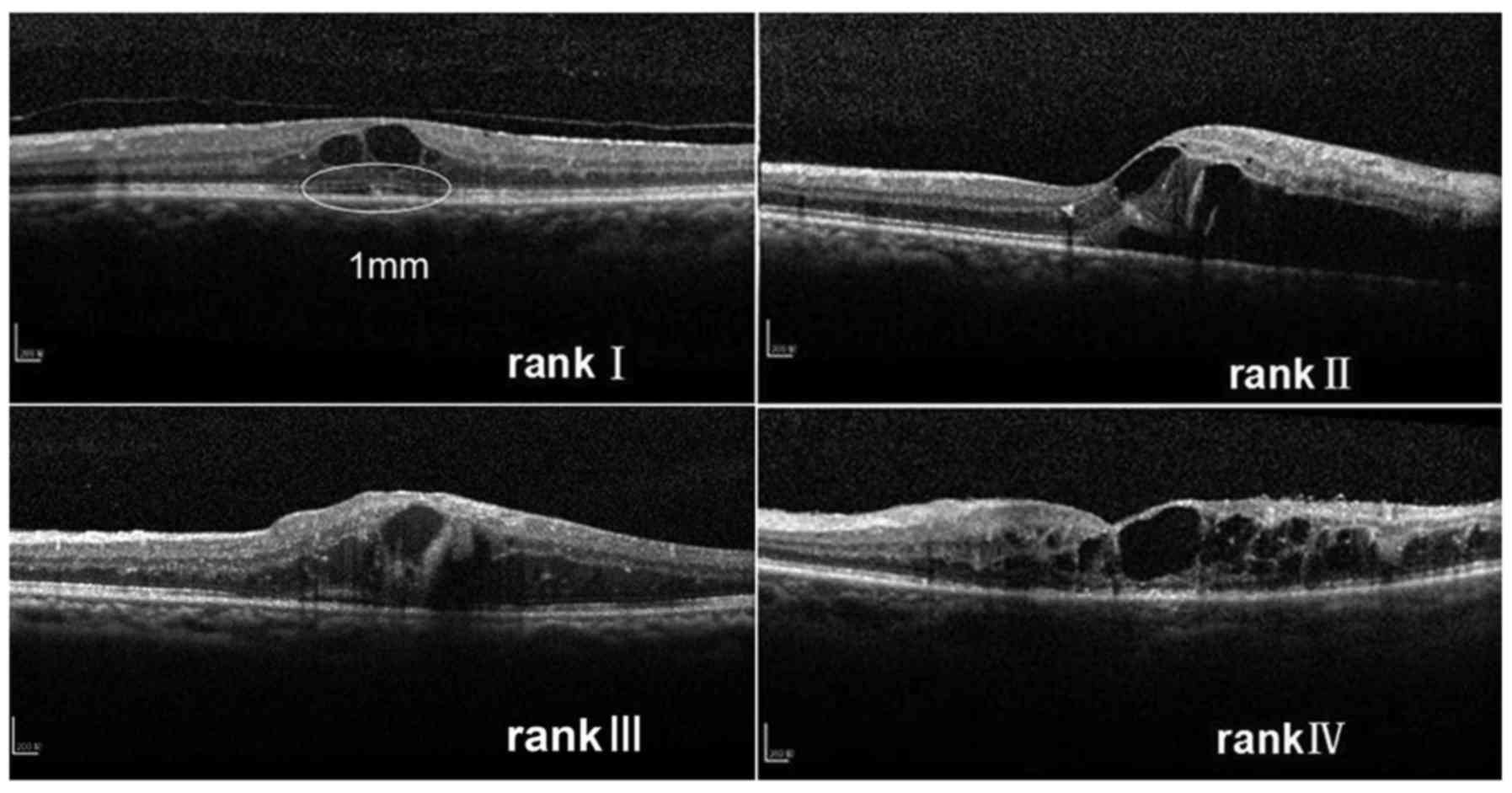
present study, an original ranking was applied that the integrity
of the ELM and the ellipsoid zone at baseline was categorized into
four ranks: i) Completely visible line; ii) partially detectable
line with undamaged center of fovea; iii) artially detectable line
with damaged center of fovea; and iv) completely invisible
line.
In this study, we applied the original ranking to
investigate the effects of clinical baseline factors of eyes with
ME secondary to RVO on post-treatment BCVA after 3 consecutive
monthly ranibizumab injections and another 3 months of follow-up in
order to find independent baseline characteristics that may predict
a positive functional therapeutic response.
Materials and methods
In this retrospective study, 31 patients (16 CRVO,
15 BRVO) with ME due to RVO received 3 monthly consecutive
intravitreal injections of 0.5 mg ranibizumab and further 3 months
of follow-up. During the follow-up period, subjects were eligible
to receive monthly intraocular ranibizumab if they had BCVA ≤20/40
or CFT ≥250 µm.
This study was approved by the Institutional Review
Board and followed the tenets of the Declaration of Helsinki.
Informed consent was obtained after patients were informed about
the possible risks.
Patients were included if they met the inclusion
criteria as follows: i) CFT on OCT was more than 300 µm; ii) the
patient had not received an intravitreal injection; iii) 3 monthly
consecutive ranibizumab injections and iv) other 3 months follow-up
were completed, and no other treatment except ranibizumab
injections was required. Patients were excluded if they had any of
the following ocular diseases: Age-related macular degeneration
(AMD), diabetic retinopathy (DR), choroidal neovascularization, a
history of ocular trauma, and a history of intraocular surgery
except cataract surgery. We also discharged patients if their
baseline OCT scan did not provide an identifiable macular
microstructure.
The patient's age, sex, and duration of RVO were
recorded. A comprehensive ophthalmologic examination was performed.
BCVA was measured with a Snellen chart and converted to a logarithm
of the minimal angle of resolution (logMAR) units for statistical
analysis. Eyes that had post-treatment BCVA of better than 0.30
logMAR were grouped in the good function group; the other eyes were
grouped in the poor function group (28). Slit-lamp and fundus examinations were
included. All patients underwent color fundus photography (Topcon
Corp., Tokyo, Japan) and fluorescein angiography (FA; Heidelberg
Engineering Inc., Heidelberg, Germany) to diagnose RVO and discover
ischemic features. In addition, we performed SD-OCT imaging at
baseline to evaluate the status of the ellipsoid zone, ELM, CFT,
and subretinal hemorrhage within a 1 mm diameter circle centered on
the fovea. All evaluations were obtained by authors masked to the
patient's BCVA.
We obtained in each study eye 2 SD-OCT (spectralis;
Heidelberg Engineering Inc.) scans 6 mm in crosshair fashion
centered on the fovea (horizontal and vertical). For horizontal and
vertical SD-OCT scans, the ART function (averaging of scans) was
activated, and 25 SD-OCT scans were averaged. For maximal
definition of the retinal layers, we used noise-reduction software
(Heidelberg Engineering Inc.). The integrity of the ELM and the
ellipsoid zone was categorized into four ranks depending on the
microstructure within the 1 mm diameter circle centered on the
fovea at baseline: i) Completely visible line, ii) partially
detectable line with undamaged center of fovea, iii) partially
detectable line with damaged center of fovea and iv) completely
invisible line. If the ranking between the horizontal and vertical
scans was different, the higher ranking was selected (Fig. 1).
All statistical analyses were performed using SPSS
ver. 18.0 (SPSS, Inc., Chicago, IL, USA). Continuous values were
compared using an independent-sample t-test or a one-way analysis
of variance (ANOVA). A paired sample t-test was used to compare the
post-treatment with the baseline values. A non-parametric test was
used if the continuous variables were abnormally distributed.
Categorical variables were assessed using the chi-squared test. To
determine the independent baseline factors that predict
post-treatment BCVA, univariate regression analysis was performed,
followed by stepwise multivariate regression analysis, logMAR BCVA
at 6 month after the first intravitreal injection was treated as a
dependent variable. Analysis of covariance was used to calculate
and compare post-treatment BCVA after adjusting for other variables
between ELM ranks. A P<0.05 was considered to indicate a
statistically significant analysis.
Results
A total of 31 eyes of 31 patients with RVO (13 men
and 18 women) were included in this study. Table I shows the baseline characteristics
of the 31 eyes. The mean age of the patients was 61.4±9.7 years. Of
all 31 RVO eyes, 16 eyes were CRVO, and 15 eyes were BRVO. The mean
interval from diagnosis to the 1st injection for the patients with
RVO was 104.0±89.0 days.
 | Table I.Baseline characteristics of patients
with ME secondary to RVO. |
Table I.
Baseline characteristics of patients
with ME secondary to RVO.
| No. of eyes
(left/right) | 31 (19/12) |
|---|
| Age (years) | 61.4±9.7 |
| Sex
(Male/Female) | 13/18 |
| CRVO/BRVO | 16/15 |
| Duration of symptoms
(days) | 104.0±89.0 |
| Baseline BCVA
(logMAR) |
0.60±0.26 |
| Optical coherence
tomography |
|
| Initial ELM
integrity | I 10, II 9, III 8, IV
4 |
| Initial ellipsoid
zone integrity | I 6, II 9, III 11, IV
5 |
| Baseline central
fovea thickness (µm) | 646.4±197.0 |
| Hemorrhage under the
fovea (yes/no) | 5/26 |
| Fluorescein
angiography |
|
|
Ischaemic/non-ischaemic type | 13/18 |
| Intact/broken foveal
capillary ring | 25/6 |
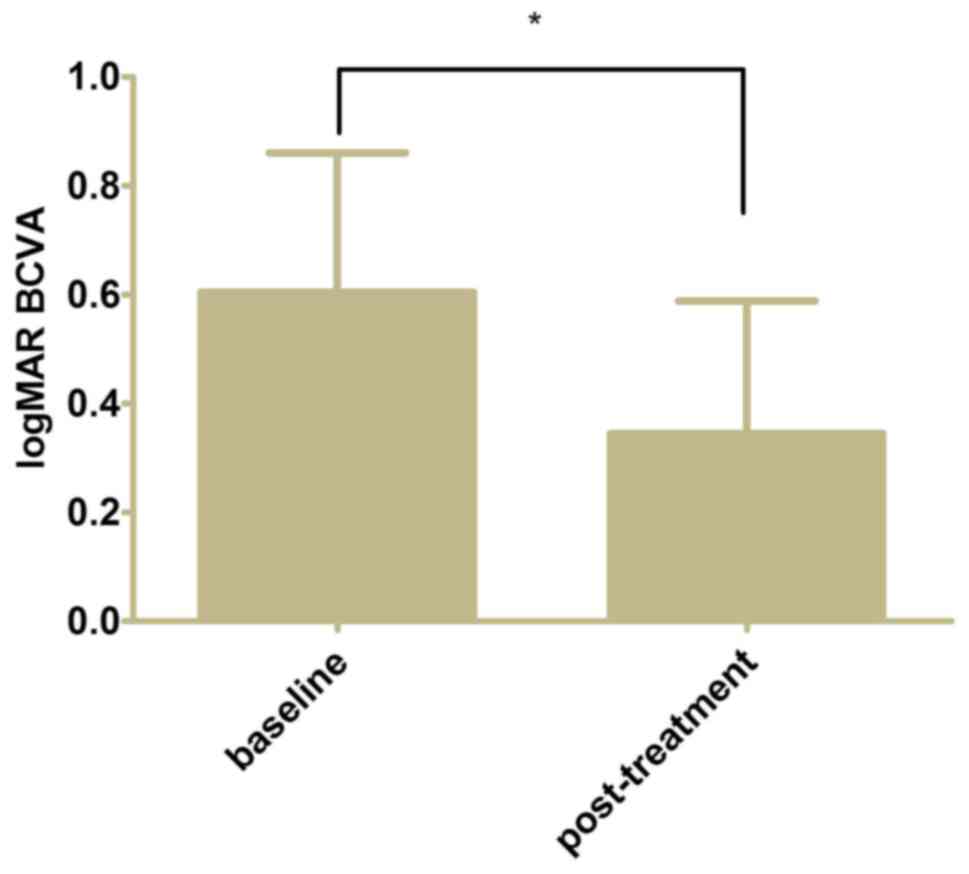
The mean post-treatment logMAR BCVA of the eyes was
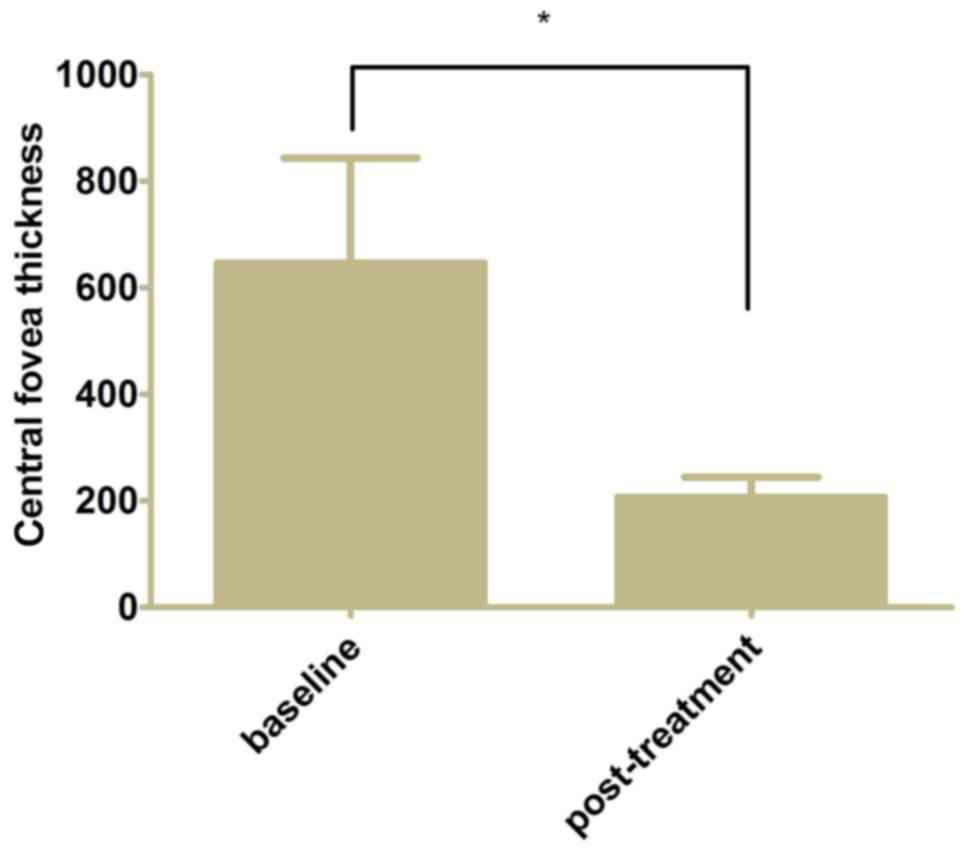
0.34±0.24 from 0.60±0.26 at baseline (Fig. 2, P<0.05). The mean CFT decreased
to 206.7±37.6 µm from 646.4±197.0 µm at baseline following 3
monthly ranibizumab injections and further 3 months of follow-up
(Fig. 3, P<0.05).
Table II shows the
general characteristics, BCVA, OCT, and FA data for the good
function and poor function groups at baseline. There was no
significant difference in the general characteristics between the
groups, while significant differences in the baseline ELM
integrity, ellipsoid zone integrity, and BCVA between the groups
were observed. The results revealed the baseline ELM integrity,
ellipsoid zone integrity, and BCVA of the good function were
significantly better than those of the poor function group
(P<0.01). Differences in the baseline CFT and FA data between
groups were found but were not statistically significant.
 | Table II.Comparison of baseline characteristics
between good function and poor function group. |
Table II.
Comparison of baseline characteristics
between good function and poor function group.
|
| Good function
group | Poor function
group |
|
|---|
|
|
|
|
|
|---|
| Baseline
predictors | N=19 | N=12 | P-value |
|---|
| Age (years) | 60.1±10.1 | 63.5±9.2 | 0.354 |
| Sex |
|
| 0.123 |
| Male | 10 | 3 |
|
|
Famale | 9 | 9 |
|
| Eye |
|
| 0.206 |
|
Left | 10 | 9 |
|
|
Right | 9 | 3 |
|
| Type |
|
| 0.179 |
|
CRVO | 8 | 8 |
|
|
BRVO | 11 | 4 |
|
| Duration of
symptoms (days) | 88.7±84.9 | 128.2±93.7 | 0.236 |
| Initial ELM
integrity | I8, II9, III2,
IV0 | I2, II0, III6,
IV4 | 0.001a |
| Initial ellipsoid
zone integrity | I5, II9, III5,
IV0 | I1, II0, III6,
IV5 |
<0.01a |
| Baseline central
fovea thickness (µm) | 640.2±143.7 | 656.2±268.1 | 0.951 |
| Baseline BCVA | 0.47±0.13 | 0.82±0.26 |
<0.01a |
| Hemorrhage under
the fovea (Yes/No) |
|
| 0.949 |
|
Yes | 3 | 2 |
|
| No | 16 | 10 |
|
|
Ischaemic/non-ischaemic type |
|
| 0.981 |
|
Yes | 8 | 5 |
|
| No | 11 | 7 |
|
| Intact/broken
foveal capillary ring |
|
| 0.762 |
|
Yes | 4 | 2 |
|
| No | 15 | 10 |
|
Univariate and multivariate regression analyses were
performed to determine the baseline factors significantly
associated with post-treatment BCVA in all patients. Univariate
regression analyses showed that the ellipsoid zone, ELM, baseline
BCVA, sex, and RVO type were associated significantly with
post-treatment BCVA (P<0.05). We then performed stepwise
multivariate regression analyses to determine the baseline factors
independently associated with post-treatment BCVA. The result
showed that the ELM integrity and the baseline BCVA were the
independent factors associated with post-treatment BCVA (B=0.149,
P<0.01; B=0.262, P=0.045, respectively). Both were positively
associated with post-treatment BCVA. Tables III and IV show the detailed results of the
regression analysis.
 | Table III.Univariate analysis results of
baseline predictors for post-treatment BCVA. |
Table III.
Univariate analysis results of
baseline predictors for post-treatment BCVA.
|
|
| Post-treatment
BCVA |
|---|
|
|
|
|
|---|
| Baseline
predictors | N/mean ± SD | B (95% CI) | P-value |
|---|
| Age (years) | 61.4±9.7 | 0.003 (−0.006,
0.013) | 0.460 |
| Sex |
| 0.188 (0.018,
0.358) | 0.032 |
|
Male | 13 |
|
|
|
Female | 18 |
|
|
| Eye |
| −0.138 (−0.317,
0.042) | 0.127 |
|
Left | 19 |
|
|
|
Right | 12 |
|
|
| Type |
| −0.174 (−0.344,
−0.004) | 0.045b |
|
CRVO | 16 |
|
|
|
BRVO | 15 |
|
|
| Duration of
symptoms (days) | 104.0±89.0 | 0.000 (−0.001,
0.001) | 0.544 |
| Initial ELM
integrity | I 10, II 9, III 8,
IV 4 | 0.189 (0.138,
0.241) |
<0.01a |
| Initial ellipsoid
zone integrity | I 6, II 9, III 11,
IV 5 | 0.186 (0.126,
0.246) |
<0.01a |
| Baseline central
fovea thickness (µm) | 646.4±197.0 | 0.000 (0.000,
0.001) | 0.124 |
| Baseline BCVA | 0.60±0.26 | 0.642 (0.374,
0.909) |
<0.01a |
| Hemorrhage under
the fovea |
| −0.024 (−0.272,
0.223) | 0.841 |
|
Yes | 5 |
|
|
| No | 26 |
|
|
|
Ischaemic/non-ischaemic type |
| −0.008 (−0.192,
0.177) | 0.932 |
|
Yes | 13 |
|
|
| No | 18 |
|
|
| Intact/broken
foveal capillary ring |
| 0.040 (−0.190,
0.270) | 0.726 |
|
Yes | 6 |
|
|
| No | 25 |
|
|
 | Table IV.Multivariate analysis results of
baseline predictors for post-treatment BCVA. |
Table IV.
Multivariate analysis results of
baseline predictors for post-treatment BCVA.
|
|
| Post-treatment
BCVA |
|---|
|
|
|
|
|---|
| Baseline
predictors | N/mean ± SD | B (95% CI) | P-value |
|---|
| Initial ELM
integrity | I 10, II 9, III 8,
IV 4 | 0.149 (0.087,
0.212) | <0.01 |
| Baseline BCVA | 0.60±0.26 | 0.262 (0.007,
0.517) | 0.045 |
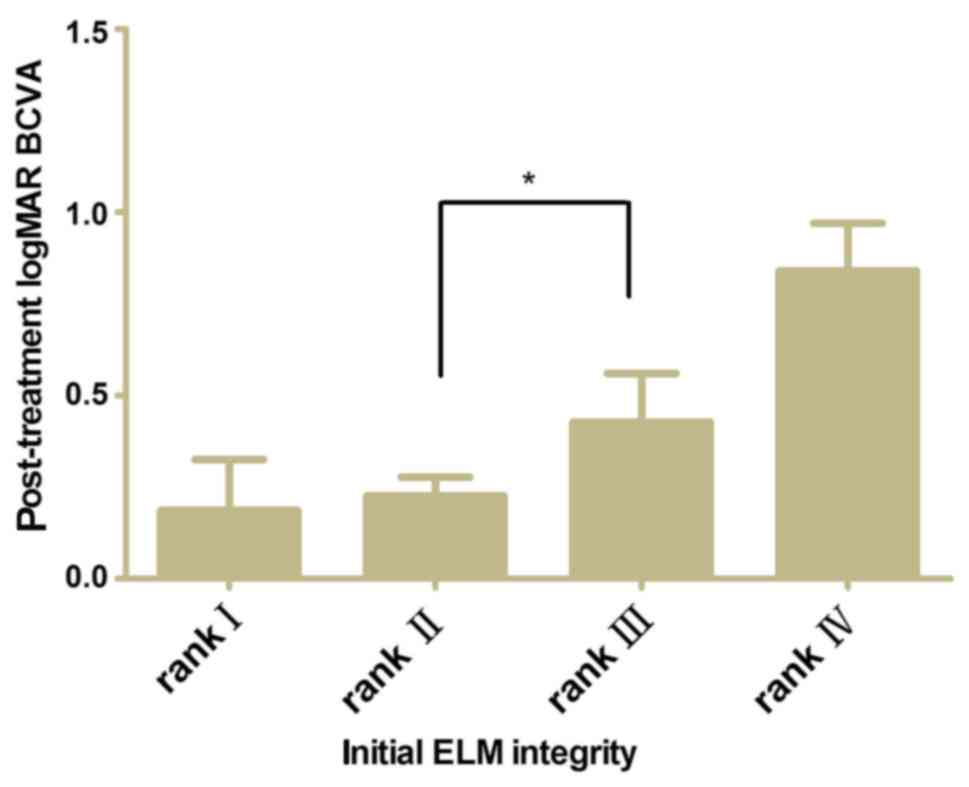
A comparison of post-treatment BCVA between the ELM
ranks after adjusting for baseline BCVA was performed. The baseline
BCVA was shown to be associated with post-treatment BCVA
independently. Fig. 4 shows the
post-treatment BCVA difference between ranks II and III was
significant (P<0.05).
Discussion
In the present study, we investigated the baseline
factors with an original ranking on OCT images to predict
post-treatment BCVA after ranibizumab treatment in patients with ME
associated with RVO. Our results showed the ELM integrity and the
baseline BCVA were the independent factors that predict
post-treatment BCVA, indicating patients with good baseline ELM
integrity in particular beneath the center of the fovea and
baseline BCVA would obtain good post-treatment BCVA after
intravitreal VEGF inhibitor therapy.
RVO is an important cause of visual impairment, and
ME secondary to RVO is the second most common major retinal
vascular disease after DR 3–5. Previously, there were no effective
treatments for ME secondary to CRVO, while only grid laser
photocoagulation was available to treat ME secondary to BRVO, but
it reduced edema very slowly and provided benefit for only a few
patients (29,30). In 2009, the Standard Care vs.
Corticosteroid for Retinal Vein Occlusion (SCORE) study recommended
1 mg intravitreal triamcinolone acetonide (TA) for ME secondary to
CRVO, although the risk of cataract and high intraocular pressure
increased. TA injections were not superior to grid laser for ME
secondary to BRVO (31). High VEGF
concentrations were present in the eyes of patients with RVO,
resulting in neovascularization and ME, and VEGF inhibitors can
block this pathogenesis, representing the safe, latest, and
effective treatment for RVO. VEGF inhibitors included bevacizumab
and ranibizumab, which were reported to be superior in BCVA gains
and CFT decrease to other treatment. Among these VEGF inhibitors,
ranibizumab have been approved in the United States and the
European Union for the treatment of ME secondary to RVO (7,14–21).
However, not all patients benefit from VEGF
inhibitors, and sometimes, BCVA does not improve even if there is a
significant decrease in CFT 2. Several studies have been conducted
to identify predictive factors for good treatment response, and
some baseline factors were thought to contribute to post-treatment
BCVA after intravitreal injections of anti-VEGF agent for patients
with ME due to RVO. The factors included age, baseline BCVA,
ischemic areas, response to first injection, duration of occlusion,
history of hypertension, hemorrhage under the fovea, and baseline
OCT findings, which were thought to be one of most important
predictors (2,10,26,32).
Today, images with high resolution of the neural
retina can be obtained in a non-invasive manner with OCT scanning,
and the microstructure of the retina such as the ellipsoid zone,
ELM, and RPE can be defined on OCT imaging (23). Changes in the macular microstructure
can be detected by OCT in most eyes with RVO during an early stage
and are believed to be important predictors for post-treatment BCVA
after intravitreal injections of an anti-VEGF agent (22,27,28,33,34).
In some studies, the CFT measured with OCT was found
to be able to predict the post-treatment BCVA outcome in ME due to
RVO after anti-VEGF agent injections (33,34).
However, some researchers concluded that the correlation between
baseline CFT and BCVA after anti-VEGF agent injections was not
significant (35). Similarly, a
contradictory conclusion regarding the association between baseline
CFT and BCVA after VEGF inhibitor injections in patients with AMD
appeared. To interpret this contradiction, Oishi et al
(36) pointed out the pattern of
correlation was V-shaped, and there was a negatively linear
correlation in eyes with CRT >203 µm and a positively linear
trend in eyes with CRT ≤203 µm. However, in the present study, the
baseline CFT was more than 203 µm, and the correlation was not
significant.
Previously, the integrity of the ellipsoid zone and
the ELM was shown to be significantly associated with
post-treatment BCVA after anti-VEGF agent injections in patients
with RVO and AMD (22,24,25,28,35,36).
Some studies demonstrated the integrity of the ellipsoid zone was
more highly associated with post-treatment BCVA than the ELM
(24,28,35);
however, some studies reported the ELM was more useful in the
prediction of post-treatment BCVA (22,25,36). In
the present study, the integrity of the ellipsoid zone and the ELM
correlated significantly with post-treatment BCVA in univariate
regression analysis, respectively, but the integrity of the
ellipsoid zone was excluded from the independent variables in
multivariate regression analysis. We found the ELM was more useful
in the prediction of post-treatment BCVA in patients with ME due to
RVO, and we agreed with the interpretation that ellipsoid zone
status may be too sensitive to evaluate diseases that cause severe
retinal damage such as AMD, retinal detachment (RD), and RVO 36.
The ELM may be more useful in the evaluation of retinal damage of
ME secondary to RVO than the ellipsoid zone.
This study revealed a significant correlation of
baseline BCVA and post-treatment BCVA after intravitreal VEGF
inhibitor injections for ME secondary to RVO in univariate and
multivariate regression analysis, in accordance with previous
studies (26).
The strengths of our study are as follows: i) The
bias resulting from the type of agents was controlled; ranibizumab
was the single anti-VEGF agent for intravitreal injections unlike
most previous studies and ii) previously, the extent of the
ellipsoid zone and ELM damage was assessed mainly by dividing the
hyperreflective line into completely visible, partially visible,
and invisible. Thus, the important effect of integrity beneath the
center of the fovea was not considered adequately. In addition, the
previous assessments were mainly based on post-treatment OCT
imaging instead of baseline OCT imaging, so they could not be real
predictors. In contrast, in the present study the integrity of the
ELM and the ellipsoid zone at baseline was categorized into four
ranks: i) Completely visible line), ii) partially detectable line
with undamaged center of fovea), iii) partially detectable line
with damaged center of fovea, and iv) completely invisible line.
The results revealed the post-treatment BCVA in ELM rank II was
significantly better than that of ELM rank III (P<0.05), which
was attributed to the important effect of the ELM integrity beneath
the center of the fovea.
In this study, we did not find a significant
association between FA data and post-treatment BCVA, perhaps
because the evaluation of the ischemia severity from baseline FA
data was difficult, and nonischemic types could be incorrectly
assessed as it would become ischemic type later. Of course, the
small sample size and the retrospective design of this study might
have affected our findings. Additional prospective investigation
especially with large samples are needed to illuminate the
predictors for BCVA after anti-VEGF agent treatment in patients
with ME secondary to RVO.
In conclusion, the ELM integrity and the baseline
BCVA may be more useful than other factors in the prediction of the
post-treatment BCVA of patients with ME associated with RVO after
intravitreal injections of ranibizumab, and the ELM integrity
beneath the center of fovea should be the focus to predict
post-treatment BCVA.
Acknowledgements
The present study was supported by Xuzhou Technology
Program (XM13B077).
References
|
1
|
Huang P, Song Z and Sun X: Predictors of
anti-vascular endothelial growth factor treatment responses in
macular edema following central vein occlusion. Chin Med J (Engl).
127:3019–3023. 2014.PubMed/NCBI
|
|
2
|
Huang P, Niu W, Ni Z, Wang R and Sun X: A
meta-analysis of anti-vascular endothelial growth factor remedy for
macular edema secondary to central retinal vein occlusion. PLoS
One. 8:e824542013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McIntosh RL, Rogers SL, Lim L, Cheung N,
Wang JJ, Mitchell P, Kowalski JW, Nguyen HP and Wong TY: Natural
history of central retinal vein occlusion: An evidence-based
systematic review. Ophthalmology. 117:1113–1123. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rogers SL, McIntosh RL, Lim L, Mitchell P,
Cheung N, Kowalski JW, Nguyen HP, Wang JJ and Wong TY: Natural
history of branch retinal vein occlusion: An evidence-based
systematic review. Ophthalmology. 117:1094–1101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rogers S, McIntosh RL, Cheung N, Lim L,
Wang JJ, Mitchell P, Kowalski JW, Nguyen H and Wong TY:
International eye disease consortium: The prevalence of retinal
vein occlusion: Pooled data from population studies from the United
States, Europe, Asia and Australia. Ophthalmology. 117:313–319.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenfeld PJ, Fung AE and Puliafito CA:
Optical coherence tomography findings after an intravitreal
injection of bevacizumab (avastin) for macular edema from central
retinal vein occlusion. Ophthalmic Surg Lasers Imaging. 36:336–339.
2005.PubMed/NCBI
|
|
7
|
Brown DM, Campochiaro PA, Singh RP, Li Z,
Gray S, Saroj N, Rundle AC, Rubio RG and Murahashi WY; Cruise
Investigators, : Ranibizumab for macular edema following central
retinal vein occlusion: Six-month primary end point results of a
phase III study. Ophthalmology. 117:1124–1133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Demir M, Oba E, Gulkilik G, Odabasi M and
Ozdal E: Intravitreal bevacizumab for macular edema due to branch
retinal vein occlusion: 12-month results. Clin Ophthalmol.
5:745–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Figueroa MS, Contreras I, Noval S and
Arruabarrena C: Results of bevacizumab as the primary treatment for
retinal vein occlusions. Br J Ophthalmol. 94:1052–1056. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallego-Pinazo R, Dolz-Marco R,
Pardo-Lopez D, Martinez-Castillo S, Lleo-Perez A, Arevalo JF and
Diaz-Llopis M: Ranibizumab for serous macular detachment in branch
retinal vein occlusions. Graefes Arch Clin Exp Ophthalmol.
251:9–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prager F, Michels S, Kriechbaum K,
Georgopoulos M, Funk M, Geitzenauer W, Polak K and Schmidt-Erfurth
U: Intravitreal bevacizumab (Avastin) for macular oedema secondary
to retinal vein occlusion: 12-month results of a prospective
clinical trial. Br J Ophthalmol. 93:452–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Spaide RF, Chang LK, Klancnik JM, Yannuzzi
LA, Sorenson J, Slakter JS, Freund KB and Klein R: Prospective
study of intravitreal ranibizumab as a treatment for decreased
visual acuity secondary to central retinal vein occlusion. Am J
Ophthalmol. 147:298–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gregori NZ, Rattan GH, Rosenfeld PJ,
Puliafito CA, Feuer W, Flynn HW Jr, Berrocal AM, Al-Attar L, Dubovy
S, Smiddy WE, et al: Safety and efficacy of intravitreal
bevacizumab (avastin) for the management of branch and hemiretinal
vein occlusion. Retina. 29:913–925. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Boyd SR, Zachary I, Chakravarthy U, Allen
GJ, Wisdom GB, Cree IA, Martin JF and Hykin PG: Correlation of
increased vascular endothelial growth factor with
neovascularization and permeability in ischemic central vein
occlusion. Arch Ophthalmol. 120:1644–1650. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Campochiaro PA, Brown DM, Awh CC, Lee SY,
Gray S, Saroj N, Murahashi WY and Rubio RG: Sustained benefits from
ranibizumab for macular edema following central retinal vein
occlusion: Twelve-month outcomes of a phase III study.
Ophthalmology. 118:2041–2049. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campochiaro PA, Heier JS, Feiner L, Gray
S, Saroj N, Rundle AC, Murahashi WY and Rubio RG; BRAVO
Investigators, : Ranibizumab for macular edema following branch
retinal vein occlusion: Six-month primary end point results of a
phase III study. Ophthalmology. 117:1102–1112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Campochiaro PA, Sophie R, Pearlman J,
Brown DM, Boyer DS, Heier JS, Marcus DM, Feiner L and Patel A;
RETAIN Study Group, : Long-term outcomes in patients with retinal
vein occlusion treated with ranibizumab: The RETAIN study.
Ophthalmology. 121:209–219. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Glanville J, Patterson J, McCool R,
Ferreira A, Gairy K and Pearce I: Efficacy and safety of widely
used treatments for macular oedema secondary to retinal vein
occlusion: A systematic review. BMC Ophthalmol. 14:72014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kinge B, Stordahl PB, Forsaa V, Fossen K,
Haugstad M, Helgesen OH, Seland J and Stene-Johansen I: Efficacy of
ranibizumab in patients with macular edema secondary to central
retinal vein occlusion: Results from the sham-controlled ROCC
study. Am J Ophthalmol. 150:310–314. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Regnier SA, Larsen M, Bezlyak V and Allen
F: Comparative efficacy and safety of approved treatments for
macular oedema secondary to branch retinal vein occlusion: A
network meta-analysis. BMJ Open. 5:e0075272015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thom HH, Capkun G, Nixon RM and Ferreira
A: Indirect comparisons of ranibizumab and dexamethasone in macular
oedema secondary to retinal vein occlusion. BMC Med Res Methodol.
14:1402014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wolf-Schnurrbusch UE, Ghanem R,
Rothenbuehler SP, Enzmann V, Framme C and Wolf S: Predictors of
short-term visual outcome after anti-VEGF therapy of macular edema
due to central retinal vein occlusion. Invest Ophthalmol Vis Sci.
52:3334–3337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keane PA and Sadda SR: Predicting visual
outcomes for macular disease using optical coherence tomography.
Saudi J Ophthalmol. 25:145–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ota M, Tsujikawa A, Murakami T, Kita M,
Miyamoto K, Sakamoto A, Yamaike N and Yoshimura N: Association
between integrity of foveal photoreceptor layer and visual acuity
in branch retinal vein occlusion. Br J Ophthalmol. 91:1644–1649.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin HJ, Chung H and Kim HC: Association
between integrity of foveal photoreceptor layer and visual outcome
in retinal vein occlusion. Acta Ophthalmol. 89:e35–e40. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jaissle GB, Szurman P, Feltgen N, Spitzer
B, Pielen A, Rehak M, Spital G, Heimann H and Meyer CH: Retinal
vein occlusion study group: Predictive factors for functional
improvement after intravitreal bevacizumab therapy for macular
edema due to branch retinal vein occlusion. Graefes Arch Clin Exp
Ophthalmol. 249:183–192. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhisitkul RB, Campochiaro PA, Shapiro H
and Rubio RG: Predictive value in retinal vein occlusions of early
versus late or incomplete ranibizumab response defined by optical
coherence tomography. Ophthalmology. 120:1057–1063. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sakamoto A, Tsujikawa A, Ota M, Yamaike N,
Kotera Y, Miyamoto K, Kita M and Yoshimura N: Evaluation of
potential visual acuity in eyes with macular oedema secondary to
retinal vein occlusion. Clin Experiment Ophthalmol. 37:208–216.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Clarkson JG, Chuang E, Gass D, Pedroso M,
Cubillas T, Duria ES, Hess DJ, Rams I, Ball M, Gutierrez A, et al:
Evaluation of grid pattern photocoagulation for macular edema in
central vein occlusion. The central vein occlusion study group M
report. Ophthalmology. 102:1425–1433. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Battaglia Parodi M, Saviano S and Ravalico
G: Grid laser treatment in macular branch retinal vein occlusion.
Graefes Arch Clin Exp Ophthalmol. 237:1024–1027. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ip MS, Scott IU, Van Veldhuisen PC, Oden
NL, Blodi BA, Fisher M, Singerman LJ, Tolentino M, Chan CK and
Gonzalez VH: A randomized trial comparing the efficacy and safety
of intravitreal triamcinolone with observation to treat vision loss
associated with macular edema secondary to central retinal vein
occlusion: The standard care vs corticosteroid for retinal vein
occlusion (SCORE) study report 5. Arch Ophthalmol. 127:1101–1114.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao L, Li B, Feng K, Han L, Ma Z and Liu
Y: Bevacizumab treatment for acute branch retinal vein occlusion
accompanied by subretinal hemorrhage. Curr Eye Res. 40:752–756.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ach T, Hoeh AE, Schaal KB, Scheuerle AF
and Dithmar S: Predictive factors for changes in macular edema in
intravitreal bevacizumab therapy of retinal vein occlusion. Graefes
Arch Clin Exp Ophthalmol. 248:155–159. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hoeh AE, Ruppenstein M, Ach T and Dithmar
S: OCT patterns of macular edema and response to bevacizumab
therapy in retinal vein occlusion. Graefes Arch Clin Exp
Ophthalmol. 248:1567–1572. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang HM, Chung EJ, Kim YM and Koh HJ:
Spectral-domain optical coherence tomography (SD-OCT) patterns and
response to intravitreal bevacizumab therapy in macular edema
associated with branch retinal vein occlusion. Graefes Arch Clin
Exp Ophthalmol. 251:501–508. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oishi A, Hata M, Shimozono M, Mandai M,
Nishida A and Kurimoto Y: The significance of external limiting
membrane status for visual acuity in age-related macular
degeneration. Am J Ophthalmol. 150:27–32. 2010. View Article : Google Scholar : PubMed/NCBI
|