Introduction
An increasing number of athletes are suffering from
sports-associated diseases in the competitive sports. For
professional female athletes, the incidence of athletic amenorrhea
(AA) is particularly high, at a rate of 65–69% in athletes such as
dancers and distance runners compared with 2–5% in normal women
(1). The high AA in female athletes
suggests that achieving pregnancy may be challenging. The current
treatment methods primarily including increasing weight and
decreasing exercise, replenishing vitamins and minerals, and
reinforcing strength training (2).
The normal menstrual cycle is regulated by the
hypothalamic-pituitary-ovarian axis, and the ovary is one of the
most important organs in this axis (3). Thus, it is important to investigate the
underlying mechanism of ovary dysfunction in AA.
The mitochondrion is the organelle of energy supply
in eukaryotic organisms. Mitochondrial abnormity or dysfunction has
been reported to be involved in various diseases (4). To some of these diseases, the
dysfunction of mitochondrion is considered as a key mechanism to
cause the dysfunction of the organ, thus it was conjectured that
the dysfunction of mitochondrion may cause the dysfunction of
ovary, which may result in AA. The number of mitochondria and the
mitochondrial DNA (mtDNA) copy number may vary among different
types of cells and tissues. Furthermore, the mitochondria and mtDNA
copy number may be altered during cell differentiation and aging.
It has also been reported in previous studies that the alteration
of mtDNA participates in the regulation of ovarian function
(5–8). Therefore, it is likely that the
dysfunction of the mitochondrion may be associated AA.
Thus, the present study aimed to describe the
alterations of the mitochondria in the ovaries of AA rats,
morphologically and at the molecular level. In addition, the study
attempted to investigate the possible mechanism underlying ovary
dysfunction by determining the mitochondrial variations.
Materials and methods
Animals and experimental design
A total of 40 healthy female Sprague Dawley rats
(3-month-old, weight, 250±20 g) with a normal menstrual cycle were
obtained from the West China Medical Experimental Animal Center of
Sichuan University (Chengdu, China). All animals used in the
present study were kept under a 12-h light/dark cycles at a
constant temperature and humidity (25±2°C, 60–75%) with free access
to chow and water. The animal procedures were approved by the
Animal Use and Care Committee of Sichuan University and conducted
according to the regulations.
The animals were randomly divided into the control
and AA groups (n=20 in each group). Rats in the control group were
maintained under a 12-h light/dark cycles at a constant temperature
and humidity (25±2°C, 60–75%), without swimming training, while
rats in the AA group were subjected to excessive swimming for 2 h
per day for 6 days per week (with 1-day break) for 3 weeks. Vaginal
exfoliative cystoscopy was conducted at 8:00-9:00 a.m. each day for
observation of any possible alterations in the menstrual cycle.
Specimen collection
After excessive swimming training for 3 weeks, the
rats were sacrificed and the ovary tissues were collected. Half of
the ovary was fixed in 4% neutral buffered paraformaldehyde at 4°C
for at least 24 h for histopathological and immunohistochemical
examinations. The samples were cut into 5-µm-thick sections.
Hematoxylin and eosin staining was performed for histopathological
exam at 25°C and slides were observed under the microscope. Images
were captured at a magnification of ×100. The remaining part of the
ovary was immediately frozen in liquid nitrogen and stored at −80°C
for mRNA and protein expression analysis. Furthermore, serum
samples were also collected and stored at −80°C for reproductive
hormones analysis. The ratio (ovary weight/body weight ×100%) was
also calculated for each rat. Samples for TEM were fixed in 2.5%
glutaraldehyde at 4°C for at least 12 h. Sections (0.05-µm-thick)
were stained with 0.5% lead citrate for 20–30 min at 20°C. Images
were captured at a magnification of ×12,000.
Detection of reproductive hormones in
the serum by radioimmunoassay
The concentration of reproductive hormones in the
serum, including estradiol (E2), progesterone (P),
follicle-stimulating hormone (FSH), luteotropic hormone (LH) and
testosterone (T), was measured and quantified using
radioimmunoassay kits (Jingmei Life Sciences Inc., Beijing, China),
according to the manufacturer's protocol.
Determination of
reproduction-associated factors in ovarian sections by
immunohistochemical assay
The deparaffinated ovarian tissue sections
(5-µm-thick) were incubated with 3% H2O2 at
37°C for 10 min to quench the endogenous peroxidase. Subsequent to
blocking by 10% goat serum at room temperature for 20 min, the
sections were incubated with primary antibodies, including 17-b
estradiol (E2; dilution: 1:300; cat. no. sc-69958; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), estrogen receptor (ER;
dilution: 1:250; cat. no. A000786; Boster Biological Technology,
Ltd., Wuhan, China), progesterone receptor (PR; dilution: 1:300;
cat. no. PB0077; Boster Biological Technology, Ltd.) overnight at
4°C, followed by incubation with horseradish-peroxidase
(HRP)-conjugated secondary antibodies (cat. nos. SP-9001 or
SP-9002; OriGene Technologies, Inc., Beijing, China) at 37°C for 30
min. Subsequently, the signals were detected using a
diaminobenzidine substrate kit (cat. nos. SP-9001 or SP-9002;
OriGene Technologies, Inc.). Positive staining in the images was
indicated by brown staining in the cytoplasm or nucleus. Five
fields-of-view were randomly selected for assessment in each tissue
section. The images were observed and captured using a white-light
microscope at a magnification of ×400.
Analysis of mtDNA copy number and NADH
dehydrogenase subunit 2 (ND2) mRNA expression in rat ovarian
tissues
The mtDNA copy number was measured by qPCR using a
SYBR™ Green PCR Master Mix (cat. no. 4309155; Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Briefly, the DNA specimens were prepared using the TIANamp Genomic
DNA kit (cat. no. DP304; Tiangen Biotech Co., Ltd., Beijing,
China). The mtDNA copy number was assessed by amplification of the
mitochondrial D-loop against the reference gene, GAPDH. The primers
used were as follows: D-loop forward, 5′-GGTTCTTACTTCAGGGCCATCA-3′
and reverse 5′-GATTAGACCCGTTACCATCGAGAT-3′; GAP DH, forward
5′-CGGGAAATCGTGCGTGACAT-3′ and reverse 5′-GAAGGAAGGCTGGAAGAGTG-3′.
The following PCR cycling conditions were performed: An activation
step at 95°C for 3 min, followed by 35 cycles of 25 sec at 95°C, 25
sec at 56°C and 30 sec at 72°C.
For ND2 mRNA detection, total RNA was extracted with
TRIzol™ Reagent (cat. no. 15596018; Thermo Fisher Scientific,
Inc.), and the concentration and quality of total RNA was measured
with a spectrophotometer. First strand cDNA was obtained using a
RevertAid First Strand cDNA Synthesis Kit (cat. no. K1622; Thermo
Fisher Scientific, Inc.). The mRNA expression levels of ND2 were
detected by qPCR using the following primers: ND2, forward
5′-TTATCCTCTTATCCGTCCTC-3′ and reverse 5′-TGTTAAGTCGAAGGGAGC-3′.
The expression levels of genes were calculated using the
2−ΔΔCq method (6). The
relative ratio of the D-loop and GAPDH expression was used as the
relative abundance of the mitochondrial genome.
Quantification of ND2 protein
expression in rat ovarian tissues by western blot analysis
Frozen ovarian tissues were homogenized and whole
proteins were extracted by ice-cold radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Nanjing, China). A BCA
kit was used to quantify the protein concentration. Equal amounts
of protein (50 µg) from each sample were resolved by 12% SDS-PAGE,
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA) and blocked with 5% non-fat powdered milk in
Tris-buffered saline/Tween 20 (containing 20 mM Tris-HCl, pH 7.5,
150 mM NaCl and 0.1% Tween-20). The membranes were then incubated
with goat anti-ND2 polyclonal antibody (dilution: 1:500; cat. no.
sc-20496; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 2 h
at room temperature, followed by incubation with an HRP-conjugated
rabbit anti-goat IgG secondary antibody (dilution: 1:10,000; cat.
no. SA00001-4; ProteinTech Group, Inc., Wuhan, China) for 1.5 h at
room temperature. For GAPDH detection, membranes were incubated
with HRP-conjugated GAPDH antibody (dilution: 1:20,000; cat. no.
HRP-60004; ProteinTech Group, Inc.) for 2 h at room temperature.
The membranes were then visualized using an enhanced
chemiluminescence detection kit (Beyotime Institute of
Biotechnology). The protein expression was normalized to that of
GAPDH, which was used as an internal control.
Statistical analysis
All data are expressed as the mean ± standard
deviation, and statistical calculations were performed with the
statistical package SPSS version 18.0 (SPSS, Inc., Chicago, IL,
USA). For comparisons between the two groups, Student's t-test was
applied. The presence of a statistically significant difference was
indicated by P<0.05.
Results
Morphological variations to ovaries in
the AA group
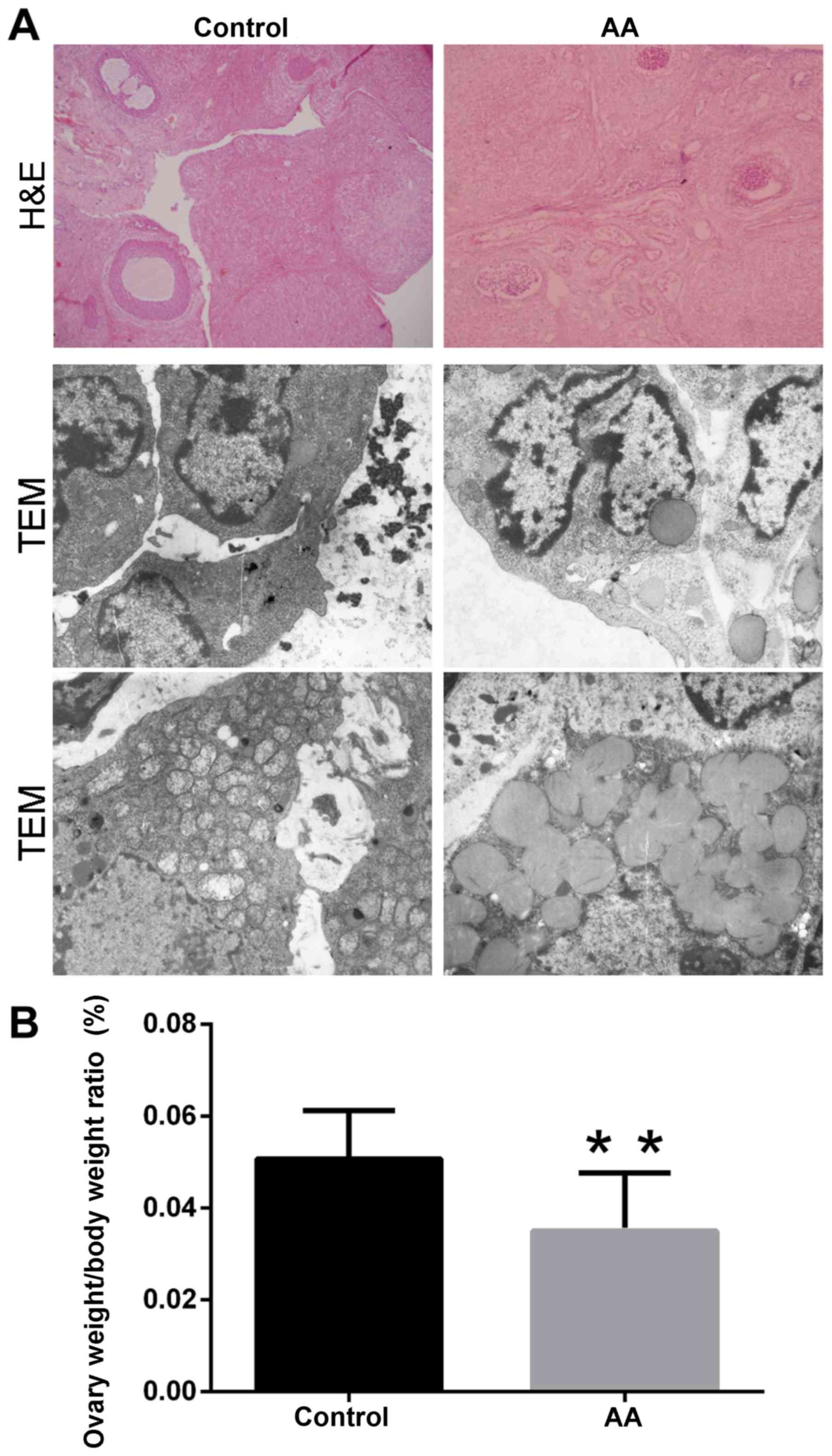
The gross morphological features of the rat ovaries
were examined by hematoxylin and eosin staining and transmission
electron microscopy (TEM) following sacrifice at week 3 (Fig. 1A). Several ovarian follicles and
corpus luteum structures were identified in the ovaries of the
control group. By contrast, evidently reduced ovarian follicles and
corpus luteum structures were observed in the AA group. These
observations were also confirmed by hematoxylin and eosin staining.
Furthermore, the ultrastructure of the mitochondria was also
observed. In the AA group, the mitochondria presented marked
abnormal alterations, including swelling of the mitochondrial
membrane, disappearance of the mitochondrial cristae and decrease
of the mitochondrial number, in comparison with the control group.
However, no evident changes in other structures were detected
between the two groups (Fig. 1A).
Notably, the ovary weight/body weight ratio of the AA group was
significantly decreased when compared with that in the control
group (0.0350±0.0127% vs. 0.0507±0.0105%, respectively; P<0.01;
Fig. 1B).
Decrease in the reproductive hormone
levels in the ovaries of AA rats
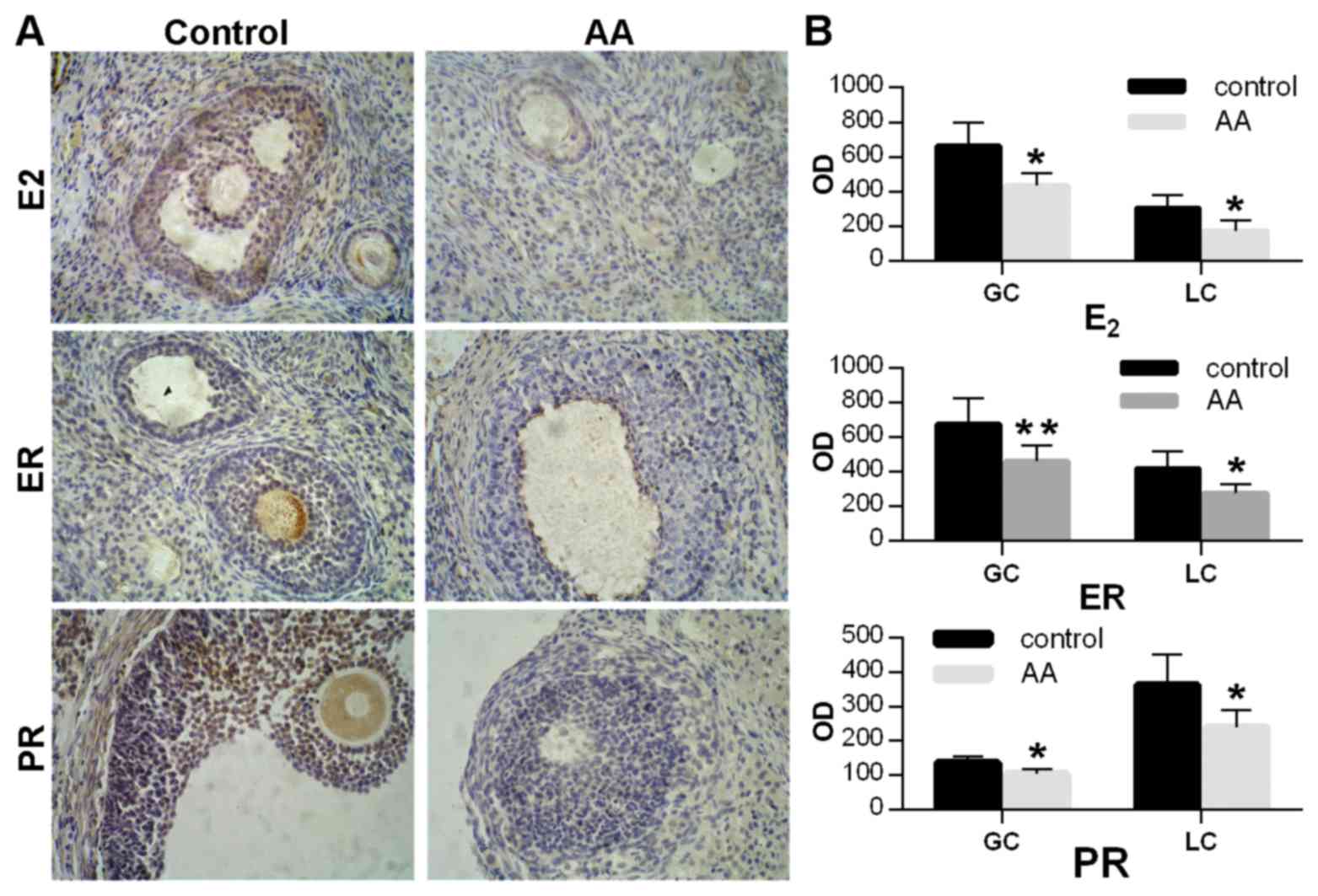
Immunohistochemical assay was applied to detect the
expression of reproductive hormones in the ovarian tissues,
including E2, ER and PR (Fig.
2A). The expression levels of these factors were calculated in
both the granular and luteal cells of the ovaries. Compared with
the control group, the optical density in the immunohistochemical
images of these hormones was significantly decreased in the AA
group in the two cell types (P<0.05 or P<0.01; Fig. 2B).
Reduction of serum reproductive
hormone levels in AA rats
Radioimmunoassay was performed in order to detect
the expression levels of five reproductive hormones in the rat
serum, including E2, P, FSH, LH and T. The levels of
these hormones were significantly decreased in the AA group when
compared with the control group (P<0.05; Table I). These results indicate depressed
ovarian function and reproductive capability in the AA rats.
 | Table I.Reproductive hormone levels in the rat
serum. |
Table I.
Reproductive hormone levels in the rat
serum.
| Group | n | E2
(ng/ml) | P (pg/ml) | FSH (mIU/ml) | LH (mIU/ml) | T (ng/dl) |
|---|
| Control | 20 |
1.56±0.48 |
73.81±23.42 |
8.57±1.22 |
2.13±0.30 |
19.41±4.51 |
| AA | 20 |
1.27±0.34a |
45.70±18.44a |
7.34±0.99a |
1.32±0.24a |
15.60±3.07a |
Decrease of relative mtDNA copy number
in AA ovarian tissues
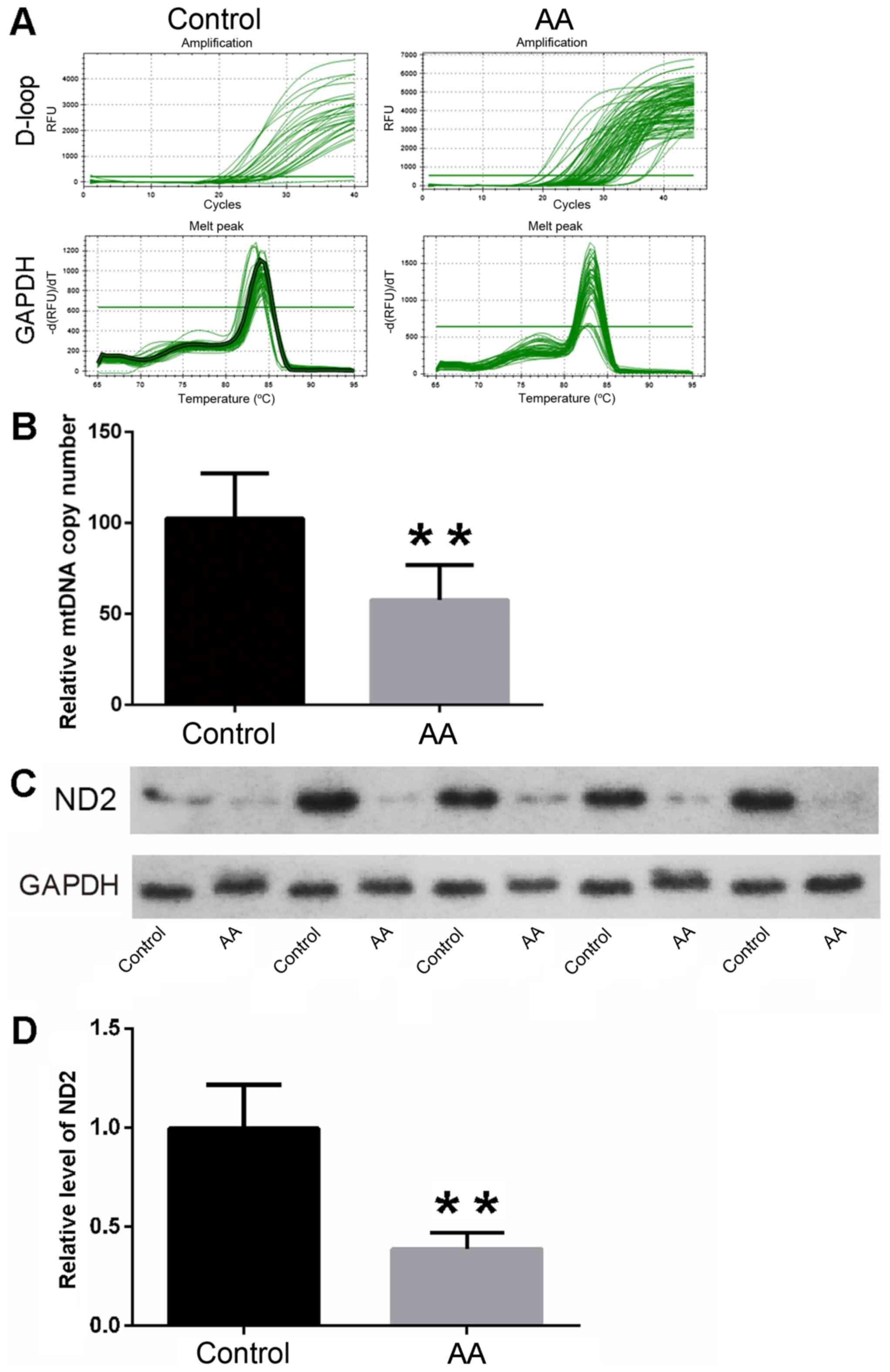
The mtDNA copy number of ovarian tissues was
quantified in the AA and control rats. Following statistical
analysis, the results revealed that the relative mtDNA copy numbers
of ovarian tissues were significantly decreased in the AA rats
compared with the control rats (57.65±19.20 vs. 102.42±24.92,
respectively; Fig. 3A and B;
P<0.01). The results indicated that the function level of the
mitochondria was higher in control group than that in AA group.
Reduction of ND2 expression in AA rat
ovarian tissues
The expression of ND2 was analyzed by western blot
analysis to evaluate the activity of mitochondria in the ovarian
tissues of AA rats. Following statistical analysis, the results
revealed that the relative protein levels of ND2 in the AA group
were markedly decreased when compared with those in the control
group. (Fig. 3C and D; P<0.01).
These findings suggested that the oxidative phosphorylase activity
was higher in the control group, which indicated the energy
metabolism level was higher in control group.
Discussion
The incidence of AA is increasing worldwide, and
this condition is one of the leading causes of infertility in
female athletes (9). However, the
underlying mechanisms and effective treatments of AA remain
unclear. In the present study, the results demonstrated a decrease
in the mtDNA copy number and protein level of ND2 in the ovaries of
AA rats. These results indicate that mitochondrial dysfunction may
be associated with the occurrence of AA.
The mitochondrion is the main organelle that
supplies energy to the cells. The mitochondrial number varies among
cells and tissues, and is associated with multiple functions.
Mitochondria contain their own DNA, and a mammalian cell contains
1,000–10,000 copies of mtDNA (10).
The mtDNA content is frequently used as a marker of mitochondrial
density, while it also reflects the oxidative or ATP-producing
capacity of tissues (11).
Alterations in the mtDNA, which are reflected by the change of its
copy number and its mutation, have been reported to be involved in
a series of diseases and in organ dysfunction (4). Several studies have reported that the
alterations of mtDNA are involved in the maturity, aging and lesion
formation in the ovary (12,13). Mutations in mtDNA can cause the
abnormally expression of oxidative phosphorylation complex and
abnormal transfer RNAs, which may be associated with polycystic
ovarian syndrome patients (12). A
previous study in pigs revealed that the copy number of mtDNA in
the ovaries increased gradually along with sexual maturity
(5). Devine et al (14) also reported that ~20% of patients
consulting for infertility presented signs of premature ovarian
aging. In addition, ovarian aging is associated with reduced oocyte
mtDNA content and mitochondrial dysfunction (13). Numerous studies have reported that
the mtDNA content in the oocytes of aged women or of women with a
diminished ovarian reserve is significantly lower when compared
with that of young patients or those with a normal ovarian reserve
(6–8). In the present study, the decreased copy
number of mtDNA in the AA rats coincided with the dysfunction of
the ovaries. The aforementioned results indicated that the decrease
of the mtDNA copy number may suppress the oxidative phosphorylation
process in ovarian cells and induce ovarian dysfunction, as well as
the maturity of oocytes. However, further studies are required to
clarify the underlying mechanisms.
NADH ubiquinone oxidoreductase (complex I) is the
largest complex of the mitochondrial respiratory chain, and is
composed by ~45 subunits. Of these, 7 subunits are directly encoded
by the mitochondrial genome, including ND1-ND6 and ND4L (15). ND2 is one of the subunits of NADH
that is a rate-limiting enzyme involved in oxidative
phosphorylation (16). In the
present study, it was observed that the protein level of ND2 was
significantly decreased in AA rats, as determined by western blot
analysis. In addition, the normal menstrual cycle and ovulation are
controlled directly by the hormones that are secreted from the
ovaries, including E2, ER, PR, P, FSH, LH and T
(17). The data of the present study
revealed that these hormones in the ovaries or serum were markedly
downregulated, which indicated the dysfunction of ovaries in the AA
group. Accordingly, it is suggested that the decrease in the mtDNA
copy number may induce the downregulation of ND2 expression and
then suppress the oxidative phosphorylation, thus resulting in a
lack of energy supply to the ovary and consequent ovarian
dysfunction.
The morphological alterations of ovaries in the AA
group observed by TEM confirmed that the mitochondria were the most
affected organelles. The current results also indicated that
mitochondrial dysfunction, particularly decreased mtDNA copy number
and ND2 expression, may be considered as an early event during the
development and/or progression of AA. Combined with the atrophy of
ovaries in AA group, it is speculated that the atrophy may be
associated with the mitochondrial dysfunction in female
athletes.
In conclusion, the findings of the present study
investigating AA rats suggest that excessive exercise may lead to
morphological variations in the ovary, particularly to
mitochondria. Furthermore, these morphological variations were
accompanied with mitochondrial function disorder through decreased
mtDNA content and ND2 expression. Thus, decrease in the mtDNA
content and ND2 expression may be considered as the possible
underlying mechanism of AA.
Acknowledgements
The authors would like to thank Mr. Xiao-Tao Zhou,
Mr. Liu-Qi Wu and Miss Cong-Cong Zhang from the Chengdu Sport
Institute (Chengdu, China), and Associate Professor Yan Fu from
Southwest University for Nationalities (Chengdu, China) for their
technical assistance.
References
|
1
|
Warren MP and Perlroth NE: The effects of
intense exercise on the female reproductive system. J Endocrinol.
170:3–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berz K and McCambridge T: Amenorrhea in
the female athlete: What to do and when to worry. Pediatr Ann.
45:e97–e102. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stafford DE: Altered
hypothalamic-pituitary-ovarian axis function in young female
athletes: implications and recommendations for management. Treat
Endocrinol. 4:147–154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Picard M, Wallace DC and Burelle Y: The
rise of mitochondria in medicine. Mitochondrion. 30:105–116. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie YM, Jin L, Chen XJ, He MN, Wang Y, Liu
R, Li MZ and Li XW: Quantitative changes in mitochondrial DNA copy
number in various tissues of pigs during growth. Genet Mol Res.
14:1662–1670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Murakoshi Y, Sueoka K, Takahashi K, Sato
S, Sakurai T, Tajima H and Yoshimura Y: Embryo developmental
capability and pregnancy outcome are related to the mitochondrial
DNA copy number and ooplasmic volume. J Assist Reprod Genet.
30:1367–1375. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duran HE, Simsek-Duran F, Oehninger SC,
Jones HW Jr and Castora FJ: The association of reproductive
senescence with mitochondrial quantity, function and DNA integrity
in human oocytes at different stages of maturation. Fertil Steril.
96:384–388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
May-Panloup P, Chrétien MF, Jacques C,
Vasseur C, Malthièry Y and Reynier P: Low oocyte mitochondrial DNA
content in ovarian insufficiency. Hum Reprod. 20:593–597. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dadgostar H, Razi M, Aleyasin A, Alenabi T
and Dahaghin S: The relation between athletic sports and prevalence
of amenorrhea and oligomenorrhea in Iranian female athletes. Sports
Med Arthrosc Rehabil Ther Technol. 1:162009.PubMed/NCBI
|
|
10
|
Falkenberg M, Larsson NG and Gustafsson
CM: DNA replication and transcription in mammalian mitochondria.
Annu Rev Biochem. 76:679–699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoeks J and Schrauwen P: Muscle
mitochondria and insulin resistance: A human perspective. Trends
Endocrinol Metab. 23:444–450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhuo G, Ding Y, Feng G, Yu L and Jiang Y:
Analysis of mitochondrial DNA sequence variants in patients with
polycystic ovary syndrome. Arch Gynecol Obstet. 286:653–659. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
May-Panloup P, Boucret L, Chao de la Barca
JM, Desquiret-Dumas V, Ferré-L'Hotellier V, Morinière C, Descamps
P, Procaccio V and Reynier P: Ovarian ageing: The role of
mitochondria in oocytes and follicles. Hum Reprod Update.
22:725–743. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Devine K, Mumford SL, Wu M, DeCherney AH,
Hill MJ and Propst A: Diminished ovarian reserve in the United
States assisted reproductive technology population: Diagnostic
trends among 181,536 cycles from the society for assisted
reproductive technology clinic outcomes reporting system. Fertil
Steril. 104:612–619 e613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Efremov RG, Baradaran R and Sazanov LA:
The architecture of respiratory complex I. Nature. 465:441–445.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wirth C, Brandt U, Hunte C and Zickermann
V: Structure and function of mitochondrial complex I. Biochim
Biophys Acta. 1857:902–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reed BG and Carr BR: The Normal Menstrual
Cycle and the Control of Ovulation. MDText.com, Inc.; South
Dartmouth MA: 2000
|