Introduction
Lung cancer is the most common tumor type in the
developed world and in 2012 it was the most commonly diagnosed
cancer (1.82 million) and the most common cause of cancer mortality
(1.6 million) worldwide (1,2). Lung cancer is classified into two major
types: Small-cell lung cancer (SCLC) and non-small cell lung cancer
(NSCLC). Most lung cancers are NSCLCs, which account for nearly 90%
of cases (2). At present, surgical
resection and chemotherapy are the major means of treating NSCLC.
Over the last few decades, significant advances in diagnostic and
therapeutic approaches have been made. However, >80% of NSCLC
patients cannot be treated with surgery due to the high prevalence
of metastasis (3). According to
statistics, the 5-year survival rate after lung cancer diagnosis is
only 17.7% (4). NSCLC commonly
develops resistance to radiation and chemotherapy, and patients are
often diagnosed at stages beyond surgical remedy (5). Therefore, the exploration of novel
drugs or combined chemotherapies for NSCLC treatment is urgently
required.
Fisetin (3′,4′,7-trihydroxyflavonol), a naturally
occurring flavonoid, is abundant in several fruits and vegetables,
including strawberry, apple, persimmon, grape, onion and cucumber
(6). At present, fisetin has shown
multiple biological activities including anti-proliferative
(7,8), pro-apoptotic (9–13),
neuroprotective (14) and
anti-oxidative activities (15).
Moreover, fisetin has been shown to suppress the proliferation of a
wide variety of tumor cell, including prostate cancer (16), liver cancer (17), colon cancer (18) and leukemia (19) cells, and inhibit the
mitogen-activated protein kinase (MAPK) and nuclear factor (NF)-κB
signaling pathways in various type of cancer cell, such as colon
and pancreatic cancer (14,20–24).
Fisetin was also reported to reduce the invasive and migratory
capacity of the A549 human lung cancer cell line via the
extracellular signal-regulated kinase (ERK) signaling pathway
(25). However, the precise impact
and associated molecular mechanisms of action of fisetin in NSCLCs
has remained to be fully elucidated, which was therefore the aim of
the present study. The results showed that fisetin had a
significant anti-tumor effect via the regulation of the
proliferation, apoptosis, cell cycle and invasion of human lung
cancer cells. These findings provide deep insight into the
anti-tumor mechanisms of fisetin and provided a theoretical basis
for its application in the clinical treatment of NSCLCs.
Materials and methods
Cell lines and culture
The A549 human NSCLC cell line was purchased from
the Cell Bank of Shanghai Institutes for Biological Sciences of the
Chinese Academy of Sciences (Shanghai, China) and maintained at
37°C in RMPI-1640 medium (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS), 10 U/ml penicillin, 10 Ag/ml streptomycin and 0.25 Ag/ml
amphotericin B (all from Gibco; Thermo Fisher Scientific, Inc.) in
the presence of 5% CO2. The medium was routinely changed
every 2 days. Cells in the logarithmic growth phase were used for
all experiments.
Drug treatment
Fisetin was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany; purity, >99% according to
high-performance liquid chromatography analysis). A stock solution
of fisetin was prepared in dimethyl sulfoxide (DMSO). A549 cells
were seeded in 6-well plates and incubated until cells were
attached to the wells. Subsequently, fisetin was added at final
concentrations of 10 and 40 µM, followed by incubation for the
indicated time periods (final DMSO concentration, <0.2%). Cells
treated with 0.1% DMSO were used as a negative control.
Total RNA extraction and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was isolated from A549 using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. RNA purity and integrity were
analyzed using an Agilent Bioanalyzer 2100 (Agilent Technologies,
Santa Clara, CA, USA).
Complementary DNA (cDNA) was synthesized using an
iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) following the manufacturer's instructions. All PCR was
performed using SYBR Premix ExTaq™ II kit (Takara, Otsu,
Japan). Total RNA was degenerated at 94°C for 3 min and amplified
for 40 cycles under the following conditions: 94°C for 5 sec for
denaturation, 52°C for 30 sec for annealing and 72°C for 15 sec for
elongation. Detection was set at 62°C. PCR amplifications were
performed in three duplicates for each sample. The relative RNA
expression was calculated using the 2−ΔΔCq method
(26). The specific primer sequences
are listed in Table I.
 | Table I.List of primers used for polymerase
chain reaction. |
Table I.
List of primers used for polymerase
chain reaction.
| Primer | Sequence (5′-3′) |
|---|
| MMP-2-F |
GGCCCTGTCACTCCTGAGAT |
| MMP-2-R |
GGCATCCAGGTTATCGGGGA |
| MMP-9-F |
AGGCCTCTACAGAGTCTTTG |
| MMP-9-R |
CAGTCCAACAAGAAAGGACG |
| CDKN1A-F |
CGGTGGAACTTTGACTTCGT |
| CDKN1A-R |
CAGGGCAGAGGAAGTACTGG |
| CDKN1B-F |
CAGAATCATAAGCCCCTGGA |
| CDKN1B-R |
TCTGACGAGTCAGGCATTTG |
| CDKN2D-F |
GCCTTGCAGGTCATGATGTTTGGA |
| CDKN2D-R |
ATTCAGGAGCTAGGAAGCTGACCA |
| Bcl-2-F |
TGCACCTGAGCGCCTTCAC |
| Bcl-2-R |
TAGCTGATTCGACCATTT |
| CXCR4-F |
TGACGGACAAGTACAGGCTGC |
| CXCR4-R |
CCAGAAGGGAAGCGTGATGA |
| CD44-F |
TATGACACATATTGCTTCAATGC |
| CD44-R |
GTGTACCATCACGGTTGACA |
| E-cadherin-F |
AGAACGCATTGCCACATACACTC |
| E-cadherin-R |
CATTCTGATCGGTTACCGTGATC |
| GAPDH-F |
CATCACCATCTTCCAGGAGCG |
| GAPDH-R |
TGACCTTGCCCACAGCCTT |
MTT assay
A549 cells were seeded at a density of
3×104 cells/ml in a 24-well plate, cultured for 24 h and
then treated with fisetin (0, 10, 20, 30 or 40 µM for 24, 48 or 72
h). The cell viability was analyzed using an MTT assay according to
a previously described method (27).
In brief, 1 µl/well of MTT (Sigma-Aldrich; Merck KGaA) was added,
followed by incubation at 37°C for an additional 4 h. The medium
was then removed and formazan was solubilized in isopropanol,
followed by spectrophotometric measurement of the absorbance at 563
nm. Each experiment was performed in triplicate and repeated three
times.
Cell cycle analysis
To determine the cell cycle distribution, A549 cells
treated as described above were collected, washed, suspended in PBS
and fixed in 75% ethanol. The fixed cells were stained with
propidium iodide (PI) supplemented with RNaseA (Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) and analyzed with a FACScan
flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data were
collected and analyzed using ModFit software 3.3 (BD
Biosciences).
Flow cytometric analysis of
apoptosis
For detection of apoptosis, cells were collected,
washed and suspended in cold PBS for analysis. Apoptosis was
detected using the Alexa Fluor® 647/7-AAD apoptosis kit
(BioLegend, Inc., San Diego, CA, USA) according to the
manufacturer's instructions. Apoptotic cells were then quantified
by flow cytometry (BD Biosciences).
Cell-matrix adhesion assay
For measurement of cell adhesion, cells were
pre-treated with fisetin (0, 10 or 40 µM) for 24 h and then seeded
in a 24-well plate coated with 150 µl type I collagen (10 µg/ml;
Coster, South Elgin, IL, USA) at a density of 5×104
cells/ml, followed by incubation for 30 min. Non-adherent cells
were then removed by PBS washes, and adherent cells were fixed in
ethanol. After a staining with 0.1% crystal violet, fixed cells
were lysed in 0.2% Triton X-100 and measured spectrophotometrically
at 550 nm.
Transwell invasion assay
The Transwell invasion assay was performed using the
Biocoat Matrigel Invasion Chamber (BD Biosciences) according to the
manufacturer's instructions. In brief, 4×104 cells were
plated in the upper chambers consisting of 8-mm membrane filter
inserts coated with Matrigel (BD Biosciences). The bottom chamber
contained RMPI-1640 medium with 10% FBS as an inducer of invasion.
After 24 h, cells on the upper surface were removed and those
attached to the lower side of the membrane were fixed and stained
with crystal violet prior to counting under a microscope in five
randomly selected fields.
Wound-healing assay
To determine cell motility, A549 cells
(1×105 cells/ml) were seeded in 6-well culture plates
and grown to 80–90% confluence. After aspirating the medium, the
center of the cell monolayer was scraped with a sterile
micropipette tip to create a denuded zone (gap) of constant width.
Subsequently, cellular debris was washed with PBS and the A549
cells were then exposed to various concentrations of fisetin (0, 10
and 40 µM) with serum-free medium. The wound closure was monitored
and images were captured at 0, 12, 24 and 48 h by an Olympus CKX-41
inverted microscope and an Olympus E410 camera (Olympus, Tokyo,
Japan). The cells migrated across the white lines were counted in
five random fields for each sample. Experiments were performed in
triplicate.
Western blot analysis
Western blotting was performed as described
previously (28). In brief, cells
were collected and lysed with complete cell lysis (Beyotime
Institute of Biotechnology, Inc., Haimen, China) with protease
inhibitor cocktail (Roche, Basel, Switzerland). A total of 10 µl
protein was loaded and separated by 12% SDS-PAGE, transferred onto
polyvinylidene difluoride membranes (Millipore, Bilerica, MA, USA)
and then incubated with the appropriate antibodies specific for
c-myc (#9402; 1:2,000) Cyclin D1 (#2922; 1:2,000), COX-2 (#4842;
1:2,000), phosphorylated (p)-ERK1/2 (#9101; 1:1,000), p-MAPK kinase
(MEK1; #9122; 1:1,000; all Cell Signaling Technology, Inc.,
Danvers, MA, USA and β-actin (1:3,000; Abcam, Cambridge, MA, USA)
at 4°C overnight. After washing with PBS containing 0.1% Tween-20,
the membranes were incubated with goat anti-rabbit IgG-horseradish
peroxidase secondary antibody (sc-2004; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h.
The immunoreactive bands were visualized using an enhanced
chemiluminescence system (Pierce; Thermo Fisher Scientific, Inc.)
and images were captured using a CCD camera system (Tanon,
Shanghai, China). The density of bands was measured by Image J
software 2.0 (National Institutes of Health, Bethesda, MD,
USA).
Caspase-3/9 fluorescent assay
Cells with appropriate treatment were collected and
washed with PBS and then resuspended in lysis buffer (pH 7.5; 25 mM
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 5 m M
MgCl2, 5 mM EDTA, 5 mM dithiothreitol, 2 mM
phenylmethane sulfonyl fluoride, 10 mg/ml pepstatin A and 10 mg/ml
leupeptin). Cell lysates were centrifuged at 12,000 × g at 4°C for
5 min, and supernatants containing 50 mg of protein were incubated
with 50 mM acetyl (Ac)-DEVD-7-amino-4-methylcoumarin (AMC; a
specific substrate for caspase-3) or Ac-LEHD-AMC (a specific
substrate for caspase-9; both Sigma-Aldrich; Merck Millipore) at
37°C for 1 h. The fluorescence of AMC was measured using a
spectrofluorometer (Hitachi F-4500; Hitachi, Tokyo, Japan) with
excitation at 360 nm and emission at 460 nm.
Statistical analysis
All experiments were performed independently for at
least three times. Values for each group are expressed as the mean
± standard deviation. Statistically significant differences were
calculated by using a two-tailed Student's t-test with SPSS
software (version 19.0; International Business Machines Corp.,
Armonk, NY, USA). The graphs were generated with GraphPad Prism 5.0
(GraphPad Inc., La Jolla, CA, USA).
Results
Fisetin inhibits A549 cell
proliferation
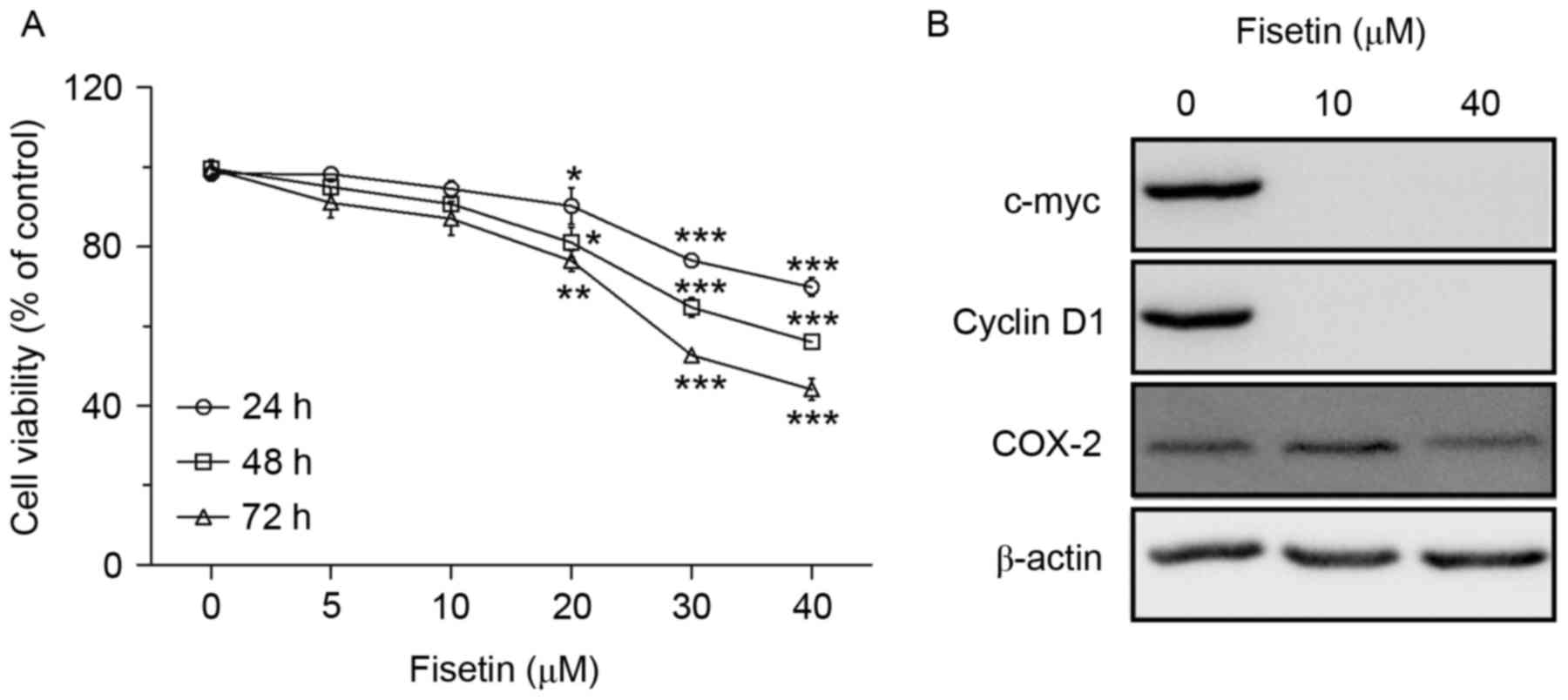
The inhibitory effects of fisetin on the growth of
the NSCLC cell line A549 were first assessed. Cells were treated
with various doses of fisetin (0, 5, 10, 20, 30 or 40 µM) for
different durations (24–72 h) and then analyzed for cell viability
by an MTT assay. As expected, the results revealed that fisetin was
effective in inhibiting the growth of A549 cells in a dose- and
time-dependent manner (Fig. 1A).
According to the growth curves, fisetin concentrations of 10 and 40
µM were used in the subsequent mechanistic experiments. The protein
expression levels of the proliferation-associated genes, cyclin D1,
c-myc and cyclooxygenase (COX)-2, were also assessed; the three
genes were downregulated by fisetin (Fig. 1B).
Fisetin causes cell cycle arrest in
A549 cells
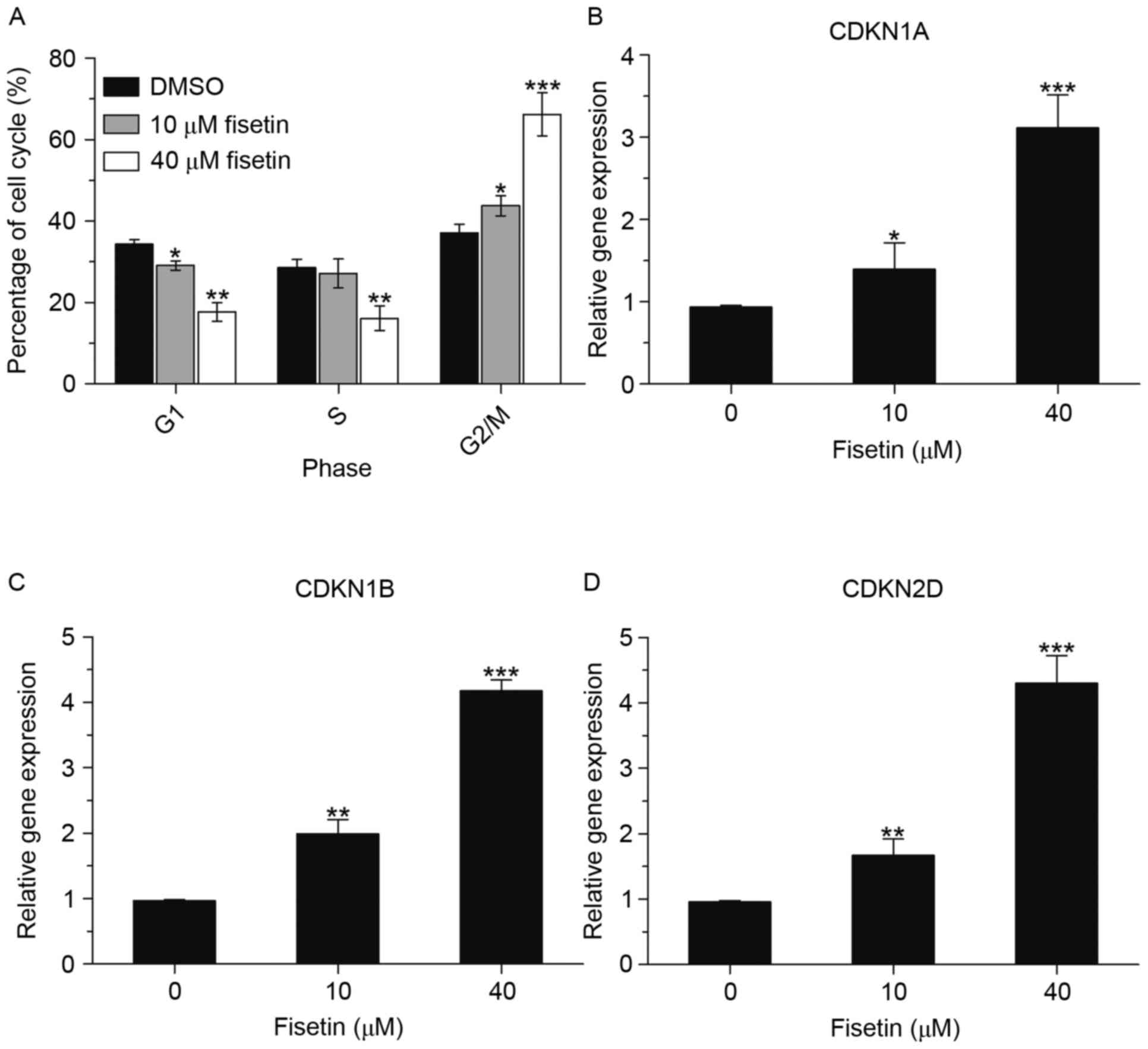
Due to the anti-proliferative effects of fisetin on
A549 cells, it was further investigated whether the growth
inhibitory effect of fisetin was mediated through cell cycle
arrest. For this purpose, the effect of fisetin on cell cycle
alterations in A549 cells was measured by flow cytometry. As shown
in Fig. 2A, fisetin caused an
accumulation of cells in G2/M phase and a reduction in G1 phase of
the cell cycle. In the 40 µM fisetin-treated group, the percentage
of cells in G1 phase decreased from 34.3 to 17.6% and the
percentage of cells in G2/M phase increased from 37.1 to 66.2%.
Furthermore, the mRNA expression levels of cell cycle-associated
genes cyclin D kinase inhibitor (CDKN) 1A, CDKN1B and CDKN2D were
significantly upregulated (Fig.
2B-D). This finding indicated that fisetin induced cell cycle
arrest in G2 phase by regulating the expression of multiple cell
cycle regulatory genes.
Fisetin induces apoptosis of A549
cells
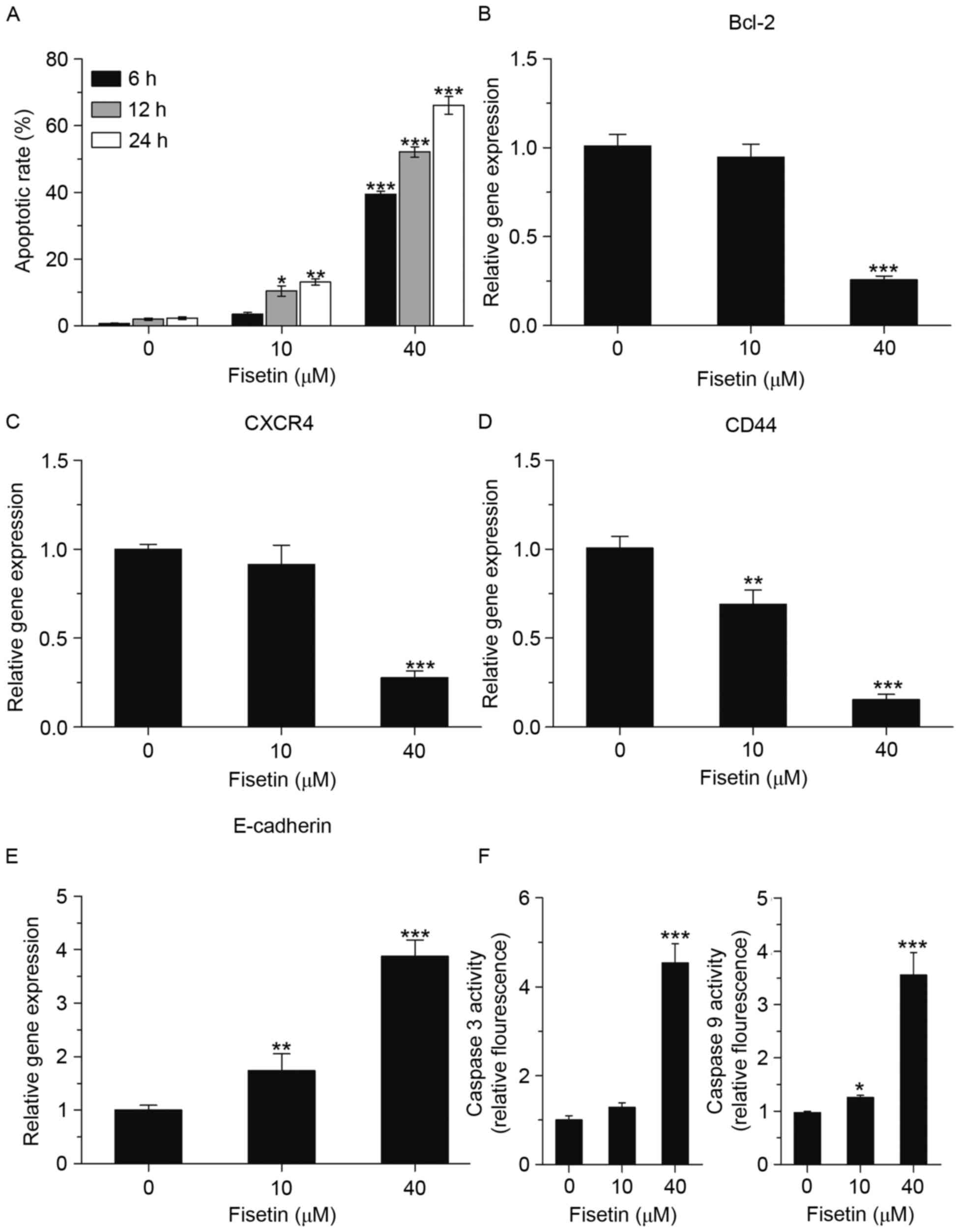
To determine whether blockage of cells in G2/M phase
by fisetin induces apoptosis of A549 cells, flow cytometric
analysis, using an Alexa Fluor 647/7-AAD apoptosis kit, was
performed. A549 cells were treated with 10 and 40 µM fisetin for 6,
12 and 24 h, and then collected for flow cytometric analysis. As
shown in Fig. 3A, the apoptotic rate
of A549 cells was markedly increased by fisetin. The mRNA
expression levels of anti-apoptotic genes (B-cell lymphoma-2, C-X-C
chemokine receptor 4, CD44 and E-cadherin) were all downregulated,
which was consistent with the increases in the apoptotic rate
(Fig. 3B-E). Moreover, the effects
of fisetin treatment on the activation of caspase-3 and −9, which
have crucial roles in apoptosis, were further investigated. Caspase
fluorescence assays were used to evaluate caspase-3 and caspase-9
activity, and the results showed that the activation of caspase-3
and caspase-9 was obviously increased by fisetin (Fig. 3F).
Fisetin suppresses cell adhesion,
invasion and migration
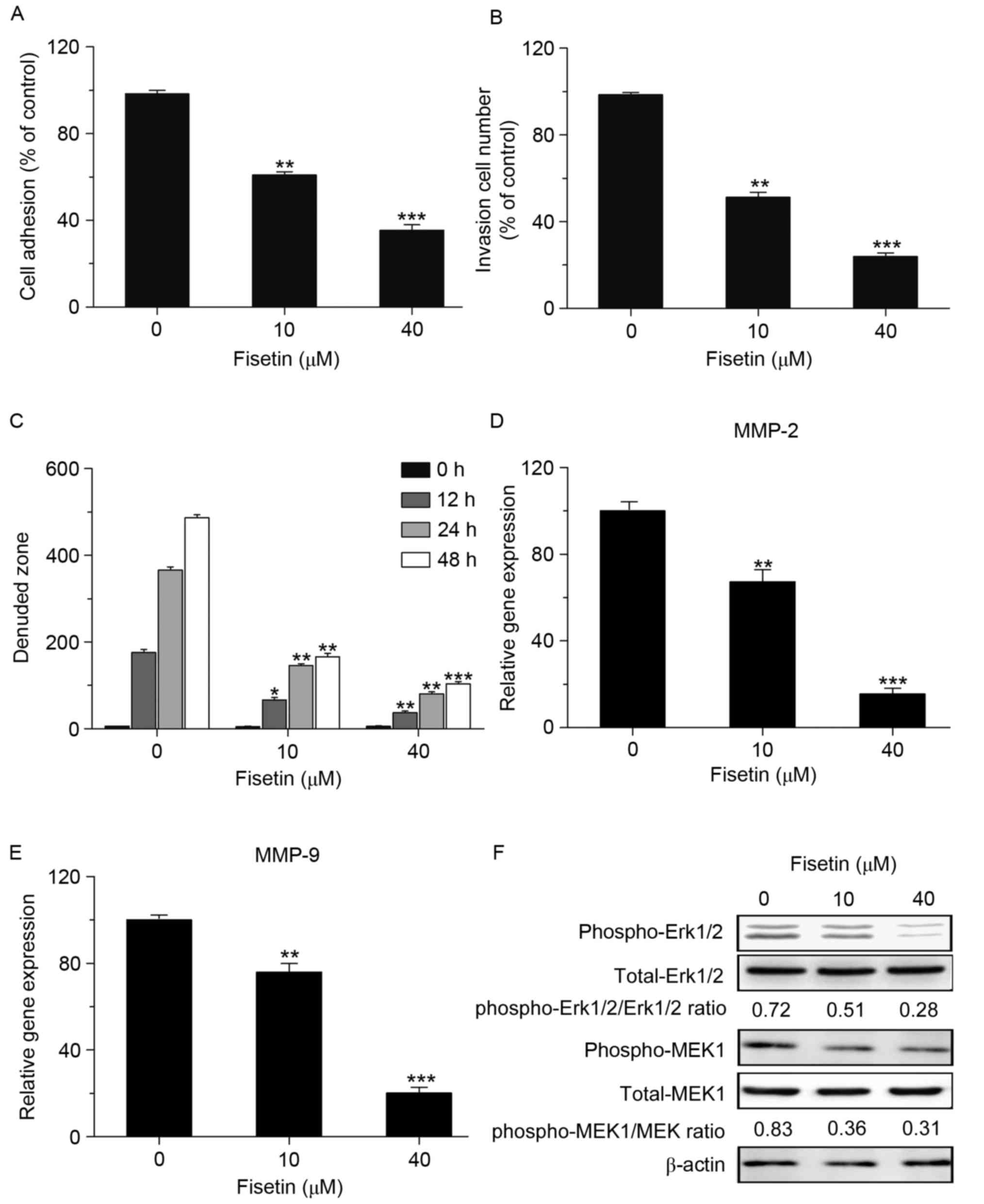
The potential anti-metastatic activity of fisetin in
A549 cells was then investigated. A549 cells were treated with 10
or 40 µM fisetin and cell adhesion, invasion and migration were
assessed. As expected, a cell-matrix adhesion assay revealed that
40 µM fisetin had a significant inhibitory effect on cell adhesion,
reducing it to 35.4% of that of the untreated control (Fig. 4A). A Transwell migration assay showed
that the invasiveness of A549 cells treated with fisetin was
suppressed compared with that of the negative control cells
(Fig. 4B). In addition, a wound
healing assay showed that the motility of A549 cells treated with
fisetin was suppressed compared with that of the negative control
cells (Fig. 4C).
The present study further examined the mRNA
expression levels of invasion-associated genes. The results were
consistent with the effect of fisetin on the adhesion, migration
and invasion of A549 cells, as the mRNA expression of matrix
metalloproteinase (MMP)-2 and MMP-9 was suppressed after fisetin
treatment. It is therefore suggested that fisetin inhibits the
adhesion, invasion and migration of lung cancer cells via
regulating the expression of associated genes, such as MMP-2 and
MMP-9 (Fig. 4D and E).
Effect of fisetin on the MAPK
signaling pathway
Since ERK1/2-associated signaling has a crucial role
in cancer biology and fisetin has been reported to inhibit melanoma
cell invasion by targeting the MAPK signaling pathway (29), the present study further investigated
its effect in lung cancer cells. First, the phosphorylation status
of ERK1/2 was determined. Western blot assays showed that p-ERK1/2
was markedly downregulated after fisetin treatment at the low as
well as the high dose (10 and 40 µM). Since activation of the ERK
pathway is regulated by MEK1, its activity of MEK1 was also
assessed. The results were similar to those on ERK, as the level of
p-MEK1 was decreased in the fisetin treatment group, which
suggested that fisetin inhibits the activation of the ERK signaling
pathway via MEK1/2 to reduce the growth and invasion in A549 cells
(Fig. 4F).
Discussion
Non-small cell lung cancer, the most common type of
cancer, remains a huge challenge to the public health sector.
Current treatments include surgical resection and chemotherapy,
which is limited by toxicity and side effects. Therefore, a growing
number of studies are aiming to find more effective anti-tumor
drugs, which may prevent carcinogenesis, curtail its progression or
even cure the disease.
Fisetin, a natural polyphenol abundantly found in
several fruits and vegetables, has attracted increasing attention
for its anti-tumor effect in certain types of cancer cell. In the
present study, the non-toxic dietary flavonoid fisetin was found to
greatly inhibit the growth of NSCLC cells in vitro via
inhibiting cell proliferation, inducing cell-cycle arrest in G2
phase, suppressing cell invasion and migration, and promoting cell
apoptosis.
The MAPK pathways serve to coordinate key cellular
processes. The ERK1/2 pathway has key roles in the regulation of
multiple biological activities and constitutive activation of the
ERK1/2 pathway contributes to tumorigenesis or cancer growth, and
increases the cell death threshold (30). ERK1/2 may be activated by MEK1
(31). The results of the present
study indicated that fisetin suppresses cell invasion by
predominantly targeting the MAPK signaling pathway as the
activation of ERK1/2 was markedly downregulated by fisetin at a
high and low dose, which is consistent with previous results
(32). Accordingly, it is suggested
that fisetin suppresses NSCLCs cell growth via the ERK1/2
pathway.
Collectively, all of these results suggested that
fisetin effectively inhibited cell proliferation and migration, and
induced apoptosis in NSCLCs. The present study demonstrated the
potential anti-cancer effects of fisetin in NSCLCs and the results
may provide a theoretical basis to support the application of
fisetin in the clinical treatment of lung cancer.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sullivan I and Planchard D: ALK inhibitors
in non-small cell lung cancer: The latest evidence and
developments. Ther Adv Med Oncol. 8:32–47. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luque M, Díez FJ and Disdier C: Optimal
sequence of tests for the mediastinal staging of non-small cell
lung cancer. BMC Med Inform Decis Mak. 16:92016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kawasaki K, Sato Y, Suzuki Y, Saito H,
Nomura Y and Yoshida Y: Prognostic factors for surgically resected
N2 non-small cell lung cancer. Ann Thorac Cardiovasc Surg.
21:217–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsu YL, Kuo PL and Lin CC: Proliferative
inhibition, cell-cycle dysregulation, and induction of apoptosis by
ursolic acid in human non-small cell lung cancer A549 cells. Life
Sci. 75:2303–2316. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arai Y, Watanabe S, Kimira M, Shimoi K,
Mochizuki R and Kinae N: Dietary intakes of flavonols, flavones and
isoflavones by Japanese women and the inverse correlation between
quercetin intake and plasma LDL cholesterol concentration. J Nutr.
130:2243–2250. 2000.PubMed/NCBI
|
|
7
|
Haddad AQ, Venkateswaran V, Viswanathan L,
Teahan SJ, Fleshner NE and Klotz LH: Novel antiproliferative
flavonoids induce cell cycle arrest in human prostate cancer cell
lines. Prostate Cancer Prostatic Dis. 9:68–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suh Y, Afaq F, Khan N, Johnson JJ, Khusro
FH and Mukhtar H: Fisetin induces autophagic cell death through
suppression of mTOR signaling pathway in prostate cancer cells.
Carcinogenesis. 31:1424–1433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pal HC, Sharma S, Elmets CA, Athar M and
Afaq F: Fisetin inhibits growth, induces G2/M arrest and apoptosis
of human epidermoid carcinoma A431 cells: Role of mitochondrial
membrane potential disruption and consequent caspases activation.
Exp Dermatol. 22:470–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Suh Y, Afaq F, Johnson JJ and Mukhtar H: A
plant flavonoid fisetin induces apoptosis in colon cancer cells by
inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways.
Carcinogenesis. 30:300–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan N, Asim M, Afaq F, Abu Zaid M and
Mukhtar H: A novel dietary flavonoid fisetin inhibits androgen
receptor signaling and tumor growth in athymic nude mice. Cancer
Res. 68:8555–8563. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khan N, Afaq F, Syed DN and Mukhtar H:
Fisetin, a novel dietary flavonoid, causes apoptosis and cell cycle
arrest in human prostate cancer LNCaP cells. Carcinogenesis.
29:1049–1056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YC, Shen SC, Lee WR, Lin HY, Ko CH,
Shih CM and Yang LL: Wogonin and fisetin induction of apoptosis
through activation of caspase 3 cascade and alternative expression
of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch
Toxicol. 76:351–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maher P, Akaishi T and Abe K: Flavonoid
fisetin promotes ERK-dependent long-term potentiation and enhances
memory. Proc Natl Acad Sci USA. 103:pp. 16568–16573. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou DX, Fukuda M, Johnson JA, Miyamori K,
Ushikai M and Fujii M: Fisetin induces transcription of NADPH:
Quinone oxidoreductase gene through an antioxidant responsive
element-involved activation. Int J Oncol. 18:1175–1179.
2001.PubMed/NCBI
|
|
16
|
Haddad AQ, Venkateswaran V, Viswanathan L,
Teahan SJ, Fleshner NE and Klotz LH: Novel antiproliferative
flavonoids induce cell cycle arrest in human prostate cancer cell
lines. Prostate Cancer Prostatic Dis. 9:68–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen YC, Shen SC, Lee WR, Lin HY, Ko CH,
Shih CM and Yang LL: Wogonin and fisetin induction of apoptosis
through activation of caspase 3 cascade and alternative expression
of p21 protein in hepatocellular carcinoma cells SK-HEP-1. Arch
Toxicol. 76:351–359. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu H, Chang DJ, Baratte B, Meijer L and
Schulze-Gahmen U: Crystal structure of a human cyclin-dependent
kinase 6 complex with a flavonol inhibitor, fisetin. J Med Chem.
48:737–743. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee WR, Shen SC, Lin HY, Hou WC, Yang LL
and Chen YC: Wogonin and fisetin induce apoptosis in human
promyeloleukemic cells, accompanied by a decrease of reactive
oxygen species, and activation of caspase 3 and Ca(2+)-dependent
endonuclease. Biochem Pharmacol. 63:225–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yao K, Zhang L, Zhang Y, Ye P and Zhu N:
The flavonoid, fisetin, inhibits UV radiation-induced oxidative
stress and the activation of NF-kappaB and MAPK signaling in human
lens epithelial cells. Mol Vis. 14:1865–1871. 2008.PubMed/NCBI
|
|
21
|
Murtaza I, Adhami VM, Hafeez BB, Saleem M
and Mukhtar H: Fisetin, a natural flavonoid, targets chemoresistant
human pancreatic cancer AsPC-1 cells through DR3-mediated
inhibition of NF-kappaB. Int J Cancer. 125:2465–2473. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim SC, Kang SH, Jeong SJ and Kim SH, Ko
HS and Kim SH: Inhibition of c-Jun N-terminal kinase and nuclear
factor κB pathways mediates fisetin-exerted anti-inflammatory
activity in lipopolysccharide-treated RAW264. 7 cells.
Immunopharmacol Immunotoxicol. 34:645–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Goh FY, Upton N, Guan S, Cheng C,
Shanmugam MK, Sethi G, Leung BP and Wong WF: Fisetin, a bioactive
flavonol, attenuates allergic airway inflammation through negative
regulation of NF-κB. Eur J Pharmacol. 679:109–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Léotoing L, Wauquier F, Guicheux J,
Miot-Noirault E, Wittrant Y and Coxam V: The polyphenol fisetin
protects bone by repressing NF-κB and MKP-1-dependent signaling
pathways in osteoclasts. PLoS One. 8:e683882013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liao YC, Shih YW, Chao CH, Lee XY and
Chiang TA: Involvement of the ERK signaling pathway in fisetin
reduces invasion and migration in the human lung cancer cell line
A549. J Agric Food Chem. 57:8933–8941. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lin R, Maeda S, Liu C, Karin M and
Edgington TS: A large noncoding RNA is a marker for murine
hepatocellular carcinomas and a spectrum of human carcinomas.
Oncogene. 26:851–858. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li S, Dong P, Wang J, Zhang J, Gu J, Wu X,
Wu W, Fei X, Zhang Z, Wang Y, et al: Icariin, a natural flavonol
glycoside, induces apoptosis in human hepatoma SMMC-7721 cells via
a ROS/JNK-dependent mitochondrial pathway. Cancer Lett.
298:222–230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pal HC, Sharma S, Strickland LR, Katiyar
SK, Ballestas ME, Athar M, Elmets CA and Afaq F: Fisetin inhibits
human melanoma cell invasion through promotion of mesenchymal to
epithelial transition and by targeting MAPK and NFκB signaling
pathways. PLoS One. 9:e863382014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ishikawa Y and Kitamura M: Dual potential
of extracellular signal-regulated kinase for the control of cell
survival. Biochem Biophys Res Commun. 264:696–701. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ellinger-Ziegelbauer H, Brown K, Kelly K
and Siebenlist U: Direct activation of the stress-activated protein
kinase (SAPK) and extracellular signal-regulated protein kinase
(ERK) pathways by an inducible mitogen-activated protein Kinase/ERK
kinase kinase 3 (MEKK) derivative. J Biol Chem. 272:2668–2674.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chou RH, Hsieh SC, Yu YL, Huang MH, Huang
YC and Hsieh YH: Fisetin inhibits migration and invasion of human
cervical cancer cells by down-regulating urokinase plasminogen
activator expression through suppressing the p38 MAPK-dependent
NF-κB signaling pathway. PLoS One. 8:e719832013. View Article : Google Scholar : PubMed/NCBI
|